Calycosin improves cognitive function in a transgenic mouse model of Alzheimer’s disease by activating the protein kinase C pathway
Lei Song, Xiaoping Li, Xiao-xue Bai, Jian Gao, Chun-yan Wang,
1 Department of Respiratory Medicine, First Hospital of Jilin University, Changchun, Jilin Province, China
2 Department of Pediatrics, First Hospital of Jilin University, Changchun, Jilin Province, China
3 Cadre’s Ward, First Hospital of Jilin University, Changchun, Jilin Province, China
4 Department of Neurosurgery, China-Japan Union Hospital of Jilin University, Changchun, Jilin Province, China
How to cite this article:Song L, Li X, Bai XX, Gao J, Wang CY (2017) Calycosin improves cognitive function in a transgenic mouse model of Alzheimer’s disease by activating the protein kinase C pathway. Neural Regen Res 12(11):1870-1876.
Funding: This work was supported by the a grant from China Postdoctoral Science Project, No. 801161020425; and the Natural Science Foundation of China, No. 8160010172.
Introduction
Alzheimer’s disease (AD) is the most common type of senile dementia, and is one of the greatest burdens of healthcare in developed countries. Its main pathological features include profuse and widespread extracellular amyloid plaques as well as intraneuronal fibrotic tangles (Sperling et al., 2014;Ramezani et al., 2016; Zhang et al., 2016). However, the underlying cause of these pathologies remains uncertain, and there is no effective disease modifying treatment. In recent years, the main focus of research on novel pharmacotherapies of AD has been the amyloidogenic hypothesis, which posits that the accumulation of amyloid beta (Aβ) peptide is responsible for cognitive impairment and neuronal death(Nasica-Labouze et al., 2015; De Strooper and Karran, 2016;Scheltens et al., 2016). Thus, there has been an effort towards the pharmacological attenuation of Aβ production by inhibiting β and γ secretase enzymes, andviaimmunotherapy targeting existing cerebral Aβ plaques. However, to date,these approaches have proven only modestly effective (Lahiri et al., 2014; Roher et al., 2014).
Radix astragaliis a traditional Chinese herbal medicine that possesses various biological functions. Three groups of active constituents have been identified in astragalus extracts: flavonoids (which impart a yellow color to the root slice), saponins (a common constituent of plants in this family), and polysaccharides (long-chain polysaccharides with potential medicinal benefit mediated by effects on leukocytes) (Cai et al., 2016; Shahzad et al., 2016). Astragaloside IV, calycosin, and formononetin are the three main bioactive compounds that are implicated in the pharmacological activities and therapeutic efficacy of radix astragali (Zhang et al., 2016). Indeed,radix astragaliis widely used in traditional Chinese medicine, with indications for the treatment of diabetes, cardiovascular diseases, nephropathy, cancer and neuropathy (Fu et al., 2014a). The main source ofradix astragaliin China is the dried root ofAstragalus membranaceusvar.mongholicus (Bge.) Hsiao, and both cultivated and wild plants are used clinically (Li et al., 2015).
Calycosin is a typical phytoestrogen that binds to estrogen receptors to produce estrogen-like effects. It is also reported to have antioxidant, anti-osteoporosis, anti-tumor and immunomodulating activities (Zhang et al., 2015). Recently,Wang and Zhao (2016) reported that calycosin had beneficial effects on the amelioration, prevention and treatment of diabetes-associated cognitive deficits, through its effects on oxidative stress, synaptic function and the PI3K/Akt/GSK-3β pathway, all of which have likewise been implicated in AD pathology. In another study, treatment with calycosin-7-O-β-D-glucoside significantly reduced infarct volume,histological damage and blood-brain barrier defects in a rat middle cerebral artery occlusion model (Fu et al., 2014b).Furthermore, calycosin-7-O-β-D-glucoside treatment inhibited the expression of matrix metalloproteinases (MMPs),and stabilized the expression of cav-1 and tight junction proteins in microvessels isolated from the ischemic rat cortex (Fu et al., 2014b). The salutogenic mechanism is unknown, but calycosin-7-O-β-D-glucoside was reported to scavenge nitric oxide radicals, inhibit MMP-2 and MMP-9 activity, and attenuate death of brain microvascular endothelial cells (Fu et al., 2014b).
Given this background, it seems appropriate to test the effects of calycosin in a model of AD. To study the pathobiology and possible pharmacological targets of AD, various transgenic mouse models have been developed, which emulate specific aspects of AD pathology in the human brain.The double mutant APP/PS1 mouse has become a well-established model for preclinical AD research, manifesting a broad range of behavioral and pathological abnormalities,notably increased brain levels of Aβ, cerebellar dysfunction,impairment of motor coordination and deficits in learning and memory, as well as increased markers of oxidative stress,inflammation, and neuronal apoptosis, all of which clearly mimic the condition of clinical AD (Gao et al., 2015; Toba et al., 2016). Thus, this study investigated the effects of calycosin treatment on the development of pathologies in the APP/PS1 transgenic mouse model of AD.
Materials and Methods
Animals
Sixty male APP/PS1 transgenic mice aged 7–8 months old and weighing 26–28 g (Nanjing Biomedical Research Institute, Nanjing, China, license No. SYXK (Su) 2010003) were used as the experimental group, and male C57BL/6 mice (n= 36) of the same age (Animal Center, Jilin University, China) were used as controls.
The study protocol was approved by the Animal Experiment Committee of Jilin University of China (approval number: 2014–014). The experimental procedure followedthe United States National Institutes of Health Guide for the Care and Use of Laboratory Animals(NIH Publication No. 85–23,revised 1986).
Drug treatment
Mice were randomized into 8 groups (n = 12 per group) as follows.
Group I: No treatment was given to C57BL/6 male mice, and they were assessed for behavioral parameters from day 64 onwards.
Group II: Dimethyl sulfoxide (0.5%; 10 mL/kg, intraperitoneally) was administered to C57BL/6 mice from day 1 to day 70.
Group III: Calycosin (40 mg/kg, intraperitoneally, daily, Sigma-Aldrich, St. Louis, MO, USA) in dimethyl sulfoxide as described for group II above, was administered to C57BL/6 mice according to a previous study (Cheng et al., 2015).
Group IV: No treatment was given to APP/PS1 transgenic mice until day 70.
Group VIII: APP/PS1 mice were administered calycosin(40 mg/kg, intraperitoneally) from day 1 until day 70, and received additional treatment with calphostin C one hour before calycosin treatment from day 64 to day 70. Calphostin C (0.1 μg in 3 mL 0.5% dimethyl sulfoxide per mouse,Sigma-Aldrich), a selective protein kinase C (PKC) inhibitor,was administered into cerebral ventricles at the following coordinates: −2.5 mm dorsal/ventral, −1.0 mm lateral, and−0.5 mm anterior/posterior from bregma), while under anesthesia (Galeotti and Ghelardini, 2011).
Behavioral assessment
Morris water maze test
The Morris water maze test was performed to evaluate learning and memory abilities in mice. The Morris water maze consisted of a circular water tank (180 cm in diameter, 70 cm in height) filled with water at 22 ± 1°C. The pool was divided into four equal quadrants labeled north, west, south and east. A colorless escape platform (10 cm in diameter)was submerged 2 cm below the surface in the east quadrant,which was designated the target quadrant. We recorded escape latency time from day 64 to day 67 of the study. On day 68, we recorded the escape latency time, time spent in the target quadrant and number of crossings in the platform area, according to a previously defined procedure (Kummer et al., 2015).
Passive avoidance test
The passive avoidance test was performed to evaluate learning and memory abilities in mice. The experimental box used for the passive avoidance test consisted of two identical 25 × 40 × 25 cm3compartments with a metal grid floor. The compartments were separated by a guillotine door. One of the compartments was white and illuminated, while the other was black and un-illuminated. Mice were trained in the passive avoidance apparatus on day 69, and 24 hours after the acquisition conditioning session (day 70). We tested passive avoidance as the retention latency in accordance with previously published methods (Liu et al., 2014).
Collection of samples and biochemical measurement
Mice were decapitated 60 minutes after the final behavioral tests. Brains were carefully removed, and hippocampi were immediately dissected on a cold plate, weighed, and quickly homogenized in ice-cold 0.9% saline. The homogenate was centrifuged at 3,000 r/min for 10 minutes at 4°C. The supernatants were collected and stored at 4°C for assays of amyloid beta, tau protein, acetylcholine (ACE), acetylcholinesterase (AchE) activity, interleukin-1beta (IL-1β), tumor necrosis factor-alpha (TNF-α), glutathione (GSH) and malondialdehyde (MDA) measurements, all according to the manufacturer’s instructions for the respective enzyme linked immunosorbent assay kits (Abcam, Hong Kong, China).
Statistical analysis
The results were expressed as the mean ± SD. All data were analyzed using SPSS 20.0 software (IBM, Armonk, NY,USA). The data were statistically analyzed using one-way analysis of variance followed by Tukey’s multiple range test.A value ofP< 0.05 was considered statistically significant.
Results
Effect of calycosin on cognitive function of APP/PS1 mice Morris water maze
The control mice had a significant reduction in escape latency time on day 67 compared with day 64 of the study, which suggests normal spatial learning capacity. However, the APP/PS1 mice had a significantly higher escape latency time on day 67 compared with control mice, suggesting impaired learning. The daily administration of calycosin significantly and dose-dependently reduced the escape latency time of APP/PS1 mice on day 67, which was significantly abolished by co-treatment with calphostin C (Figure 1A). Furthermore, on day 68 of the study, control mice spent a significantly longer time in the target quadrant compared with the other quadrants, which suggest normal memory. APP/PS1 mice showed a significant reduction in the time spent in the target quadrant on day 68 and performed fewer crossings in the platform area compared with control mice, indicating impaired memory. The daily administration of calycosin significantly and dose-dependently increased the time spent in the target quadrant on day 68 and the number of crossings in the platform area of APP/PS1 mice, which were significantly abolished by treatment with calphostin C (Figure 1A−C).
Passive avoidance test
All mice showed a normal mean initial latency on day 32,but significant changes were observed in retention latency on day 33, as assessed in the passive avoidance test. APP/PS1 mice had a significant decrease in retention latency on day 70 of the study compared with control mice, which suggests impaired cognitive function. The daily administration of calycosin significantly and dose-dependently increased the retention latency of APP/PS1 mice, which was significantly abolished by co-treatment with calphostin C (Figure 2).
Effect of calycosin on the levels of hippocampal amyloid beta, tau protein, inflammation, and oxidative stress in APP/PS1 mice
APP/PS1 mice showed a significant increase in hippocampal amyloid beta, tau protein, AChE activity, ACh, TNF-α,IL-1β and MDA along with a reduction in GSH levels. The daily administration of calycosin significantly and dose-dependently decreased the hippocampal amyloid beta, tau protein, TNF-α, IL-1β, AChE and MDA levels, but increased Ach and GSH levels in APP/PS1 mice (P< 0.05 orP< 0.01).These beneficial effects of calycosin were significantly abolished by the treatment of calphostin C (Figures 3–6).
Discussion
APP/PS1 mice in this study had significantly impaired learning and memory characterized by increased levels of hippocampal amyloid beta, tau protein, pro-inflammatory mediators (IL-1β and TNF-α), and oxidative stress (increased TBARS and decreased GSH) compared with age-matched C57BL/6 mice. The daily administration of calycosin at three different doses significantly and dose-dependently corrected the impaired spatial learning and memory, and normalized the levels of hippocampal amyloid beta, tau protein, inflammation and oxidative stress in APP/PS1 animals. These bene ficial effects of calycosin in APP/PS1 animals were significantly abolished by the co-administration of a selective inhibitor of PKC, calphostin C, suggesting the neuroprotective effects of calycosin were dependent on activation of the PKC pathway.
APP/PS1 mice are a widely used transgenic model of AD to research the mechanistic processes involved in AD.It was reported that 39 species of Aβ, of both murine and human origin, were detectable in APP/PS1 mouse brain,demonstrating a higher abundance than other widely used transgenic models of AD such as Tg2576 (Allue et al., 2016).This supports the use of APP/PS1 to study disease-altering treatments for AD. The amyloid-beta hypothesis remains the most widely accepted notion to explain the induction of impaired learning, memory and the cardinal neuropathological features of AD. A vast array of possible pathways has been reported for the induction of AD by Aβ. Bian and colleagues recently reported evidence for increased intracerebral levels of Tau and phosphorylated Tau, along with increased neuronal death, and altered morphology in the hippocampus of APP/PS1 mice, which were associated with learning and memory deficits (Bian et al., 2016). This suggests that tau-pathology is initiated by Aβ. Higher levels of pro-inflammatory cytokines and inducible nitric oxide synthase along with reduced anti-inflammatory cytokines and arginase-1 have been found in the brain of APP/PS1 animals, suggesting the activation of pro-inflammatory pathways (Wan et al., 2016).In addition, Tian and colleagues observed a reduction of superoxide dismutase activity along with increased levels of MDA in APP/PS1 mice compared with wild-type C57 mice,which suggests higher levels of oxidative stress (Tian et al.,2016). Thus, our results with APP/PS1 mice are consistent with previously published reports.
We found that the daily administration of calycosin to APP/PS1 mice corrected their impaired learning and memory, and reduced the hippocampal levels of Aβ, tau protein,and oxidative stress markers and inflammatory cytokine levels. Among theradix astragaliconstituents, calycosin, a typical phytoestrogen, induces estrogen-like effects. In addition,calycosin was reported to have antioxidant, anti-osteoporosis, anti-tumor and immunomodulating activities (Zhang et al., 2015). The potential pharmaceutical properties of calycosin for the treatment of tumors, inflammation, stroke,and cardiovascular diseases have gained increasing attention in recent years (Wang et al., 2014; Cheng et al., 2015; Su et al., 2016; Zhao et al., 2016). Indeed, the potential use of calycosin to treat diseases is attributed to its isoflavonoid and phytoestrogenic properties. The beneficial effects of calycosin most likely result from its interaction with endoplasmic reticulum receptors on the cell membrane, and modulation of the mitogen-activated protein kinase signaling pathway(Gao et al., 2014).
Wang and Zhao (2016) reported that calycosin ameliorated and prevented diabetes-associated cognitive deficits,oxidative stress, and tau phosphorylation (Wang and Zhao,2016); however, no other study has reported the effects of calycosin in learning, memory and dementia models, even thoughradix astragaliis considered a possible treatment for vascular dementia (Man et al., 2012). A mixture ofradix astragaliwith other herbs (Buyuan Congnaodecoction) has been used to improve learning and memory in a rat model of AD, while reducing hippocampal Aβ accumulation (Chen et al., 2012), and remains the only study of the potential utility of calycosin, an important constituent ofradix astragali, to rescue behavioral and neurochemical pathology in a transgenic model of AD.
As noted above, the glycoside of calycosin was reported to significantly reduce infarct volume, histological damage and blood-brain-barrier permeability in a middle cerebral artery occlusion stroke model, and the glycoside remarkably inhibited the expression and activities of MMPs, nitric oxide and stabilized the expression of cav-1 and tight junction proteins in microvessels isolated from ischemic rat cortex(Fu et al., 2014b). Calycosin treatment decreased the expression of synapsin and post-synaptic density protein, as well as brain-derived neurotrophic factor, in diabetic rats (Wang and Zhao, 2016). Calycosin was also reported to downregulate the expression levels of NFATc1 and c-Fos by suppressing the activation of nuclear factor-κB and mitogen-activated protein kinases (Quan et al., 2015) and to reduce neutrophil in filtration and myeloperoxidase levels in myocardial infarction (Cheng et al., 2015). These studies indicate the anti-inflammatory potential of calycosin.
The proinflammatory cytokines, TNF-α and IL-1β, are thought to be involved in the progression of AD pathology(Quintanilla et al., 2012). The present study showed that calycosin normalized the levels of TNF-α and IL-1β in the hippocampus of AD model mice, suggesting the rescue of functions by attenuating inflammatory signaling. Free radicals are the products of normal cellular metabolism and are involved in the development of AD (Quintanilla et al., 2012).The increased production of free radicals and decreased endogenous antioxidant systems may accelerate membrane phospholipid breakdown, leading to lipid peroxidation and cellular dysfunction. In the present study, increased MDA and decreased GSH levels were found in the hippocampus of APP/PS1 mice and these were reversed by calycosin to potentially rescue lipid peroxidation in the AD model hippocampus. Similarly, calycosin treatment was reported to decrease MDA levels, and increase SOD levels and GSHPx activity in the hippocampus of diabetic animals (Wang and Zhao, 2016). The glycoside of calycosin significantly increased the activities of antioxidant enzymes, scavenged reactive oxygen species and reduced MDA production during thioacetamide-induced oxidative stress in BRL-3A cells (Jian et al., 2015), suggesting free radical scavenging and anti-oxidative stress effects of calycosin. Here, we showed that prolonged treatment with calycosin rescued spatial memory and normalized a whole range of neuropathological markers in the APP/PS1 mouse model of AD. PKC, a family of protein kinase enzymes involved in controlling the function of other proteins through phosphorylation of these proteins, plays a key role in modulating signal transduction cascades (Newton and Brognard, 2017). A previous study demonstrated that PKC signaling is upstream of the mitogen-activated protein kinase pathway, which promotes Aβ degradation and reduces the accumulation of Aβ in the brain (Cheng et al., 2016).In this study, we found the beneficial effect of calycosin on APP/PS1 animals was significantly abolished by the co-administration of a selective PKC inhibitor, calphostin C, indicating calycosin may provide benefit to AD mice specifically through the PKC pathway.
Taken together, we conclude that APP/PS1 mice have impaired learning and memory along with increased hippocampal Aβ, tau protein, proinflammatory mediators and oxidative stress. The administration of calycosin significantly and dose-dependently corrected all of these markers in APP/PS1 mice. These beneficial effects of calycosin were significantly abolished by the administration of PKC inhibitor, calphostin C. Thus, calycosin, an active constituent ofradix astragali, may represent a novel therapeutic agent for the treatment of AD. Further research should be targeted towards the understanding of its various pharmacologicalmechanisms, which might be involved in the beneficial effects of calycosin in AD.
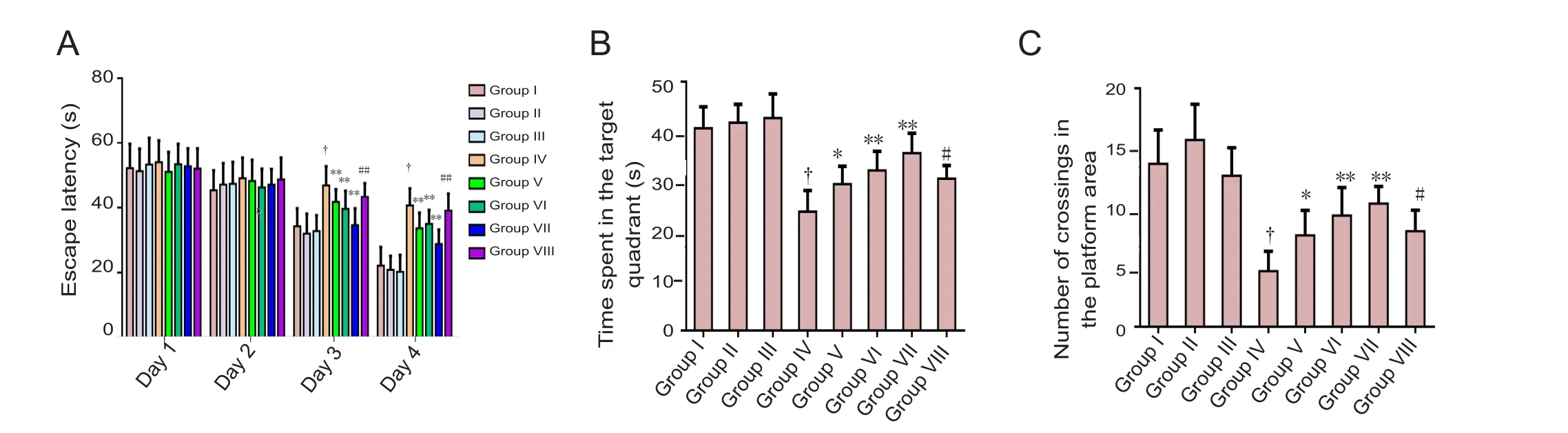
Figure 1 Effect of calycosin on learning and memory abilities of APP/PS1 mice assessed by the Morris water maze.(A) Escape latency time during an acquisition trial in the Morris water maze. (B) Mean time spent in the target quadrant on day 68 during a retrieval trial in the Morris water maze. (C) Number of platform crossings in the target quadrant on day 68 during a retrieval trial in the Morris water maze. Results are expressed as the mean ± SD (n = 12; one-way analysis of variance followed by Tukey’s multiple range test). *P < 0.05, **P< 0.01, vs. group IV; #P < 0.05, ##P < 0.01, vs. group VII; †P < 0.01, vs. groups I, II and III. Group I: Untreated C57BL/6 mice; group II: C57BL/6 with daily vehicle; group III: C57BL/6 mice treated with calycosin 50 mg/kg; group IV: APP/PS1 mice; groups V, VI, VII: APP/PS1 mice treated with calycosin 10, 20 and 30 mg/kg daily, respectively; group VIII: calycosin 40 mg/kg + calphostin C (a selective protein kinase C inhibitor, 0.1 μg in 3 μL 0.5% dimethyl sulfoxide per mouse). S: Second.

Figure 3 Effect of calycosin on the levels of hippocampal amyloid beta (Aβ) and tau protein levels assessed by enzyme linked immunosorbent assay.
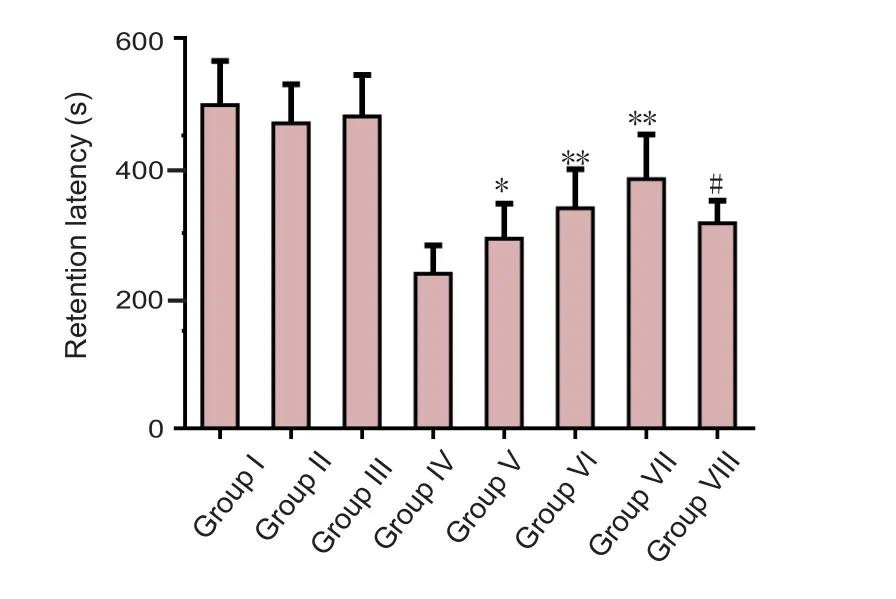
Figure 2 Effect of calycosin on the cognitive function of APP/PS1 mice assessed by the passive avoidance test.Results are expressed as the mean ± SD (n = 12; one-way analysis of variance followed by Tukey’s multiple range test). *P < 0.05, **P < 0.01,vs. group IV; #P < 0.01, vs. group VII. Group I: Untreated C57BL/6 mice; group II: C57BL/6 with daily vehicle; group III: C57BL/6 mice treated with calycosin 50 mg/kg; group IV: APP/PS1 mice; groups V,VI, VII: APP/PS1 mice treated with calycosin 10, 20 and 30 mg/kg daily, respectively; group VIII: calycosin 40 mg/kg + calphostin C (a selective protein kinase C inhibitor, 0.1 μg in 3 μL 0.5% dimethyl sulfoxide per mouse). S: Second.

Figure 4 Effect of calycosin on the hippocampal cholinergic dysfunction in APP/PS1 mice assessed by enzyme linked immunosorbent assay.(A) Levels of hippocampal acetylcholine and (B) acetylcholinesterase activity. Results are expressed as the mean ± SD (n = 12; one-way analysis of variance followed by Tukey’s multiple range test). *P < 0.05, **P < 0.01, vs. group IV; #P < 0.05, ##P < 0.01, vs. group VII. Group I: Untreated C57BL/6 mice; group II: C57BL/6 with daily vehicle; group III: C57BL/6 mice treated with calycosin 50 mg/kg; group IV: APP/PS1 mice; groups V, VI, VII: APP/PS1 mice treated with calycosin 10, 20 and 30 mg/kg daily, respectively; group VIII: calycosin 40 mg/kg + calphostin C (a selective protein kinase C inhibitor, 0.1 μg in 3 μL 0.5% dimethyl sulfoxide per mouse).

Figure 5 Effect of calycosin on hippocampal inflammatory factor in APP/PS1 mice assessed by enzyme linked immunosorbent assay.(A) Levels of interleukin-1 (IL-1) protein and (B) levels of tumor necrosis factor-alpha (TNF-α) protein. Results are expressed as the mean ± SD (n= 12; one-way analysis of variance followed by Tukey’s multiple range test). *P < 0.05, **P < 0.01, vs. group IV; ##P < 0.01, vs. group VII. Group I:Untreated C57BL/6 mice; group II: C57BL/6 with daily vehicle; group III: C57BL/6 mice treated with calycosin 50 mg/kg; group IV: APP/PS1 mice;groups V, VI, VII: APP/PS1 mice treated with calycosin 10, 20 and 30 mg/kg daily, respectively; group VIII: calycosin 40 mg/kg + calphostin C (a selective protein kinase C inhibitor, 0.1 μg in 3 μL 0.5% dimethyl sulfoxide per mouse).

Figure 6 Effect of calycosin on hippocampal oxidative stress in APP/PS1 mice assessed by enzyme linked immunosorbent assay.(A) Levels of hippocampal malondialdehyde (MDA) and (B) levels of hippocampal glutathione (GSH). **P < 0.01, vs. group IV; #P < 0.05, vs. group VII. Results are expressed as the mean ± SD (n = 12; one-way analysis of variance followed by Tukey’s multiple range test). Group I: Untreated C57BL/6 mice; group II: C57BL/6 mice with daily vehicle; group III: C57BL/6 mice treated with calycosin 50 mg/kg; group IV: APP/PS1 mice;groups V, VI, VII: APP/PS1 mice treated with calycosin 10, 20 and 30 mg/kg daily, respectively; group VIII: calycosin 40 mg/kg + calphostin C (a selective protein kinase C inhibitor, 0.1 μg in 3 μL 0.5% dimethyl sulfoxide per mouse).
Author contributions:We declare that this work was done by the authors named in this article and all liabilities pertaining to claims relating to the content of this article will be borne by the authors. LS and CYW conceived and designed the study. JG and XPL collected the data. XXB analyzed the data. All authors approved the final version of the paper.
Conflicts of interest:None declared.
Research ethics:The study protocol was approved by the Animal Experimentation Committee of Jilin University of China (approval number: 2014-014). The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1986).
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under identical terms.
Allue JA, Sarasa L, Izco M, Perez-Grijalba V, Fandos N, Pascual-Lucas M,Ogueta S, Pesini P, Sarasa M (2016) Outstanding phenotypic differences in the profile of amyloid-beta between Tg2576 and APPswe/PS1dE9 transgenic mouse models of Alzheimer’s disease. J Alzheimers Dis 53:773-785.
Bian H, Bian W, Lin X, Ma Z, Chen W, Pu Y (2016) RNA interference silencing of glycogen synthase kinase 3beta inhibites tau phosphorylation in mice with Alzheimer disease. Neurochem Res 41:2470-2480.
Cai YM, Zhang Y, Zhang PB, Zhen LM, Sun XJ, Wang ZL, Xu RY, Xue RL(2016) Neuroprotective effect of Shenqi Fuzheng injection pretreatment in aged rats with cerebral ischemia/reperfusion injury. Neural Regen Res 11:94-100.
Chen M, Wang J, Ming C (2012) Buyuan Congnao decoction decreases hippocampal beta-amyloid expression in a rat model of Alzheimer’s disease. Neural Regen Res 7:664-668.
Cheng XD, Gu JF, Yuan JR, Feng L, Jia XB (2016) Suppression of A549 cell proliferation and metastasis by calycosin via inhibition of the PKC-alpha/ERK1/2 pathway: an in vitro investigation. Mol Med Rep 13:3709-3710.
Cheng Y, Zhao J, Tse HF, Le XC, Rong J (2015) Plant natural products calycosin and gallic acid synergistically attenuate neutrophil infiltration and subsequent injury in isoproterenol-induced myocardial infarction:a possible role for leukotriene B4 12-hydroxydehydrogenase? Oxid Med Cell Longev 2015:434052.
De Strooper B, Karran E (2016) The cellular phase of alzheimer’s disease.Cell 164:603-615.
Fu S, Gu Y, Jiang JQ, Chen X, Xu M, Chen X, Shen J (2014) Calycosin-7-O-beta-D-glucoside regulates nitric oxide /caveolin-1/matrix metalloproteinases pathway and protects blood-brain barrier integrity in experimental cerebral ischemia-reperfusion injury. J Ethnopharmacol 155:692-701.
Galeotti N, Ghelardini C (2011) Antidepressant phenotype by inhibiting the phospholipase Cbeta(1) -protein kinase Cgamma pathway in the forced swim test. Neuropharmacology 60:937-943.
Gao J, Liu ZJ, Chen T, Zhao D (2014) Pharmaceutical properties of calycosin, the major bioactive isoflavonoid in the dry root extract of Radix astragali. Pharm Biol 52:1217-1222.
Gao Y, Hu YZ, Li RS, Han ZT, Geng Y, Xia Z, Du WJ, Liu LX, Zhang HH,Wang LN (2015) Cattle encephalon glycoside and ignotin injection improves cognitive impairment in APPswe/PS1dE9 mice used as multitarget anti-Alzheimer’s drug candidates. Neuropsychiatr Dis Treat 11:537-548.
Jian L, Xin L, Yufang M, Yifan H (2015) Protective effect of calycosin-7-O-beta-D-glucopyranoside against oxidative stress of BRL-3A cells induced by thioacetamide. Pharmacogn Mag 11:524-532.
Kummer MP, Schwarzenberger R, Sayah-Jeanne S, Dubernet M, Walczak R, Hum DW, Schwartz S, Axt D, Heneka MT (2015) Pan-PPAR modulation effectively protects APP/PS1 mice from amyloid deposition and cognitive deficits. Mol Neurobiol 51:661-671.
Lahiri DK, Maloney B, Long JM, Greig NH (2014) Lessons from a BACE1 inhibitor trial: of f-site but not offbase. Alzheimers Dement 10:S411-419.
Li AP, Li ZY, Sun HF, Li K, Qin XM, Du GH (2015) Comparison of two different astragali radix by a (1)H NMR-based metabolomic approach. J Proteome Res 14:2005-2016.
Liu MY, Wang S, Yao WF, Zhang ZJ, Zhong X, Sha L, He M, Zheng ZH,Wei MJ (2014) Memantine improves spatial learning and memory impairments by regulating NGF signaling in APP/PS1 transgenic mice.Neuroscience 273:141-151.
Man SC, Chan KW, Lu JH, Durairajan SS, Liu LF, Li M (2012) Systematic review on the efficacy and safety of herbal medicines for vascular dementia. Evid Based Complement Alternat Med 2012:426215.
Nasica-Labouze J et al. (2015) Amyloid beta protein and alzheimer’s disease: when computer simulations complement experimental studies.Chem Rev 115:3518-3563.
Newton AC, Brognard J (2017) Reversing the paradigm: protein kinase c as a tumor suppressor. Trends Pharmacol Sci 38:438-447.
Quan GH, Wang H, Cao J, Zhang Y, Wu D, Peng Q, Liu N, Sun WC (2015)Calycosin Suppresses RANKL-mediated osteoclastogenesis through inhibition of MAPKs and NF-kappaB. Int J Mol Sci 16:29496-29507.
Quintanilla RA, Orellana JA, von Bernhardi R (2012) Understanding risk factors for Alzheimer’s disease: interplay of neuroinflammation, connexin-based communication and oxidative stress. Arch Med Res 43:632-644.
Ramezani M, Darbandi N, Khodagholi F, Hashemi A (2016) Myricetin protects hippocampal CA3 pyramidal neurons and improves learning and memory impairments in rats with Alzheimer’s disease. Neural Regen Res 11:1976-1980.
Roher AE, Maarouf CL, Kokjohn TA, Whiteside CM, Kalback WM, Serrano G, Belden C, Liebsack C, Jacobson SA, Sabbagh MN, Beach TG (2014)Neuropathological and biochemical assessments of an Alzheimer’s disease patient treated with the gamma-secretase inhibitor semagacestat.Am J Neurodegener Dis 3:115-133.
Scheltens P, Blennow K, Breteler MM, de Strooper B, Frisoni GB, Salloway S,Van der Flier WM (2016) Alzheimer’s disease. Lancet 388:505-517.
Sperling R, Mormino E, Johnson K (2014) The evolution of preclinical Alzheimer’s disease: implications for prevention trials. Neuron 84:608-622.
Su X, Huang Q, Chen J, Wang M, Pan H, Wang R, Zhou H, Zhou Z, Liu J,Yang F, Li T, Liu L (2016) Calycosin suppresses expression of pro-inflammatory cytokines via the activation of p62/Nrf2-linked heme oxygenase 1 in rheumatoid arthritis synovial fibroblasts. Pharmacol Res 113:695-704.
Tian X, Ji C, Luo Y, Yang Y, Kuang S, Mai S, Ma J, Yang J (2016) PGE2-EP3 signaling pathway contributes to protective effects of misoprostol on cerebral injury in APP/PS1 mice. Oncotarget 7:25304-25314.
Toba J, Nikkuni M, Ishizeki M, Yoshii A, Watamura N, Inoue T, Ohshima T (2016) PPARgamma agonist pioglitazone improves cerebellar dysfunction at pre-Abeta deposition stage in APPswe/PS1dE9 Alzheimer’s disease model mice. Biochem Biophys Res Commun 473:1039-1044.
Wan W, Zhang C, Danielsen M, Li Q, Chen W, Chan Y, Li Y (2016) EGb761 improves cognitive function and regulates inflammatory responses in the APP/PS1 mouse. Exp Gerontol 81:92-100.
Wang X, Zhao L (2016) Calycosin ameliorates diabetes-induced cognitive impairments in rats by reducing oxidative stress via the PI3K/Akt/GSK-3beta signaling pathway. Biochem Biophys Res Commun 473:428-434.
Wang Y, Dong X, Li Z, Wang W, Tian J, Chen J (2014) Downregulated RASD1 and upregulated miR-375 are involved in protective effects of calycosin on cerebral ischemia/reperfusion rats. J Neurol Sci 339:144-148.
Zhang DQ, Wang HB, Wang SF, Wang DQ (2015) Research achievements on biological activities of calycosin. Zhongguo Zhong Yao Za Zhi 40:4339-4345.
Zhang G, Ou R, Li F, Wu J, Zheng L, Tong Y, Liu Y, Liu Z, Lu L (2016)Regulation of drug-metabolizing enzymes and efflux transporters by Astragali radix decoction and its main bioactive compounds: Implication for clinical drug-drug interactions. J Ethnopharmacol 180:104-113.
Zhang S, Huang XY, Liu S, Li YJ, Zhao JC (2016) Effects of amyloid-beta 25-35 on expression of synapse-associated proteins in PC12 neurons Effects of amyloid-beta 25-35 on expression of synapse-associated proteins in PC12 neurons. Zhongguo Zuzhi Gongcheng Yanjiu 20:224-229.
Zhao X, Li X, Ren Q, Tian J, Chen J (2016) Calycosin induces apoptosis in colorectal cancer cells, through modulating the ERbeta/MiR-95 and IGF-1R, PI3K/Akt signaling pathways. Gene 591:123-128.
- 中国神经再生研究(英文版)的其它文章
- The role of general anesthetics and the mechanisms of hippocampal and extra-hippocampal dysfunctions in the genesis of postoperative cognitive dysfunction
- Saponins from Panax japonicus attenuate age-related neuroinflammation via regulation of the mitogenactivated protein kinase and nuclear factor kappa B signaling pathways
- Taking out the garbage: cathepsin D and calcineurin in neurodegeneration
- MicroRNAs as diagnostic markers and therapeutic targets for traumatic brain injury
- Interferon regulatory factor 2 binding protein 2: a new player of the innate immune response for stroke recovery
- Endogenous retinal neural stem cell reprogramming for neuronal regeneration

