Ramulus Cinnamomi extract attenuates neuroin-flammatory responses via downregulating TLR4/MyD88 signaling pathway in BV2 cells
Huan Yang, Xiao Cheng, Ying-lin Yang, Yue-hua Wang,, Guan-hua Du,
1 Beijing Key Laboratory of Drug Target Identification and Drug Screening, Institute of Materia Medica, Chinese Academy of Medical Sciences &Peking Union Medical College, Beijing, China
2 State Key Laboratory of Bioactive Substance and Function of Natural Medicines, Institute of Materia Medica, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China
How to cite this article:Yang H, Cheng X, Yang YL, Wang YH, Du GH (2017) Ramulus Cinnamomi extract attenuates neuroin-flammatory responses via downregulating TLR4/MyD88 signaling pathway in BV2 cells. Neural Regen Res 12(11):1860-1864.
Funding: This study was supported by a grant from the National Natural Science Foundation of China, No.81473383; a grant from the Medical and Health Innovation Project of Chinese Academy of Medical Sciences, No. 2016-I2M-3-007; and a grant from Key Project of New-Drugs Creation of Science and Technology of China, No. 2012ZX09103101-078 and 2017ZX09101003-003-019.
Introduction
Microglia are the tissue macrophages of the brain and are crucial for maintaining tissue homeostasis and for the scavenging of pathogens, dying cells, and molecules through microbial-associated molecular pattern receptor dependent and independent mechanisms (Sid et al., 2014). Activated microglia induced by stimuli release various pro-inflammatory factors, such as interleukin-6 (IL-6), interleukin-1β (IL-1β),and tumor necrosis factor (TNF)-α (Innamorato et al., 2008;Lu et al., 2011; Lafrenaye, 2016). The excessive production of these inflammatory mediators can act as neurotoxins and damage the brain (Chao et al., 1992; McGuire et al., 2001). Therefore, for the treatment of many neuroinflammation-mediated diseases, such as stroke, Alzheimer’s disease, and Parkinson’s disease, controlling microglial activation and inhibiting the release of pro-inflammatory factors are of great significance.
Lipopolysaccharide (LPS) stimulates the release of pro-inflammatory cytokines and nitric oxide (NO) production in BV2 microglial cells (Kang et al., 2004; Piao et al., 2004; Lyu et al., 2006; Lu et al., 2007). Toll-like receptors (TLRs) play important roles in initiating immune responses; for example,TLR4 in the immune system binds to LPS to stimulate pro-inflammatory cytokine release (Jack et al., 2005; Takeuchi and Akira, 2010). Myeloid differentiation factor 88 (MyD88) plays a crucial role in signal transduction in the TLR4 signaling pathway (O’Neill and Bowie, 2007) and it was reported that the TLR4/MyD88 signaling pathway plays an important role in neuroinflammation (Qin et al., 2013).
Ramulus Cinnamomi(RC, GuiZhi in Chinese), a traditional Chinese herb is used to treat inflammation based on its purgative, antipyretic, anti-inflammatory, and antineoplastic activities (Zheng et al., 2015; Kwon et al., 2016). However, how RC extract affects neuroinflammation in microglial cells is poorly understood. Here, the effect and possible mechanisms of RC extract against LPS-induced inflammation in BV2 microglial cells were investigated.
Materials and Methods
Preparation of RC extract
Dried RC extract was extracted from GuiZhi and identified by Professor Hai-lin Qin (Department of Phytochemistry, Institute of Materia Medica, Chinese Academy of Medical Sciences& Peking Union Medical College). In brief, dried RC (30 g)was soaked in 95% aqueous ethanol (500 mL) for 2 hours and then refluxed by water-bath heating for 1 hour. The extracts were filtered and concentrated by a rotator evaporator (Heidolph, Schwabac, Germany). Then, they were placed in a 500 mL of separatory funnel, and extracted with petroleum ether three times. The extract was stored at 4°C.
Cell culture
BV2 microglial cells, an immortalized murine microglial cell line, have been used as a valid substitute for primary microglia(Hennet al., 2009). In the current study, BV2 cells were purchased from Cell Resource Center, Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences & Peking Union Medical College (Beijing, China) and maintained in Dulbecco’s modified Eagle’s medium (Gibco BRL, Gaithersburg USA) containing 100 U/mL penicillin, 100 μg/mL streptomycin (Gibco BRL), and 10% fetal bovine serum (FBS;Gibco BRL) at 37°C in a 5% CO2humidified cell incubator.
RC extract treatment and cell viability assay
BV2 cells were cultured in a 96-well plate (2 × 104cells/well).At 70–80% confluence, BV2 cells were pre-incubated with RC extract 30 or 100 μg/mL for 1 hour and then exposed to 200 ng/mL LPS (Sigma-Aldrich, St. Louis, MO, USA) for another 24 hours. After treatment, BV2 cell morphology was observed and photographed under an optical microscope (Olympus X71, Tokyo, Japan). Cell viability was measured using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolin bromide(MTT; Sigma, St. Louis, MO, USA) (Han et al., 2013). Briefly,10 µL of the MTT solution was added to each well of the 96-well plate and incubated at 37°C for 4 hours. After removing the medium, the formazan product was dissolved in 200 µL dimethyl sulfoxide in each well. Absorbance values were measured at 560 nm with a microplate reader (Spectramax M5 microplate reader, Molecular Devices, Sunnyvale, CA, USA).Cell supernatant collection
BV2 cells were added to a 6-well plate (4 × 105cells/well). At 70–80% confluence, the medium was replaced with DMEM free of FBS and cells were pre-incubated with different concentrations of RC extract for 1 hour and then exposed to LPS(200 ng/mL) for another 24 hours. Then, the BV2 cells were collected and centrifuged at 4,000 ×gfor 5 minutes, and the supernatant was collected.
NO assay
Nitrite in the cell culture media was measured as an index of NO production using the Griess assay kit (Promega, Madison,WI, USA) (de Oliveira et al., 2014). Then, 50 μL of supernatant was mixed with the Griess regents for 10 minutes at room temperature. Absorbance values at 540 nm were measured using a microplate reader (Spectramax M5 microplate reader, Molecular Devices) and nitrite concentrations were determined by comparison to the Nitrite Standard reference curve.
Cytokine quantification by ELISA
The levels of IL-6, IL-1β and TNF-α in the supernatant were determined using ELISA kits (Genetimes, ExCell Biology,Shanghai, China) in accordance with the manufacturer’s instructions. First, the ELISA plate was coated with anti-mouse antibody. After overnight incubation at 4°C, the plates were washed three times with sterile PBS, and the uncoated sites were blocked with 200 μL of PBS containing 10% FBS for 2 hours at 37°C. After subsequent washing, 100 μL of the supernatant obtained from the culture samples was added,and the plates were incubated for 2 hours at 37°C. The plates were subsequently washed and the biotinylated anti-mouse antibody solution was added to each well except for the blank control, and incubated for 1 hour at 37°C. After incubation and additional washing, the horseradish peroxidase(HRP)-streptavidin conjugated secondary antibody was added to each well and incubated for 30 minutes at 37°C. After washing, 3,3′,5,5′-tetramethybenzidine (TMB) solution was added and incubated at room temperature, followed by the addition of stop solution to each well. The absorbance value at 450 nm was measured using a microplate reader. The cytokine concentration was determined using a standard curve.
Western blot assay

Figure 1 Effect of RC extract on cell morphology and cell viability of BV2 cells.(A) Morphology of cells treated with normal medium (a), LPS (b), LPS + RC extract 30 μg/mL (c), LPS + RC extract 100 μg/mL (d) under an optical microscope (original magnification, 40×)(B) Viability of BV2 cells determined by MTT assay. Data are expressed as the mean ± SD. Experiments were performed in triplicate. RCE 30: RC extract at 30 μg/mL; RCE 100: RC extract at 100 μg/mL; LPS: lipopolysaccharide;RC: Ramulus Cinnamomi.
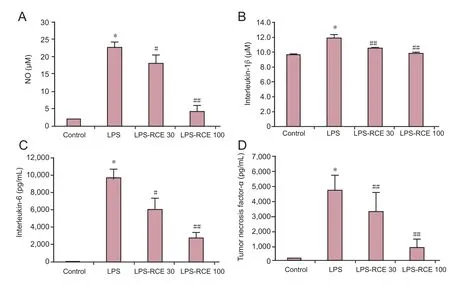
Figure 2 Effect of RC extract on NO production and pro-inflammatory factors in the supernatants of BV2 cells as detected by ELISA.(A) NO level; (B) interleukin-1β level; (C) interleukin-6 level; (D) tumor necrosis factor-α level. Data are expressed as the mean± SD. Experiments were performed in triplicate. Intergroup comparisons were conducted using one-way analysis of variance and Student-Newman-Keuls test.*P < 0.01, vs. control; #P< 0.05, ##P < 0.01, vs. LPS group. RCE 30: RC extract at 30 μg/mL; RCE 100: RC extract at 100 μg/mL; LPS:lipopolysaccharide; RC:Ramulus Cinnamomi; NO:nitric oxide.
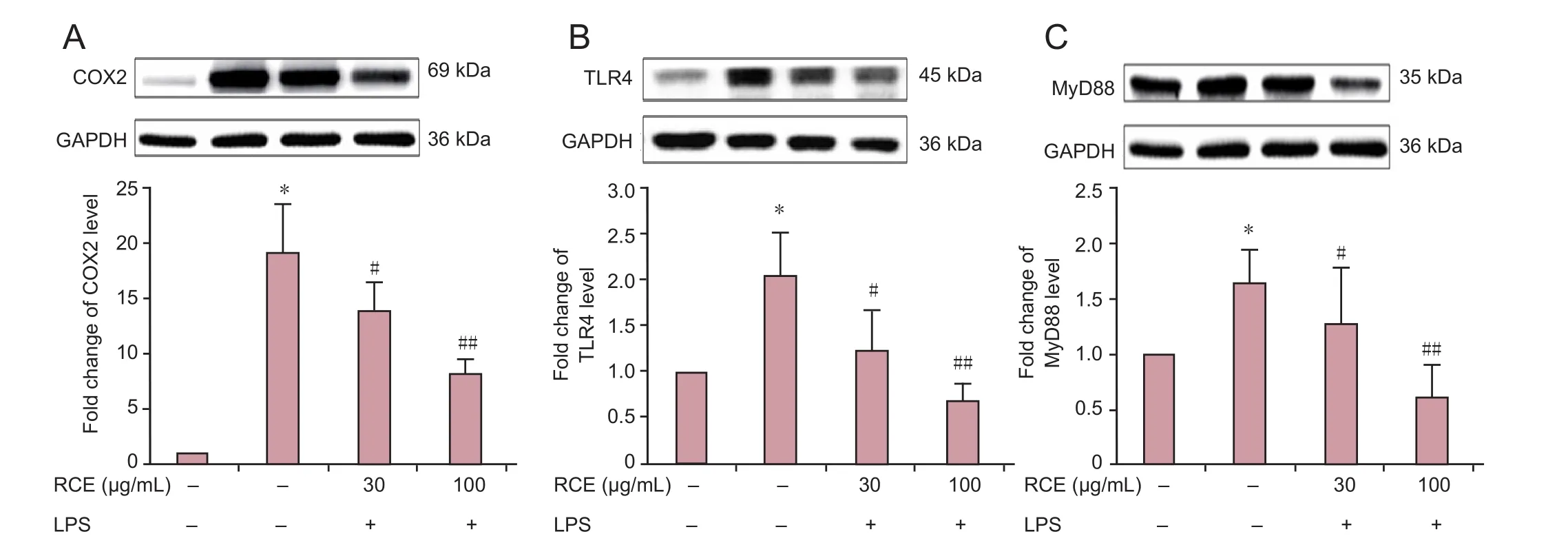
Figure 3 Effect of RC extract on the expression levels of COX2, TLR4, and MyD88 in LPS-treated BV2 cells detected by western blotting.(A–C) COX2, TLR4, and MyD88 protein expression levels. Data are expressed as the mean ± SD. Experiments were performed in triplicate. Intergroup comparisons were conducted using one-way analysis of variance and the Student-Newman-Keuls test. *P < 0.01 vs. control group; #P < 0.05,##P < 0.01, vs. LPS group. RCE 30: RC extract 30 μg/mL; RCE 100: RC extract 100 μg/mL; LPS: lipopolysaccharide; RC: Ramulus Cinnamomi; COX2:cycloxygenase-2; TLR4: Toll-like receptor 4; MyD88: myeloid differentiation factor 88; GAPDH: glyceraldehyde-3-phosphate dehydrogenase.
After treatment, the cells were lysed with Radio Immunoprecipitation Assay (RIPA) buffer in the presence of cocktail protease inhibitors (pepstatin, leupeptin, aprotinin) in an iced water bath for 30 minutes and then centrifuged at 4°C at 12,000 ×gfor 15 minutes. The supernatant was collected and the protein concentration was determined using BCA assay.Then, loading buffer was added into samples, boiled for 5 minutes and used for the following assay.
Total protein from the supernatant was separated by 10%SDS-polyacrylamide gel electrophoresis, and the protein bands were transferred to nitrocellulose membranes. The membranes were blocked by incubation with 5% bovine serum albumin(BSA) in TBS-T buffer (10 mM Tris-HCl, 150 mM NaCl, and 0.5% Tween-20) for 1 hour at room temperature and then incubated with different primary antibodies: rabbit anti-COX-2(Rt, 1:1,000, Abcam, Cambridge, UK), rabbit anti-TLR4 (Rt,1:1,000, Abcam), rabbit anti-MyD88 (Rt, 1:1,000, Abcam),and anti-GAPDH (Rt, 1:1,000, Abcam) overnight at 4°C. After washing, the membranes were incubated with HRP-conjugated rabbit anti-goat secondary antibody (1:2,000, Abcam) for 2 hours at 37°C, followed by washing. The bands were revealed using the ECL system (Beijing ComWin Biotech Co., Ltd., Beijing, China). The signal densities on the blots were measured with Gel-pro software (Molecular Imager ChemiDoc XRS+ System, Bio-Rad, CA, USA) and normalized using anti-GAPDH as an internal control (optical density detected protein/optical density internal control).
Statistical analysis
Statistical analysis was performed using GraphPad Prism 6.02(GraphPad Software Inc., CA, USA) Data are expressed as the mean ± SD. Measurement data between groups were compared using one-way analysis of variance and the Student-Newman-Keuls test.P< 0.05 was considered statistically significant.
Results
Effect of RC extract on BV2 cell viability
After treatment, BV2 cell morphology was observed under an optical microscope. As shown inFigure 1, RC extract at concentrations of 30 and 100 μg/mL had no significant effect on the viability of BV2 cells following LPS induction. Therefore,RC extract at concentrations of 30 and 100 μg/mL was used for all subsequent experiments.
RC extract reduced NO, IL-6, IL-1β, and TNF-α production in the supernatants of LPS-induced BV2 cells
As shown inFigure 2, 200 ng/mL LPS induction for 24 hours significantly increased the levels of NO, IL-1β, IL-6, and TNF-α in the supernatants of BV2 cells compared with the control group (allP< 0.01). However, after treatment with RC extract for 24 hours, both 30 and 100 μg/mL RC extract significantly decreased the levels of NO (P< 0.05), as well as IL-6, IL-1β and TNF-α (allP< 0.05) in the supernatants of LPS-induced BV2 cells.
RC extract downregulated protein expression levels of COX2, TLR4, and MyD88 in LPS-induced BV2 cells
As shown inFigure 3, western blot assay results showed that LPS stimulation significantly increased the protein expression levels of COX2, TLR4, and MyD88 in BV2 cells (allP< 0.01).However, treatment with 30 and 100 μg/mL RC extract inhibited the protein expression of COX2, TLR4, and MyD88 in LPS-induced BV2 cells (P< 0.05 orP< 0.01).
Discussion
RC is commonly used for gastritis, dyspepsia, blood circulation disturbances and inflammation (Liao et al., 2012).RC extract was reported to relax vascular smooth muscle by inhibiting Ca2+influxviaL-type Ca2+channels and inositol triphosphate-induced Ca2+release from the sarcoplasmic reticulum (Kang and Shin, 2012). Moreover, Jung et al. (2011)found that RC extract exhibited antioxidant activityin vitroand protected against gastric damagein vivoby the stimulation of mucus secretion. Hwang et al. (2009) reported that RC exhibited anti-inflammatory effects by downregulating the expression of various genes related to inflammatory responses in LPS-stimulated BV2 cells. In the current study, we report for the first time that RC extract inhibits NO, IL-6, IL-1β, and TNF-α production in BV2 microglial cells, possibly by inhibiting the TLR4/MyD88 signaling pathway.
During central nervous inflammation, levels of the inducible form of NOS are increased, and the persistent overproduction of NO is mediated by appropriate stimuli such as LPS or pro-inflammatory cytokines (MacMicking et al., 1997; Lyu et al., 2006). The overproduction of NO may induce nerve injury. Furthermore, pro-inflammatory factors (IL-1β, IL-6 and TNF-α) in the ischemic brain are upregulated from resident brain cells and infiltrating immune cells after ischemic insult (Waje-Andreassen et al., 2005; Zeng et al., 2012; Jin et al., 2013). In our study, we found that the levels of NO, IL-1β,IL-6, and TNF-α were increased after LPS induction, and that RC extract significantly reduced the LPS-induced NO, IL-6,IL-1β, and TNF-α production.
COX2, the key enzyme responsible for the synthesis of inflammation-related prostaglandin, is closely associated with chronic inflammation (Lee et al., 2002; Kim et al., 2004; Guo et al., 2006). Notably, RC extract was reported to suppress COX2 expression and decrease LPS-induced PGE2 production in RAW 264.7 macrophages (Park et al., 2005). Our results indicate that the COX2 protein expression level was also increased in BV2 microglial cells by the effect of LPS. However, RC extract treatment significantly decreased the protein expression level of COX2.
TLRs are widely expressed in a variety of immune cells and brain resident cells, such as microglia, cerebral endothelium,neurons, and astrocytes (Bsibsi et al., 2002; Jack et al., 2005;Marsh et al., 2009). Activation of TLR signaling pathways regulates cytokine and chemokine production. TLR4 is a specific pattern recognition receptor for LPS (Park et al., 2009; Takeuchi and Akira, 2010). TLR signaling involves MyD88-dependent and -independent pathways. It was reported that mice lacking either TLR2 or TLR4 were less susceptible to cerebral ischemic damage (Cao et al., 2007; Ziegler et al., 2007). Furthermore, TLR4-/-mice were protected from brain injury induced by global or permanent focal cerebral ischemia (Casoet al., 2007; Hua et al., 2007). In this study, our results indicated that RC extract treatment significantly decreased the protein expression levels of TLR4 and MyD88, suggesting that RC extract alleviates inflammation in BV2 microglial cells by inhibiting the TLR4/MyD88 signaling pathway.
In summary, RC extract effectively inhibites neuroinflammation induced by LPS in BV2 microglial cells by downregulating the TLR4/MyD88 signaling pathway. This comprehensive understanding of RC extract in nervous system will provide novel insight into the development of therapeutic approaches against neuroinflammation-mediated diseases.
Author contributions:HY, XC, and YLY performed the research and analyzed the data. HY and YHW wrote the paper. YHW and GHD designedthe research and revised the paper. All authors read and approved the final manuscript.
Con flicts of interest:None declared.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under identical terms.
Bsibsi M, Ravid R, Gveric D, van Noort JM (2002) Broad expression of Tolllike receptors in the human central nervous system. J Neuropathol Exp Neurol 61:1013-1021.
Cao CX, Yang QW, Lv FL, Cui J, Fu HB, Wang JZ (2007) Reduced cerebral ischemia-reperfusion injury in Toll-like receptor 4 deficient mice. Biochem Biophys Res Commun 353:509-514.
Chao CC, Hu S, Molitor TW, Shaskan EG, Peterson PK (1992) Activated microglia mediate neuronal cell injury via a nitric oxide mechanism. J Immunol 149:2736-2741.
de Oliveira RG, Mahon CP, Ascêncio PG, Ascêncio SD, Balogun SO, de Oliveira Martins DT (2014) Evaluation of anti-inflammatory activity of hydroethanolic extract of Dilodendron bipinnatum Radlk. J Ethnopharmacol 155:387-395.
Dugoua JJ, Seely D, Perri D, Cooley K, Forelli T, Mills E, Koren G (2007)From type 2 diabetes to antioxidant activity: a systematic review of the safety and efficacy of common and cassia cinnamon bark. Can J Physiol Pharmacol 85:837-847.
Guo JY, Huo HR, Zhao BS, Liu HB, Li LF, Ma YY, Guo SY, Jiang TL (2006)Cinnamaldehyde reduces IL-1beta-induced cyclooxygenase-2 activity in rat cerebral microvascular endothelial cells. Eur J Pharmacol 537:174-180.
Han Y, Jung HW, Bae HS, Kang JS, Park YK (2013) The extract of Cinnamomum cassia twigs inhibits adipocyte differentiation via activation of the insulin signaling pathway in 3T3-L1 preadipocytes. Pharm Biol 51:961-967.
Henn A, Lund S, Hedtjärn M, Schrattenholz A, Pörzgen P, Leist M (2009)The suitability of BV2 cells as alternative model system for primary microglia cultures or for animal experiments examining brain inflammation. ALTEX 26:83-94.
Hwang SH, Choi YG, Jeong MY, Hong YM, Lee JH, Lim S (2009) Microarray analysis of gene expression profile by treatment of Cinnamomi Ramulus in lipopolysaccharide-stimulated BV-2 cells. Gene 443:83-90.
Innamorato NG, Rojo A, García-Yagüe AJ, Yamamoto M, de Ceballos ML,Cuadrado A (2008) The transcription factor Nrf2 is a therapeutic target against brain inflammation. J Immunol 181:680-689.
Jack CS, Arbour N, Manusow J, Montgrain V, Blain M, McCrea E, Shapiro A,Antel JP (2005) TLR signaling tailors innate immune responses in human microglia and astrocytes. J Immunol 175:4320-4330.
Jin R, Liu L, Zhang S, Nanda A, Li G (2013) Role of inflammation and its mediators in acute ischemic stroke. J Cardiovasc Transl Res 6:834-851.
Jung J, Lee JH, Bae KH, Jeong CS (2011) Anti-gastric actions of eugenol and cinnamic acid isolated from Cinnamomi Ramulus. Yakugaku Zasshi 131:1103-1110.
Kang G, Kong PJ, Yuh YJ, Lim SY, Yim SV, Chun W, Kim SS (2004) Curcumin suppresses lipopolysaccharide-induced cyclooxygenase-2 expression by inhibiting activator protein 1 and nuclear factor kappab bindings in BV2 microglial cells. J Pharmacol Sci 94:325-328.
Kang YH, Shin HM (2012) Cinnamomi ramulus ethanol extract exerts vasorelaxation through inhibition of Ca influx and Ca release in rat aorta.Evid Based Complement Alternat Med 2012:513068.
Kwon OJ, Kim MY, Shin SH, Lee AR, Lee JY, Seo BI, Shin MR, Choi HG,Kim JA, Min BS, Kim GN, Noh JS, Rhee MH, Roh SS (2016) Antioxidant and Anti-inflammatory effects of Rhei Rhizoma and Coptidis Rhizoma mixture on reflux esophagitis in Rats. Evid Based Complement Alternat Med 2016:2052180.
Lafrenaye AD (2016) Physical interactions between activated microglia and injured axons: do all contacts lead to phagocytosis? Neural Regen Res 11:538-540.
Lee HS, Kim BS, Kim MK (2002) Suppression effect of Cinnamomum cassia bark-derived component on nitric oxide synthase. J Agric Food Chem 50:7700-7703.
Liao JC, Deng JS, Chiu CS, Hou WC, Huang SS, Shie PH, Huang GJ (2012)Anti-inflammatory activities of Cinnamomum cassia constituents in vitro and in vivo. Evid Based Complement Alternat Med 2012: 429320.
Lu DY, Tang CH, Liou HC, Teng CM, Jeng KC, Kuo SC, Lee FY, Fu WM(2007) YC-1 attenuates LPS-induced proinflammatory responses and activation of nuclear factor-kappaB in microglia. Br J Pharmacol 151:396-405.
Lu MJ, Zhu Y, Sun J, Yang XR (2011) Microglia mediates inflammation injury in mouse models of Parkinson’s disease. Zhongguo Zuzhi Gongcheng Yanjiu 15:1945-1948.
Lyu SA, Lee SY, Lee SJ, Son SW, Kim MO, Kim GY, Kim YH, Yoon HJ, Kim H, Park DI, Ko WS (2006) Seungma-galgeun-tang attenuates proinflammatory activities through the inhibition of NF-kappaB signal pathway in the BV-2 microglial cells. J Ethnopharmacol 107:59-66.
MacMicking J, Xie QW, Nathan C (1997) Nitric oxide and macrophage function. Annu Rev Immunol 15:323-350.
Marsh BJ, Williams-Karnesky RL, Stenzel-Poore MP (2008) Toll-like receptor signaling in endogenous neuroprotection and stroke. Neuroscience 158:1007-1020.
McGuire SO, Ling ZD, Lipton JW, Sortwell CE, Collier TJ, Carvey PM (2001)Tumor necrosis factor alpha is toxic to embryonic mesencephalic dopamine neurons. Exp Neurol 169:219-230.
Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO (2009) The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature 458:1191-1195.
Piao HZ, Jin SA, Chun HS, Lee JC, Kim WK (2004) Neuroprotective effect of wogonin: potential roles of inflammatory cytokines. Arch Pharm Res 27:930-936.
Qin X, Sun ZQ, Zhang XW, Dai XJ, Mao SS, Zhang YM (2013) TLR4 signaling is involved in the protective effect of propofol in BV2 microglia against OGD/reoxygenation. J Physiol Biochem 69:707-718
Sid B, Glorieux C, Valenzuela M, Rommelaere G, Najimi M, Dejeans N, Renard P, Verrax J, Calderon PB (2014) AICAR induces Nrf2 activation by an AMPK-independent mechanism in hepatocarcinoma cells. Biochem Pharmacol 91:168-180.
Suppapitiporn S, Kanpaksi N, Suppapitiporn S (2006) The effect of cinnamon cassia powder in type 2 diabetes mellitus. J Med Assoc Thai 89 Suppl 3:S200-205.
Takeuchi O, Akira S (2010) Pattern recognition receptors and inflammation. Cell 140:805-820.
Waje-Andreassen U, Kråkenes J, Ulvestad E, Thomassen L, Myhr KM,Aarseth J, Vedeler CA (2005) IL-6: an early marker for outcome in acute ischemic stroke. Acta Neurol Scand 111:360-365.
Zheng FH, Wei P, Huo HL, Xing XF, Chen FL, Tan XM, Luo JB (2015)Neuroprotective effect of gui zhi (ramulus cinnamomi) on ma huang-(herb ephedra-) induced toxicity in rats treated with a ma huang-gui zhi herb pair. Evid Based Complement Alternat Med 2015:913461.
Ziegler G, Harhausen D, Schepers C, Hoffmann O, Röhr C, Prinz V, König J,Lehrach H, Nietfeld W, Trendelenburg G (2007) TLR2 has a detrimental role in mouse transient focal cerebral ischemia. Biochem Biophys Res Commun 359:574-579.
- 中国神经再生研究(英文版)的其它文章
- The role of general anesthetics and the mechanisms of hippocampal and extra-hippocampal dysfunctions in the genesis of postoperative cognitive dysfunction
- Saponins from Panax japonicus attenuate age-related neuroinflammation via regulation of the mitogenactivated protein kinase and nuclear factor kappa B signaling pathways
- Taking out the garbage: cathepsin D and calcineurin in neurodegeneration
- MicroRNAs as diagnostic markers and therapeutic targets for traumatic brain injury
- Interferon regulatory factor 2 binding protein 2: a new player of the innate immune response for stroke recovery
- Endogenous retinal neural stem cell reprogramming for neuronal regeneration

