植物还原法制备钯纳米单质的还原率研究
贺媛媛,傅吉全
(北京服装学院材料科学与工程学院,北京 100029)
植物还原法制备钯纳米单质的还原率研究
贺媛媛,傅吉全*
(北京服装学院材料科学与工程学院,北京 100029)
以PdCl2为钯前驱体、银杏叶提取液为还原剂,采用植物还原法将Pd2+从PdCl2溶液中还原出来,制备钯纳米单质;采用等离子体发射光谱分析法(ICP-AES)测定反应溶液中残留的Pd2+浓度,计算反应的还原率;采用正交实验探讨PdCl2浓度、反应温度、反应时间及银杏叶提取液体积对还原率的影响。结果表明,钯纳米单质最佳制备条件为:PdCl2浓度0.005 mol·L-1、反应温度80 ℃、反应时间24 h、银杏叶提取液体积60 mL,在此条件下,反应的平均还原率为91.74%。证实,可以用ICP-AES对植物还原法进行定量研究。
植物还原法;钯纳米单质;正交实验;还原率
Palladium(Pd),a noble metal,has attracted much attention due to its unique small size effect[1].The catalytic potential of Pd nanoparticles(NPs) has been applied in many areas,including organic synthesis,catalytic degradation,chemical/biological sensors,hydrogen generation/storage,methane combustion,supercapacitors,and lithium ion batteries[2-5].In the past,Pd NPs have been prepared by a variety of physical and chemical methods,which require sophisticated equipment and excess chemicals,and easily cause environment contamination[6].The increased demand for preparation of Pd NPs and the emerging economic and environmental issues make people aware of the need to develop a simple,environmentally friendly,and cost-effective method.In this case,the biological processes using plants,microorganisms,enzymes,and biochemicals have been used to prepare Pd NPs as green substitutes.Among these methods,plant reduction is a method for the preparation of metal NPs under mild conditions using plant materials,which has unique properties and many advantages,including extensive availability,sustainability,chemical function,biocompatibility,and biodegradability[7].In addition to eco-friendly,the method can also control the morphology of NPs better,and provide a variety of precursor selections as reducing agents.In the biosynthesis of plant extracts,the biochemical components of leaves,flowers,bark,and roots,such as reducing sugars,terpenoids,flavonoids,swan glycosides,polyols,and amino acids,are involved in the reduction of metal ions into zero valent metals and nucleation processes,and they are treated as NPs with different sizes and shapes[8].
For Pd NPs,the plant reduction process involves the introduction of biogenic substances into Pd2+solution leading to the reduction of Pd0and stabilizes the NPs with controlled morphological and structural characteristics.The preparation of Pd NPs by a plant reduction method is shown in Tab.1.

Tab.1 The preparation of Pd NPs by a plant reduction method
As shown in Tab.1,the size of the obtained Pd NPs is in the range of 3-100 nm,the products are stable and difficult to aggregate.In the above reports,the prepared Pd NPs were characterized by X-ray diffraction(XRD),scanning electron microscope(SEM) or transmission electron microscopy(TEM),and they focused on qualitative analysis,but quantitative analysis was not performed.At present,only Li[16]had reported the measurement of reduction rate of this method,but there was no in-depth study on the influencing factors of reduction rate.
In this study,using PdCl2as a palladium precursor,Ginkgobilobaleaves extract as a reducing-agent,we prepared palladium nano-element through reducing Pd2+from PdCl2solution by a plant reduction method.We measured the residual Pd2+concentration in the reaction solution by inductively coupled plasma atomic emission spectroscopy(ICP-AES),and calculated the reduction rate of the reaction.Moreover,we investigated the effects of PdCl2concentration,reaction temperature,reaction time,and volume ofGinkgobilobaleaves extract on the reduction rate by an orthogonal experiment.
1 Experiment
1.1 Materials and reagents
Gingkgobilobaleaves were picked by ourselves.
PdCl2was supplied by Tianjin Guangfu Fine Chemical Research Institute.Anhydrous ethanol,hydrochloric acid(HCl),and nitric acid(HNO3) were got from Beijing Chemical Plant.All reagents used in this experiment were of analytical grade.Experimental water was deionized(DI) water.
1.2 Experimental method
Ginkgobilobaleaves dry powder preparation:the collectedGinkgobilobaleaves were cleaned and dried,then placed in the oven at 80 ℃ for 12 h.Finally the leaves were crushed with high-speed universal crusher,and then sieved.The size of the particles was around 100 mesh,and the powders were stored in the fresh bag at room temperature for use in the future.
Ginkgobilobaleaves extract preparation:weighting 5 g ofGinkgobilobaleaves dry powders in a conical flask,then 100 mL of DI water and 150 mL of anhydrous ethanol were added.The solution was boiled at 60 ℃ for 24 h.After it cooled down,the insoluble matter was removed by suction filtration,and the resulting supernatant was 20 g·L-1ofGinkgobilobaleaves extract.
Pd nano-element preparation:20 g·L-1ofGinkgobilobaleaves extract was used as the reducing-agent.20 mL,40 mL,and 60 mLGinkgobilobaleaves extract were mixed with 0.01 mol·L-1,0.005 mol·L-1,and 0.001 mol·L-1PdCl2solution,respectively.Then the reaction carried out in a water bath shaker at a temperature range of 40-80 ℃.
1.3 Characterization of Pd nano-element
1.3.1 XRD analysis
XRD(D8 advance model) was used for qualitative analysis of Pd nano-element.XRD is widely used in the phase analysis of catalyst,crystal structure analysis,and composition analysis.By XRD analysis,the formation of Pd nano-element single crystal can be verified.The reaction solution was heated in a crucible,and after the liquid was completely evaporated the black material obtained was used to prepare a test sample for XRD characterization and analysis.
1.3.2 TEM analysis
The Pd nano-element particle size and distribution were observed by TEM(Tecnai F30) directly.The Pd nano-element samples were dispersed in the ethanol solution and then the solution was dropped onto a copper grid for TEM.The size,morphology,distribution,and crystallinity of Pd nano-element were observed.
1.4 Calculation of Pd nano-element reduction rate
The quantitative analysis of prepared gold(Au) and silver(Ag) NPs by a plant reduction method can be studied by UV-Vis spectroscopy,because the UV-Vis spectra of the NPs have a significant surface plasmon resonance absorption peak(SPR peak),and the position,half height,and peak intensity of the absorption peak are strongly related to particle size,particle size distribution,and product concentration[17].However,there is no obvious SPR peak in Pd NPs and few quantitative studies on the preparation of Pd nano-element by a plant reduction method have been reported.In this paper,we used ICP-AES(CIROSEOP 120-800 nm) to quantitatively analyze Pd nano-element,measured the residual Pd2+concentration in the reaction solution and calculated the corresponding reduction rate.The specific operations were as follows:
(1)Preparation of the samples for standard curve:0.01 g PdCl2powders were measured accurately and added in the beaker.After dissolved completely,the solution was transferred to 50 mL volumetric flask.5 mL of the solution was accurately quantified from the volumetric flask,then 5 mL of 0.5% HNO3was added,finally 10 mL of 100 mg·L-1PdCl2solution was prepared.The PdCl2standard solutions with concentration gradient of 1 mg·L-1,2 mg·L-1,3 mg·L-1,4 mg·L-1,5 mg·L-1were prepared in the same way using 0.5% HNO3again.
(2) Preparation of the test sample:10 mL of Pd sol was put in a centrifuge tube and centrifuged for 10 min at 10 000 r·min-1.The appropriate supernatant was taken and added in another centrifuge tube,then it was diluted with 0.5% HNO3twice to 5 mg·L-1level,and finally the residual Pd2+concentration in the solution was measured by ICP-AES.
The reduction rate(Q) of Pd is calculated by the following equation,wherenis the concentration of PdCl2precursor(mmol·L-1),M is the atomic weight(g·mol-1) of Pd,Nis the dilution factor,cfis the residual Pd2+concentration(mg·L-1).
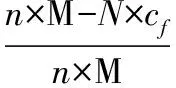
1.5 Design of orthogonal experiment
In the preparation of Pd nano-element by a plant reduction method,many factors affect the reduction rate.In order to investigate the influencing factors comprehensively,PdCl2concentration,reaction temperature,reaction time,and volume ofGinkgobilobaleaves extract were selected as four factors.Through an orthogonal experiment method,we could explore the effects of these factors on the reduction rate,and determine a relatively high reduction rate of the process.
2 Results and discussion
2.1 Quantitative analysis of Pd nano-element
ICP-AES was used for quantitative analysis.The residual Pd2+concentration in the reaction solution was measured,and the reduction rate of the reaction was calculated.The preparation conditions were optimized according to aL9(34) orthogonal experiment at the level of 4 factors and 3 levels.The results were shown in Tab.2.

Tab.2 Results of orthogonal experiment
As shown in Tab.2,the maximum reduction rate was 97.94% and the minimum reduction rate was 65.47%;with the increase of reaction temperature,reaction time,and volume ofGinkgobilobaleaves extract,the reduction rate increased.The reason could be that the higher temperature leaded to the faster movement rate of Pd2+in the reaction solution,it was easier to collide with the effective reduction groups inGinkgobilobaleaves extract,then the reduction reaction was accelerated,and the reduction rate increased;with the increase of volume ofGinkgobilobaleaves extract,the plant components that acted as reducing agents in solution increased,and more Pd2+were reduced,which resulted in an increase of the reduction rate;with the extension of reaction time,more and more effective reduction groups were exposed,so that the reducing-agent increased and the reduction rate increased as well.As reported in the previous paper[18],the reduction rate was not directly proportional to the precursor concentration,and the reaction rate increased with the increase of concentration;in contrast,when the concentration increased to a certain value,it would gradually inhibit the reduction reaction to generate Pd NPs.
The results of the orthogonal experiment showed that the order of the factors that affect the reduction rate was:B>C>D>A,that was reaction temperature had the greatest effect on the reduction rate,reaction time had the second effect,and volume ofGinkgobilobaleaves extract and PdCl2concentration had little effect.Considering the change of A,B,C,and D factors in 3 levels,the optimal conditions were A2B3C3D3,that was PdCl2concentration was 0.005 mol·L-1,reaction temperature was 80 ℃,reaction time was 24 h,and volume ofGinkgobilobaleaves extract was 60 mL.
The residual Pd2+concentration was measured by ICP-AES.The results showed that the reduction rate was 92.49% and 90.98%,respectively,and the average value was 91.74%.
The reduction rate was not the maximum,and the reason could be that before each experiment the standard solution for standard line was not re-prepared.It also could result from the influence of the interaction of each factor in the orthogonal experiment.
2.2 Qualitative analysis of Pd nano-element
2.2.1 XRD analysis(Fig.1)
As shown in Fig.1,the XRD pattern of the product showed four obvious diffraction peaks(1,2,3,and 4),which appeared at 2θ=40.5°,47.0°,66.5°,and 81.5°,respectively.Compared with Pd standard XRD pattern,the four peaks were the characteristic peaks of Pd element,and corresponded to(111),(200),(220),and(311) crystal planes of elemental Pd crystals,respectively,which proved the formation of Pd element.
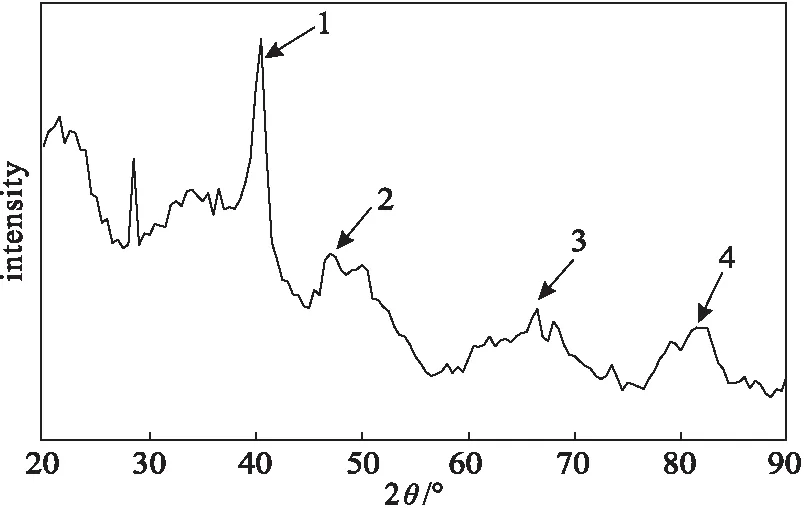
Fig.1 XRD pattern of product
2.2.2 TEM analysis(Fig.2)
The morphology of Pd NPs was observed from low magnification to high magnification.As shown in

Fig.2 TEM images of product 1(a,b) and product 2(c,d)
Fig.2a,although the particle size of Pd NPs was various,the overall distribution was relatively uniform and only a small amount of Pd NPs aggregated.As shown in Fig.2b,some Pd NPs were large and some were small.The size difference was large and the shape of Pd NPs were spherical.As shown in Fig.2c,the produced Pd NPs appeared in a rod-like structure,and a larger flattened crystal face of the particles with a relatively regular shape was amplified and obtained Fig.2d.From the high resolution lattice images above the square(square) and lower left(rod) NPs,it could be seen that there was a clear spacing between the crystal faces and two crystals formed.Through the TEM images of product 1 and product 2,the products prepared under different reduction conditions have different morphologies,which are worthy of further study.
3 Conclusion
Pd nano-element was prepared by reduction volume ofGinkgobilobaleaves extract successfully.The residual Pd2+concentration in the reaction solution was measured by ICP-AES.Then the reduction rate was calculated to complete the quantitative analysis.The optimal preparation conditions were obtained by an orthogonal experiment as follows:PdCl2concentration was 0.005 mol·L-1,reaction temperature was 80 ℃,reaction time was 24 h,volume ofGinkgobilobaleaves extract was 60 mL.The corresponding average reduction rate was 91.74%.The reason of the deviation might be the interaction between the factors.The influencing factors of reduction rate were as follows:reaction temperature>reaction time>volume ofGinkgobilobaleaves extract>PdCl2concentration,that is,reaction temperature had the greatest effect on the reduction rate,reaction time was the second,and volume ofGinkgobilobaleaves extract and PdCl2concentration had little effect on the reduction rate.
[1] VISHNUKUMAR P,VIVEKANANDHAN S,MUTHURAMKUMAR S.Plant-mediated biogenic synthesis of palladium nanoparticles:recent trends and emerging opportunities[J].ChemBioEng Reviews,2017,4(1):18-36.
[2] LEE S,LEE B,MEHMOOD F,et al.Oxidative decomposition of methanol on subnanometer palladium clusters:the effect of catalyst size and support composition[J].The Journal of Physical Chemistry C,2010,144(23):10342-10348.
[3] ZHANG Y,XIANG Q,XU J Q,et al.Self-assemblies of Pd nanoparticles on the surfaces of single crystal ZnO nanowires for chemical sensors with enhanced performances[J].Journal of Materials Chemistry,2009,19(27):4701-4706.
[4] ZENG Q,CHENG J S,LIU X F,et al.Palladium nanoparticle/chitosan-grafted graphene nanocomposites for construction of a glucose biosensor[J].Biosensors & Bioelectronics,2011,26(8):3456-3463.
[5] STROBEL R,GRUNWAIDT J D,CAMENZIND A,et al.Flame-made alumina supported Pd-Pt nanoparticles:structural properties and catalytic behavior in methane combustion[J].Catalysis Letters,2005,104(1/2):9-16.
[6] KANCHANA A,DEVARAJAN S,AYYAPPAN S R.Green synthesis and characterization of palladium nanoparticles and its conjugates fromSolanumtrilobatumleaf extract[J].Nano-Micro Letters,2010,2(3):169-176.
[7] MOHAMMADINEJAD R,KARIMI S,IRAVANI S,et al.Plant-derived nanostructures:types and applications[J].Green Chemistry,2016,18(1):20-52.
[8] DAUTHAL P,MUKHOPADHYAY M.Biosynthesis of palladium nanoparticles usingDelonixregialeaf extract and its catalytic activity for nitro-aromatics hydrogenation[J].Industrial & Engineering Chemistry Research,2013,52(51):18131-18139.
[9] NADAGOUDA M N,VARMA R S.Green synthesis of silver and palladium nanoparticles at room temperature using coffee and tea extract[J].Green Chemistry,2008,10(8):859-862.
[10] SATHISHKUMAR M,SNEHA K,KWAK I S,et al.Phyto-crystallization of palladium through reduction process usingCinnamomzeylanicumbark extract[J].Journal of Hazardous Materials,2009,171(1/2/3):400-404.
[11] JIA L S,ZHANG Q,LI Q B,et al.The biosynthesis of palladium nanoparticles by antioxidants inGardeniajasminoidesEllis:long lifetime nanocatalysts forp-nitrotoluene hydrogenation[J].Nanotechnology,2009,20(38):385601-385610.
[12] YANG X,LI Q B,WANG H X,et al.Green synthesis of palladium nanoparticles using broth ofCinnamomumcamphoraleaf[J].Journal of Nanoparticle Research,2010,12(5):1589-1598.
[13] SHENY D S,PHILIP D,MATHEW J.Rapid green synthesis of palladium nanoparticles using the dried leaf ofAnacardiumoccidentale[J].Spectrochimica Acta Part A:Molecular and Biomolecular Spectroscopy,2012,91:35-38.
[14] PETLA R K,VIVEKANANDHAN S,MISRA M,et al.Soybean(Glycinemax) leaf extract based green synthesis of palladium nanoparticle[J].Journal of Biomaterials and Nanobiotechnology,2012,3:14-19.
[15] MOHAM K K,MANDAL B K,SIVA K K,et al.Biobased green method to synthesise palladium and iron nanoparticles usingTerminaliachebulaaqueous extract[J].Spectrochimica Acta Part A:Molecular and Biomolecular Spectroscopy,2013,102:128-133.
[16] 李学亮.基于生物还原的钯纳米颗粒的可控合成[D].厦门:厦门大学,2013.
[17] 王文塔.还原制备金/银纳米颗粒的生物质筛选及还原成分的初步探索[D].厦门:厦门大学,2009.
[18] 王易展.基于植物还原法的金纳米颗粒的可控合成及其分离纯化初探[D].厦门:厦门大学,2012.
O614.82
A
1672-5425(2017)12-0053-06

