MAPKs and acetyl-CoA are associated with Curvularia lunata pathogenicity and toxin production in maize
NI Xuan, GAO Jin-xin, YU Chuan-jin, WANG Meng, SUN Jia-nan, LI Ya-qian, CHEN Jie
School of Agriculture and Biology, Shanghai Jiao Tong University/State Key Laboratory of Microbial Metabolism, Shanghai Jiao Tong University/Key Laboratory of Urban Agriculture (South), Ministry of Agriculture, Shanghai 200240, P.R.China
RESEARCH ARTICLE
MAPKs and acetyl-CoA are associated with Curvularia lunata pathogenicity and toxin production in maize
NI Xuan, GAO Jin-xin, YU Chuan-jin, WANG Meng, SUN Jia-nan, LI Ya-qian, CHEN Jie
School of Agriculture and Biology, Shanghai Jiao Tong University/State Key Laboratory of Microbial Metabolism, Shanghai Jiao Tong University/Key Laboratory of Urban Agriculture (South), Ministry of Agriculture, Shanghai 200240, P.R.China
Mitogen-activated protein kinase (MAPK) cascades play an important role in extracellular signal transduction and are involved in the pathogenicity of fungal pathogens to host plants. In Curvularia lunata, the roles of two MAPK genes, Clk1 and Clm1,have already been studied. Clk1 is involved in conidia formation and pathogenicity, and Clm1 is closely related to pathogen cell wall formation and pathogenicity to maize leaves. In this study, a third C. lunata MAPK gene, Clh1, which is homologous to hog1, was successfully cloned. We found that a Clh1 deletion mutant had lower intracellular glycerol accumulation than the wild-type stain and was unable to grow normally under osmotic stress conditions. Furthermore, the deletion mutants of three C. lunata MAPK genes (Clk1, Clm1 and Clh1) had lower levels of acetyl-CoA, which is an important intermediate product in the synthesis of melanin and furan toxin, and down-regulated expression of pathogenicity-associated genes.Furthermore, pathogenicity and the ability to produce toxin were restored after adding acetyl-CoA to the culture medium,suggesting that acetyl-CoA is closely involved in the pathogen MAPK signaling pathway.
Curvularia lunata, MAP kinase, acetyl-CoA, pathology
1. Introduction
Curvularia lunata (Wakker) Boed, is a fungal pathogen of maize and belongs to Deuteromycotina. Curvularia leaf spot is the most important maize disease in China and caused a great loss in maize production in northern China in the 1990s (Rizner et al. 2003). Although it has been shown that melanin and furan toxin are the most important factors affecting the pathogenicity of C. lunata (Liu et al. 2009; Gao et al. 2014), less research has focused on identifying the genes that are responsible for their production.
Mitogen-activated protein kinase (MAPK) cascades play an important role in regulating the pathogenicity of phytopathogenic fungi (Moriwaki et al. 2007; Ding et al.2009). Changes in signals have been closely linked to pathogen virulence factors, stress response, nutrient metabolism, differentiation and cell survival (Bardwell 2007;McClean et al. 2007). The Fus3/Kss1 homologous gene Pmk1 controls the formation of melanized appressoria and plant infection in Magnaporthe grisea (Sesma and Osbourn 2004). The Slt2 homologous gene, Mgslt2, is sensitive to glucanase and several fungicides, and in Mycosphaerella graminicola Mgslt2 gene deletion results in the formation of unmelanized colonies with short aerial hyphae (Mehrabi et al. 2006). And the Hog1 homologous gene cpmk1 in Cryphonectria parasitica is involved in osmosensitivity, and deletion of this gene causes poor conidiation, a reduction in laccase and cryparin production and, most signi ficantly,an obvious decline in pathogenicity to chestnut trees (Park et al. 2004). In our previous research, we identi fied Clk1,the Fus3/Kss1 homologous gene, and Clm1, the Slt2 homologous gene, in C. lunata and showed that Clk1 is involved in conidia formation and pathogenicity, and Clm1 is involved in pathogen cell wall formation and pathogenicity to maize leaves (Wang et al. 2011; Gao et al. 2014). Therefore,C. lunata MAPKs play a signi ficant role in the generation of melanin and virulence.
Acetyl coenzyme A (acetyl-CoA) is generated by the β-oxidation of fatty acids, and acetyl-CoA originating from peroxisomal fatty acid β-oxidation is considered to be essential for the biosynthesis of melanin in appressorial cells. In C. orbiculare, the melanin biosynthetic pathway starts with the synthesis of 1,3,6,8-T4HN from malonyl-CoA(Asakura et al. 2012), which is derived from acetyl-CoA, and melanin synthesis in the appressoria is largely dependent upon acetyl-CoA originating from MFE1-dependent fatty acid β-oxidation (Wang et al. 2007). The generation of acetyl-CoA in M. grisea is required for completion of the prepenetration phase of host plant infection (Bhadauria et al. 2012). These findings suggest that acetyl-CoA and β-oxidation are essential to virulence and melanin synthesis and that defects in the production of acetyl-CoA would affect the pathogenicity of C. lunata. However, so far there is no evidence that directly links acetyl-CoA level to the generation of C. lunata virulence factors.
Based on previous work, it has been hypothesized that there is some relationship between MAPK signaling and acetyl-CoA in building-up C. lunata pathogenicity. In order to understand this relationship, we conducted a series of gene knockout experiments to uncover the contribution of acetyl-CoA to the expression of Clh1, toxin production and pathogenicity in C. lunata.
2. Materials and methods
2.1. Fungal strains, plasmid, bacterial strain and host plant
C. lunata strain CX-3 is a wild-type strain that causes typical leaf spots in maize. The ΔClk1 and ΔClm1 strains have deletions in the C. lunata Clk1 and Clm1 MAPK genes,respectively (Wang and Chen 2011; Gao et al. 2013). The plasmid 1300qh, which carries a hygromycin B resistance gene selective marker, was used for gene disruption.Escherichia coli DH5α maintained in Luria-Bertani medium was used for cloning and propagating the plasmid. Huangzao 4 maize was used as the host plant for pathogenicity assays.
2.2. DNA/RNA extraction and cDNA synthesis
To obtain mycelia for DNA and RNA extraction, C. lunata was cultured in potato-dextrose medium with shaking at 120 r min–1for 72 h at 28°C, and then filtered through two layers of sterile gauze, washed with sterilized Mili-Q water,and ground in liquid nitrogen. Genomic DNA was isolated using the CTAB method (Sambrook et al. 1983). Total RNA was extracted using the RNAprep Pure Plant Kit (TianGen Biotech (Beijing) Co., Ltd., China). The concentration of total RNA was determined using the NANODROP2000 (Thermo Scienti fic, USA). First-strand cDNA was synthesized using the PrimeScriptTMRT Reagent Kit according to the manufacturer’s instructions (TaKaRa, Japan).
2.3. Cloning and disruption of the Clh1 gene
All primers used in this study (Table 1) were designed using Primer 5.0 and synthesized by Biosune Biotech (Shanghai,China). The whole sequences of C. lunata were obtained.The full-length Clh1 gene sequence, including the introns,was obtained by Blastn searches against GenBank. The 660-bp up-stream flanking sequence, Clh1_u, of Clh1 was ampli fied with the Clh1_u_f/Clh1_u_r primer pair from C. lunata CX-3 genomic DNA, and the 874-bp down-stream flanking sequence, Clh1_d, was ampli fied with the Clh1_d_f and Clh1_d_r primer pair (Fig. 1-A). The PCR products were con firmed by DNA sequencing.
To generate the Clh1 disruption construct, the One Step Cloning Kit (QcbioScience&Technologies Co., Shanghai)was used (Fig. 2). The up- and down-stream sequences of the Clh1 gene were inserted into the 1300qh vector to generate the plasmid 1300qh::Clh1. The final disruption construct was transformed into E. coli DH5α competent cells.
2.4. Transformation
Preparation of E. coli DH5α competent cells and transformation of C. lunata were performed according to previously described methods (Liu et al. 2010). Transfor mants were selected on CYA medium supplemented with 300 μg mL-1hygromycin B. To identify the gene-deletion mutants, the Hyg_F/Hyg_R and Clh1_4F/Clh1_4R primer pairs were used.
2.5. Southern blot analysis
Southern blot analysis was performed on genomic DNA isolated from C. lunata wild-type strain CX-3 and putative Clh1 disruption mutants. DNA aliquots of 5 μg were digested with HindIII at 37°C for 24 h, separated by agarose gel electrophoresis. To generate the probe, the regioncorresponding to the Clh1 deletion was ampli fied from CX-3 genomic DNA with the primer pair Clh1_F7/Clh1_R7 and was labeled with digoxigenin. Probes were hybridized with the digested DNA bound to filters.
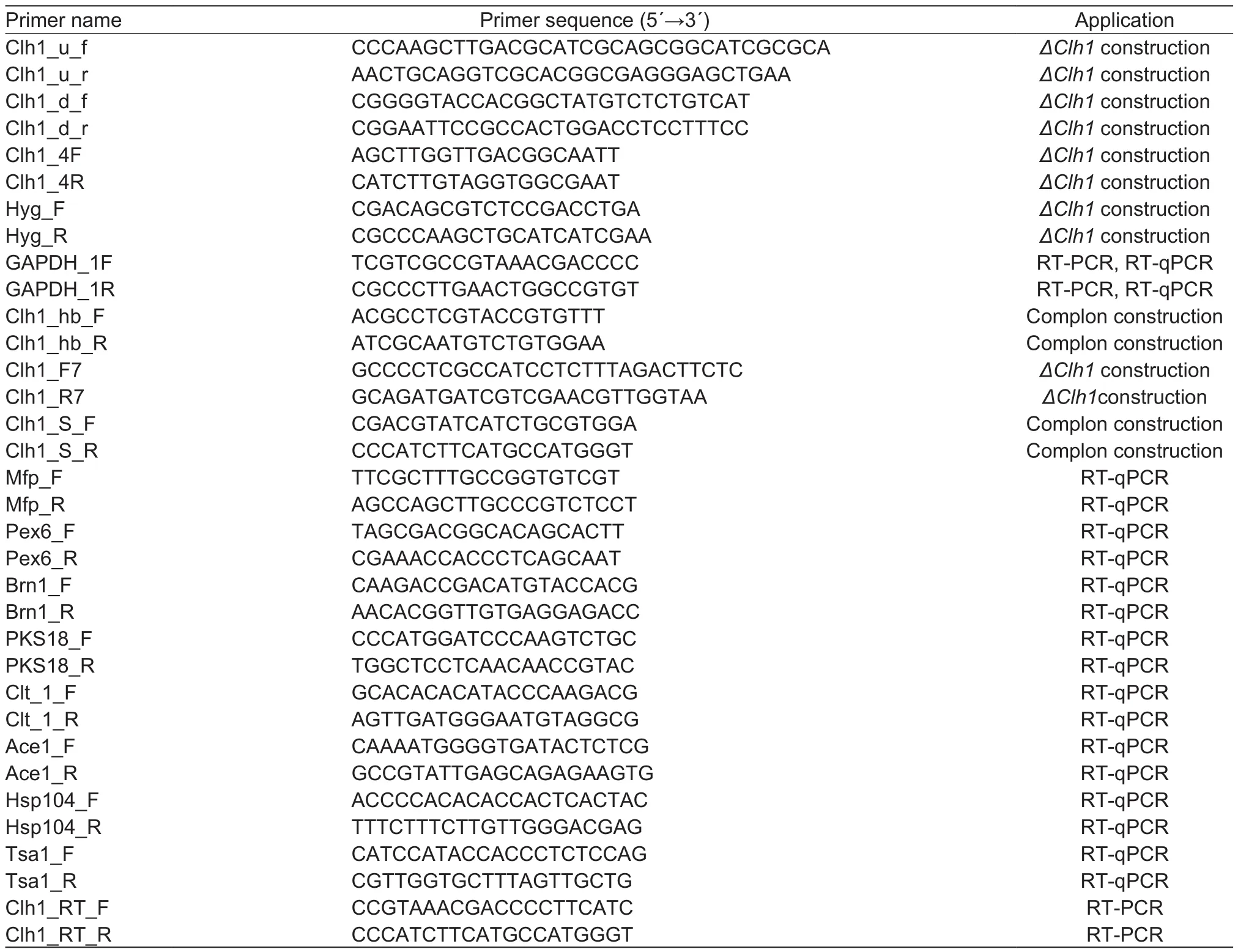
Table 1 Primer sequences used in this study
2.6. Construction of sod complon
A 1 134-bp fragment used for construction of the complementa tion construct containing the Clh1 open reading frame (ORF) was ampli fied from CX-3 genomic DNA using the primer pair Clh1_hb_F/R. The Clh1_hb_F/R primers contain a HindIII and EcoRI digestion site, respectively. The complementation fragment and the pCcmac1 vector were digested with FastDigest®HindIII and FastDigest®EcoRI(Fermentas). The digested complementation fragment was ligated into the digested pCcmac1 vector using T4 DNA ligase (Fermentas). The resulting plasmid, pCcmac1::Clh1,was first transformed into Agrobacterium tumefaciens AGL-1 competent cells using the above method and then into ΔClh1 cells by ATMT (Liu et al. 2010). The putative transformants were transferred to CYA plates containing 300 μg mL–1G418 and 200 μg mL–1of geneticin and then subcultured on CYA plates containing 300 μg mL–1G418 for 5 generations to test their mitotic stabilities. The positive complementation complons were identi fied by PCR using the primer pair Clh1_S_F/Clh1_S_R.
2.7. Determination of intracellular glycerol accumulation
Each strain was grown in potato dextrose for 2 days at 28°C in a shaker. After harvesting the mycelia and grinding in liquid nitrogen, 100 mg powerdered mycelia were transferred to a 2-mL microcentrifuge tube containing 0.1 mL glycerol extraction buffer (Shanghai Chaoyan Biotechnology Co.).After vortexing three times for 30 s each, the tubes were centrifuged at 5 000×g for 20 min. For each sample, the resulting supernatant was transferred to a new tube, and a 10-μL aliquot was mixed with 190 μL glycerol detection buffer (Shanghai Chaoyan Biotechnology Co.). After the mixture was incubated at 37°C for 15 min, the amount of intracellular glycerol was determined as described (Jiang et al. 2012). All tests were conducted with three replications.

Fig. 1 Construction of Clh1 deletion mutant. A, ampli fication of the 5´ flanking sequence of the Clh1 gene. Lane M, DNA molecular standard; lane 1, the 5´ flanking sequence Clh1 (780 bp containing 15 bp of restriction sites and protective bases) was ampli fied with the Clh1_u_f/r primer pair. B, ampli fication of the 3´ flanking sequence of the Clh1 gene. Lane M, DNA molecular standard;lanes 1 and 2, the 3´ flanking sequence of Clh1 (880 bp containing 15 bp of restriction sites and protective bases) was ampli fied with the Clh1_d_f/r primer pair. C, recombinant plasmid 1300qh::Clh1:3 was identi fied by digesting with FastDigest® BamHI and FastDigest® EcoRI. D, positive Escherichia coli DH5α clones containing plasmid 1300qh::Clh1:53 were identi fied by colony PCR with the Clh1_d_f/r primer pair. Lanes 1–6, positive clones. E, recombinant plasmid 1300qh::Clh1:53 was identi fied by digesting with FastDigest® HindIII and FastDigest® PstI. F, Southern blot analysis of transformant No. 5 (see G). 1, blot hybridized with a hyg gene probe; 2, blot hybridized with a Clh1 probe. G, putative Curvularia lunata transformants were identi fied by amplifying the hyg gene with the Hyg_F/R primer pair and the Clh1 gene with the Clh1_hb_F/R primer pair. Lane 1, wild-type CX-3; lanes 2–6,positive transformants No. 1–5; lane 7, knockout vector. Transformant No. 5 was selected for further study. H, transformant No. 5 was further validated by the assaying the transcription of the Clh1 gene using semi-quantitative RT-PCR and the gapdh gene as the reference. Lanes 1–2, wild-type CX-3 cDNA was used as the semi-quantitative RT-PCR template; lanes 3–4, transformant No. 5 cDNA was used as the semi-quantitative RT-PCR template.
2.8. Quanti fication of acetyl-CoA
Overnight cultures of CX-3, ΔClh1, ΔClk1 and ΔClm1 in PDA were diluted to 106cells mL–1, rinsed twice with PBS,and flash frozen in liquid N2. Cells were resuspended in 500 μL of water, and this was followed by beating for 5 min. Next, 200 μL cell lysate was centrifuged to remove cell debris, and 100 μL clari fied lysate was used for acetyl-CoA quanti fication with a PicoProbeTM Acetyl-CoA Quanti fication Kit (BioVision, San Francisco, CA, USA)as per the manufacturer’s instructions. Acetyl-CoA levels were determined based on fluorescence measurements at 589 nm and levels in the lysates were extrapolated from an acetyl-CoA standard curve after correcting for background.The assays were performed in triplicate.
2.9. Pathogenicity assay
Brie fly, the CX-3, ΔClh1, ΔClk1 and ΔClm1 strains were cultured on PDA medium in the dark at 28°C for 7 d. The spores were washed and diluted to 1.0×106spores mL–1.This spore suspension served as one inoculum. To test the function of acetyl-CoA, 1 mmol L–1acetyl-CoA was added to the spore suspension. The two kinds of inocula were separately inoculated on maize seedling leaf surfaces at the 4–5 leaf stage (Huangzao 4). The level of infection of leaves was ranked 3, 5 and 7 days after inoculation. This test was repeated for three independent biological replicates.
2.10. Real-time quantitative PCR (RT-qPCR)

Fig. 2 Working chart of the 1300qh::Clh1:53 vector construction.
Total RNA extraction and cDNA synthesis were carried out using the methods described above. Primers used for RT-qPCR were designed by Primer 5.0 and synthesized at Biosune Co. (China). The Power SYBR Green PCR Master Mix (2×) (TaKaRa, Japan) was used according to the manufacture’s recommendations. To each reaction 900 nmol L–1forward and reverse primers, 1–100 ng cDNA template, and nuclease-free water were added.Ampli fication was done with the following conditions:incubation at 95°C for 5 min followed by 35 cycles of 95°C for 15 s, 60°C for 15 s and 72°C for 15 s. GAPDH was used as the reference gene.
2.11. Extraction and comparison of the toxin produced by wild-type and mutant C. lunata strains
The wild-type CX-3 and its derivatives were activated on PDA plates in the dark at 28°C for 7 days. For each strain, a 7-mm diameter PDA disk containing mycelia was inoculated in 150 mL PD medium and cultured in the dark at 28°C with shaking at 120 r min–1for 7 days. The extraction of crude toxin and HPLC analysis of the crude extracts and puri fied toxin were done as previously described (Liu et al. 2009).
3. Results
3.1. Obtaining of Clh1 deletion mutants and complons
In order to analyze the role of Clh1 in C. lunata, Clh1 was disrupted by homologous recombination. Using ATMT, a 1 134-bp Clh1 DNA sequence was replaced by the hph gene sequence included the disruption vector, 1300qh::Clh1:53,via homologous recombination between the plasmid and CX-3 chromosomal DNA. After successive rounds of selection on PDA plates containing 300 µg mL–1hygromycin B, five transformants were obtained. Only one transformant was proven to be a Clh1-deletion mutant by PCR (Fig. 1-H).The deletion was further con firmed by semi-quantitative RT-PCR and Southern blot analysis (Fig. 1-F–G). To further con firm the function of the Clh1 gene, a Clh1 complon was constructed by transforming plasmid pCcmac1::Clh1 into ΔClh1 by ATMT. After successive rounds of selection, four positive transformants were selected for further con firmation.A 1 134-bp Clh1 fragment was ampli fied from the four transformants by PCR suggesting that they contain the fulllength Clh1 gene. One gene knockout transformant and one complon transformant (CP) were selected for subsequent study of Clh1 function.
3.2. Sensitivity of the Clh1 deletion mutant to osmotic stress
The ability of fungal pathogens to mechanically penetrate host plants is closely related to the increase in glycerol accumulation and the resulting increase in osmotic pressure of the appressoria and also to melanin accumulation in the appressoria cell wall. Saccharomyces cerevisiae cells produce a large amount of glycerol to counterbalance the osmotic pressure when they are subjected to hyper-osmotic stress (Posas et al. 1996). To determine whether a similar system operates in C. lunata, and whether the Clh1 gene regulates the response to osmotic stress, the intracellular glycerol content of the wild-type CX-3, ΔClh1 mutant and complone strains was determined. As illustrated in Fig. 3,the ΔClh1 mutant produced much lower amounts of glycerol than the wild-type strain CX-3, and the complone strain produced less glycerol than CX-3 but much more compared with ΔClh1. To determine whether the Clh1 gene was sensitive to osmotic stress, the growth rates of CX-3, ΔClh1 and CP strains were analyzed on PDA plates containing 1 mol L–1NaCl, 1.5 mol L–1NaCl, 2 mol L–1NaCl, 5 mmol L–1H2O2or 10 mmol L–1H2O2. In the presence of 2 mol L–1NaCl or 10 mmol L–1H2O2, the mycelial growth of the Clh1 mutant was reduced in comparison with that of the wild-type strain and CP (Fig. 4).
3.3. Effect of MAPK genes on the expression of genes related to pathogenicity
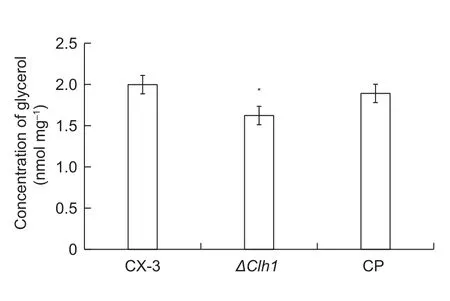
Fig. 3 Changes in intracellular glycerol content due to osmotic stress. Wild-type strain CX-3, ΔClh1 and complon (CP) mutants were grown on PD medium for 2 d. All mycelia were harvested,and the intracellular glycerol content was determined. The means of at least three independent experiments and the standard errors are shown. * indicates that change folds are signigicantly different (P<0.05).

Fig. 4 Response of the Cscherichia lunata ΔClh1 strain to osmotic stress. A, colonies of the wild-type CX-3, ΔClh1 and complon(CP) strains were grown on PDA plates with or without 1, 1.5, or 2 mmol L–1 NaCl, 2.5, 5, or 10 mmol L–1 H2O2. All strains were incubated at 28°C for 7 d and photographed. Scale bar=5 cm. B, the growth rates of the CX-3, ΔClh1 and CP mutants were determined following growth of the cultures on PDA plates with or without 1, 1.5, or 2 mol L–1 NaCl, 2.5, 5, or 10 mmol L–1 H2O2.Data are means and standard deviation. Signi ficant differences among ΔClh1, wild type and complon are indicated with asterisks(*, P<0.05; **, P<0.01).
We selected six genes known to be involved in toxin or melanin production, including clt1, pks18, Brn1, ace1,hsp104 and tsa1 (Liu et al. 2011; Gao et al. 2013), as hallmarks to evaluate the effect of the MAPK genes, Clh1,Clk1 and Clm1, on pathogenicity gene expression. In comparison with CX-3, the abundances of these genes were at least two-fold lower in all three MAPK mutants (Fig. 5),suggesting that these MAPK genes play important roles in the generation of melanin and toxin and thereby affect pathogenicity.
3.4. The level of acetyl-CoA is decreased in the MAPK mutants
The levels of acetyl-CoA in the Clh1, Clk1 and Clm1 deletion mutants and wild-type CX-3 strains were quanti fied, and the expression levels of acetyl-CoA related genes were determined. We found that the levels of acetyl-CoA in the mutants were lower than those in CX-3 (Fig. 6-A).Levels in the ΔClk1 were almost two times lower than those in CX-3. Mfe1 and Pex6 are genes that are related to peroxisomes, which belong to the microbody class of single-membrane bound organelles and perform distinct functions in lipid metabolism, such as β-oxidation of fatty acids and synthesis of cholesterol. The β-oxidation of fatty acids is a well-conserved metabolic process that results in the degradation of fatty acids to acetate. So the expression level of Mfe1 and Pex6 in the MAPK mutants can indirectly indicate the expression level of acetyl-CoA. We found that the expression levels of Mfe1 and Pex6 were downregulated by at least two-fold (P<0.05) in ΔClh1 and ΔClk1 compared with the wild-type strain CX-3 (Fig. 6-B). Based on our results, we conclude that the MAPK deletion mutants have less acetyl-CoA for toxin or melanin biosynthesis, and other pathways cannot fully compensate the loss.
3.5. Addition of acetyl-CoA increases toxin production in MAPK mutants
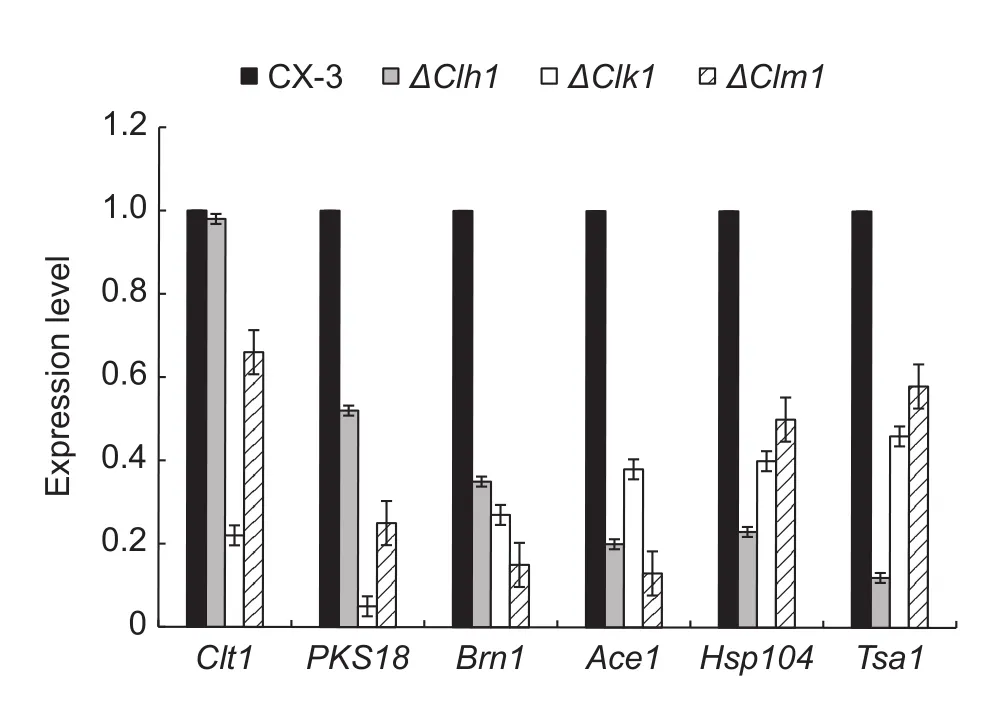
Fig. 5 RT-qPCR analysis of the transcriptional levels of pathogenicity-associated genes in CX-3 and mitogen-activated protein kinase (MAPK) mutants. A housekeeping gene, gapdh,was used as the reference gene. The transcriptional levels in the wild-type strain CX-3 were used as standards. The columns represent mean fold-change, and the error bars show the standard deviation around the mean (n=9). The entire analysis was conducted with three biological replicates and technological replicates.

Fig. 6 The production of acetyl-CoA and expression of acetyl-CoA related genes in the wild-type CX-3 and mitogen-activated protein kinase (MAPK) mutant strains. A, intracellular acetyl-CoA levels of CX-3 and MAPK mutants at 72 h. The entire analysis was conducted with three biological replicates and technological replicates. B, RT-qPCR analysis of the transcriptional levels of acetyl-CoA-related genes (Mfp and Pex6) in CX-3 and MAPK mutants. A housekeeping gene, gapdh, was used as the reference gene. The transcriptional levels in the wild-type strain CX-3 were used as standards. The columns represent indicate fold-change,and the error bars show the standard deviation around the mean (n=12). Signi ficant differences are indicated with asterisks(*P<0.05; **P<0.01).
Consistent with earlier reports, the deletion of Clh1, Clk1 and Clm1 strains showed much lower pathogenicity to maize leaves than the wild-type strain (Fig. 7-A). This result is also consistent with the signi ficantly decreased levels of acetyl-CoA in the MAPK mutants (Fig. 6). Because acetyl-CoA is important for pathogen virulence, we hypothesized that acetyl-CoA might play a compensating role in the production of toxin in the MAPK mutants in some unknown way. To test the contribution of acetyl-CoA to toxin production, we added 10 mmol L–1acetyl-CoA to the mutant spore suspensions in toxin-induced medium. We found that the production of the toxin, methyl-5-(hydroxymethyl)-furan-2-carboxylate,in the MAPK mutants was signi ficantly increased and even restored to wild-type levels after adding acetyl-CoA.In addition, the pathogenicity of MAPK mutants to maize leaves was restored after adding acetyl-CoA to the spore suspension (Figs. 7-B and 8).
4. Discussion
Mitogen-activated protein kinase (MAPK) cascades are highly conserved signaling modules that function downstream of receptors in eukaryotic cells and are heavily involved in cell responses to environmental stress stimuli(Meng and Zhang 2013). Compared with the wild-type strain, the pathogenicity of MAPK deletion mutants was weakened. In particular, ΔClk1 lost almost all pathogenicity,which is similar to the findings in studies of other pathogen mutants, such as the CtPMK1 mutant of M. oryzae(Xiong et al. 2015) and the Chk1 mutant of Cochliobolus heterostrophus (Urban et al. 2003).
Clh1 is one of MAP kinase genes and it has high similarity to the yeast HOG1 gene. We demonstrated that Clh1 is indeed involved in the accumulation of glycerol and mycelium tolerance to osmotic stress. Previous studies have shown that the pathogenicity of fungal appressoria to host plants is highly associated with the extent of glycerol accumulation,which increases turgidity of the appressoria, and allows the pathogen to more easily break through host cell wall barrier. Our data demonstrated that the accumulation of glycerol in the mycelium was regulated by Clh1 gene, and revealed another, indirect, way that a MAPK gene can affect pathogen pathogenicity. We also further con firmed that three MAPK genes are involved in the regulation of the production of pathogenicity-related factors, such as toxin and melanin and also of tolerance to osmotic stress, etc.In our previous study, we con firmed that the furanoid toxin methyl-5-(hydroxymethyl)furan-2-carboxylat produced by C. lunata is a pathogenicity factor for Curvularia leaf spot in maize. Here, we found that the furan toxin concentrations of MAPK mutants were much lower than the wild-type strain.Moreover, there were no furan toxins detected when Clk1,a homolog of yeast FUS3/KSS1, was deleted. Strong tolerance to osmotic stress is usually viewed as an indicator of pathogen survival over stress from host crop resistance and natural environments. Therefore, MAPKs could improve pathogen virulence by increasing tolerance of pathogen cells to stress conditions. We observed complete attenuation of virulence and pathogenicity in the three C. lunata MAPK mutants, Clh1, Clk1, and Clm1 (Liu et al. 2009).

Fig. 7 Disease phenotype of corn leaves after inoculation with conidial suspension. Disease symptoms (A) and lesion size (B)were evaluated in detached leaf inoculation assays. A single conidial suspension (×106 spores mL–1) and a conidial suspension containing 10 mmol L–1 acetyl-CoA were dropped on detached leaves of corn plants (a minimum of 15 leaves for each experiment),and lesion size was measured at 3 d after inoculation. Data are means and standard deviation. Signi ficant differences are indicated change folds with asterisks (**, P<0.01).
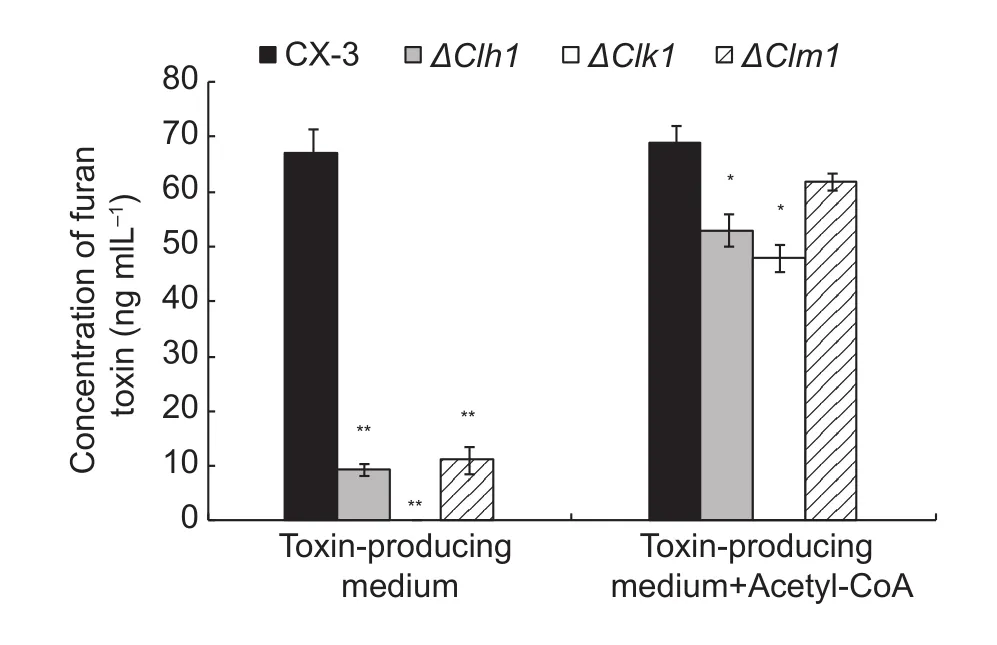
Fig. 8 Absolute quanti fication of furan toxin in mitogenactivated protein kinase (MAPK) mutants. The wild-type and mutant strains were grown in toxin-producing medium with or without acetyl-CoA for 30 d. After purifying by 0.45 μm filtration and ethyl acetate extraction, HPLC (high-performance liquid chromatography) was used to determine the toxin concentration.The entire test was conducted with three biological replicates.Data are means and standard deviation. Signi ficant differences are indicated with asterisks (*P<0.05; **P<0.01).
Intriguingly, we found that increasing acetyl-CoA increased toxin production and pathogenicity even when the Clh1, Clk1, and Clm1 genes were deleted (Li et al.2010; Raffaello et al. 2012; Wang et al. 2016). Acetyl-CoA, together with malonyl-CoA and SAM (S-adenosyl methionine), has been reported to contribute to pathogen virulence in different ways, particularly as a precursor of fungal pathogen toxin biosynthesis in Fusarium verticilloides. The F. verticilloides (Alexander et al. 2009)toxin biosynthetic pathway includes steps that are similar to those in the biosynthesis of C. lunata furan toxin.The main pathways for producing cytosolic acetyl-CoA in eukaryotic cells are β-oxidation of fatty acids, from acetate through the action of acetate synthetase (Acs1),and from citrate through the action of Acl1 (Grif fiths et al.2012). Deletion of ACL1 completely attenuates virulence,whereas the mfe2 and acs1 mutants exhibit differing degrees of reduced virulence (Hu et al. 2008; Kretschmer et al. 2012). In the current study, we showed that addition of acetyl-CoA could recover toxin production in MAPK deletion mutants, indicating that there is a close positive relationship between MAPK and acetyl-CoA during toxin biosynthesis and infection. Deletions of three MAPK genes led to the reduction of acetyl-CoA production,which probably resulted in the lack of toxin biosynthesis precursor. However, we still don’t understand the details of how MAPK signaling is related to acetyl-CoA production.
5. Conclusion
We have identi fied an important role of C. lunata Clh1 in osmotic stress and virulence to maize. In addition, we have demonstrated for the first time that acetyl-CoA is involved in MAPK-mediated signaling pathways and could restore the virulence of MAPK deletion mutants and affect pathogenicity.Therefore, we conclude that acetyl-CoA may be a precursor of toxin biosynthesis and thereby affect the infection of maize leaves by C. lunata.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31471734 and 31672072) and the earmarked fund for China Agriculture Research System(CARS-02). All authors have declared no con flict of interest.
Alexander N J, Proctor R H, Mccormick S P, Abbas H K. 2009.Genes, gene clusters, and biosynthesis of trichothecenes and fumonisins in fusarium. Toxin Reviews,28, 198–215.
Asakura M, Yoshino K, Hill AM, Kubo Y, Sakai Y, Takano Y.2012. Primary and secondary metabolism regulates lipolysis in appressoria of colletotrichum orbiculare. Fungal Genetics& Biology,49, 967–975.
Bardwell L, Zou X, Nie Q, Komarova N L. 2007. Mathematical models of speci ficity in cell signaling. Biophysical Journal,92, 3425–3441.
Bhadauria V, Banniza S, Vandenberg A, Selvaral G, Wei Y.2012. Peroxisomal alanine: Glyoxylate aminotransferase agt1 is indispensable for appressorium function of the rice blast pathogen, Magnaporthe oryzae. PLOS ONE,7,e36266.
Ding S, Zhou X, Zhao X, Xu J R. 2009. The PMK1 MAP kinase pathway and infection-related Morphogenesis advances in genetics. In: Genomics and Control of Rice Blast Disease.pp. 13–21.
算法的具体实现可参考的有Minimax算法和Alpha-beta剪枝。Minimax算法是一种悲观算法,即每步都假设对方选择最优的情况下,己方进行选择;而Alpha-beta剪枝则可以简化计算量,大体思路为我们不需要构建完整的树,其中当前格局无法找到最好情况下,我们应该返回父节点,而舍弃当前节点。两者的结合可以完成2048游戏的多步分析,使胜率达到较高水平。
Gao J X, Liu T, Chen J. 2014. Insertional mutagenesis and cloning of the gene required for the biosynthesis of the non-host-speci fic toxin in Cochliobolus lunatus that causes maize leaf spot. Phytopathology,104, 332–339.
Gao S G, Li Y Q, Gao J X, Suo Y J, Fu K H, Li Y Y, Chen J.2014. Genome sequence and virulence variation-related transcriptome pro files of Curvularia lunata, an important maize pathogenic fungus. BMC Genomics,15, 1–18.
Gao S G, Zhou F H, Liu T, Li Y Y, Chen J. 2013. A MAP kinase gene, Clk1, is required for conidiation and pathogenicity in the phytopathogenic fungus Curvularia lunata. Journal of Basic Microbiology,53, 214–223.
Grif fiths E J, Hu G, Fries B, Caza M, Wang J, Gsponer J, Gates-Hollingsworth M A, Kozel T R, De Repentigny L, Kronstad J W. 2012. A defect in ATP-citrate lyase links acetyl-CoA production, virulence factor elaboration and virulence in Cryptococcus neoformans. Molecular Microbiology,86,1404–1423.
Hans T, Zhang Z, Wei Y, Collinge D B. 1997. Subcellular localization of H2O2, in plants. H2O2, accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. The Plant Journal,11, 1187–1194.
Hu G, Cheng PY, Sham A, Perfect J R, Kronstad J W. 2008.Metabolic adaptation in Cryptococcus neoformans,during early murine pulmonary infection. Molecular Microbiology,69, 1456–1475.
Jiang J, Yun Y, Liu Y, Ma Z. 2012. FgVELB is associated with vegetative differentiation, secondary metabolism and virulence in Fusarium graminearum. Fungal Genetics and Biology,49, 653–662.
Kretschmer M, Wang J, Kronstad J W. 2012. Peroxisomal and mitochondrial β-oxidation pathways in fluence the virulence of the pathogenic fungus Cryptococcus neoformans. Eukaryotic Cell,11, 1042–1054.
Li A, Wang Y, Tao K, Dong S, Huang Q, Dai T, Zheng X,Wang Y. 2010. Pssak1, a stress-activated map kinase of phytophthora sojae, is required for zoospore viability and infection of soybean. Molecular Plant-Microbe Interactions,23, 1022–1031.
Liu T, Liu L, Jiang X, Hou J, Fu K, Zhou F, Chen J. 2010.Agrobacterium-mediated transformation as a useful tool for the molecular genetic study of the phytopathogen Curvularia lunata. European Journal of Plant Pathology,126, 363–371.
Liu T, Liu L X, Jiang X, Huang X L, Chen J. 2009. A new furanoid toxin produced by Curvularia lunata, the causal agent of maize Curvularia leaf spot. Canadian Journal of Plant Pathology,31, 22–27.
Liu T, Xu S, Liu L, Zhou F, Hou J, Chen J. 2011. Cloning and characteristics of Brn1 gene in Curvularia lunata causing leaf spot in maize. European Journal of Plant Pathology,131, 211–219.
Mcclean M N, Mody A, Broach J R, Ramanathan S. 2007. Crosstalk and decision making in MAP kinase pathways. Nature Genetics,39, 567.
Mehrabi R, Van der Lee T, Waalwijk C, Gert H J. 2006. MgSlt2,a cellular integrity MAP kinase gene of the fungal wheat pathogen Mycosphaerella graminicola, is dispensable for penetration but essential for invasive growth. Molecular Plant-Microbe Interactions,19, 389–398.
Meng X, Zhang S. 2013. MAPK cascades in plant disease resistance signaling. Annual Review of Phytopathology,51,245–266.
Moriwaki A, Kihara J, Mori C, Arase S. 2007. A MAP kinase gene, bmk1, is required for conidiation and pathogenicity in the rice leaf spot pathogen Bipolaris oryzae. Microbiological Research,162, 108–114.
Park S M, Choi E S, Kim M J, Cha B J, Yang M S, Kim D H.2004. Characterization of HOG1 homologue, CpMK1,from Cryphonectria parasitica and evidence for hypovirusmediated perturbation of its phosphorylation in response to hypertonic stress. Molecular Microbiology,51, 1267–1277.
Posas F, Wurgler-Murphy S M, Maeda T, Witten E A, Thar T C, Saito H. 1996. Yeast HOG1 MAP kinase cascade is regulated by a multistep phosphorelay mechanism in the SLN1-YPD1-SSK1 “two-component” osmosensor. Cell,86,865–875.
Raffaello T, Keriö S, Asiegbu F O. 2012. Role of the HaHOG1 MAP kinase in response of the conifer root and but rot pathogen (Heterobasidion annosum) to osmotic and oxidative stress. PLOS ONE,7, e31186.
Rižner T L, Wheeler M H. 2003. Melanin biosynthesis in the fungus Curvularia lunata (teleomorph: Cochliobolus lunatus). Canadian Journal of Microbiology,49, 110–119.
Sesma A, Osbourn A E. 2004. The rice leaf blast pathogen undergoes developmental processes typical of root-infecting fungi. Nature,431, 582–586.
Urban M, Mott E, Farley T, Hammond-Kosack K. 2003 The Fusarium graminearum MAP1 gene is essential for pathogenicity and development of perithecia. Molecular Plant Pathology,4, 347–359.
Wang J Y, Chen J. 2011. Cloning and functional analysis of Clm1 in Curvularia lunata. Acta Phytopathologica Sinica,41, 464–472. (in Chinese)
Wang Y, Tian L, Xiong D, Klosterman S J, Xiao S X, Tian C M.2016. The mitogen-activated protein kinase gene, VdHog1,regulates osmotic stress response, microsclerotia formation and virulence in Verticillium dahliae. Fungal Genetics and Biology,88, 13–23.
Wang Z Y, Soanes D M, Kershaw M J, Talbot N J. 2007. Functional analysis of lipid metabolism in Magnaporthe grisea reveals a requirement for peroisomal fatty acid β-oxidation during appressorium-mediated plant infection. Molecular Plant-Microbe Interactions,20, 475–491.
Xiong Q, Xu J, Zhao Y, Wang K. 2015. CtPMK1, a mitogenactivated-protein kinase gene, is required for conidiation,appressorium formation, and pathogenicity of Colletotrichum truncatum on soybean. Annals of Applied Biology,167,63–74.
13 February, 2017 Accepted 22 June, 2017
NI Xuan, E-mail: xuanni16@163.com; Corresponsence CHEN Jie, E-mail: Jiechen59@sjtu.edu.cn
© 2018 CAAS. Publishing services by Elsevier B.V. All rights reserved.
10.1016/S2095-3119(17)61697-6
Section editor WAN Fang-hao
Managing editor ZHANG Juan
 Journal of Integrative Agriculture2018年1期
Journal of Integrative Agriculture2018年1期
- Journal of Integrative Agriculture的其它文章
- Climate change and agriculture: Impacts and adaptive responses in Iran
- Development of elite restoring lines by integrating blast resistance and low amylose content using MAS
- Evaluation of stability and yield potential of upland rice genotypes in North and Northeast Thailand
- ldenti fication of the resistance gene to powdery mildew in Chinese wheat landrace Baiyouyantiao
- New clues concerning pigment biosynthesis in green colored fiber provided by proteomics-based analysis
- Constitutive expression of feedback-insensitive cystathionine γ-synthase increases methionine levels in soybean leaves and seeds
