First report of field resistance to cyantraniliprole, a new anthranilic diamide insecticide, on Bemisia tabaci MED in China
WANG Ran, WANG Jin-da, CHE Wu-nan, LUO Chen
1 Institute of Plant and Environment Protection, Beijing Academy of Agriculture and Forestry Sciences, Beijing 100097, P.R.China
2 National Engineering Research Center of Sugarcane, Fujian Agriculture and Forestry University, Fuzhou 350002, P.R.China
3 Department of Pesticide Sciences, Shenyang Agricultural University, Shenyang 110866, P.R.China
RESEARCH ARTICLE
First report of field resistance to cyantraniliprole, a new anthranilic diamide insecticide, on Bemisia tabaci MED in China
WANG Ran1*, WANG Jin-da2*, CHE Wu-nan3, LUO Chen1
1 Institute of Plant and Environment Protection, Beijing Academy of Agriculture and Forestry Sciences, Beijing 100097, P.R.China
2 National Engineering Research Center of Sugarcane, Fujian Agriculture and Forestry University, Fuzhou 350002, P.R.China
3 Department of Pesticide Sciences, Shenyang Agricultural University, Shenyang 110866, P.R.China
The Bemisia tabaci (Gennadius) cryptic species complex comprises important insect pests that cause devastating damage to agricultural crops worldwide. In China, the B. tabaci Mediterranean (MED) (or biotype Q) species is threatening agricultural production all over the country as resistance to commonly used insecticides has increased. This situation highlights the need for alternative pest control measures. Cyantraniliprole, a novel anthranilic diamide insecticide, has been widely employed to control Hemipteran pests. To monitor the levels of resistance to cyantraniliprole in B. tabaci field populations in China, bioassays were conducted for 18 field samples from nine provinces over two years. Compared with median lethal concentration (LC50) for the MED susceptible strain, all field samples had signi ficantly higher resistance to cyantraniliprole.Furthermore, resistance factors (RFs) increased signi ficantly in samples from Shanxi (from 5.62 in 2015 to 25.81 in 2016),Hunan (3.30 in 2015 to 20.97 in 2016) and Hubei (from 9.81 in 2015 to 23.91 in 2016) provinces. This study indicates a considerable decrease in the ef ficacy of cyantraniliprole against B. tabaci and establishes a baseline of susceptibility that could serve as a reference for future monitoring and management of B. tabaci resistance to cyantraniliprole.
Bemisia tabaci, cyantraniliprole, anthranilic diamides, baseline susceptibility, resistance development
1. lntroduction
The tobacco white fly Bemisia tabaci (Gennadius), also known as cotton white fly or sweet potato white fly, is a cryptic species complex of small insects with sucking mouthparts.This species complex is distributed globally in tropical,subtropical, and low-latitude temperate regions. B. tabaci has been recorded feeding on more than 600 plants (De Barro et al. 2011; Wang et al. 2014) including cotton, tomato,tobacco, cassava, sweet potato, and many other flower and vegetable crops. In addition, B. tabaci is an important disease vector, transmitting more than 200 species of plant viruses (Hogenhout et al. 2008). The two most invasive and destructive cryptic species of B. tabaci are Middle East-Asia Minor I (MEAM1 or biotype B) and Mediterranean (MED or biotype Q). MEAM1 is widely distributed in China on vegetable and cotton crops (Luo et al. 2002; Wang et al. 2010), and MED was first detected in China in 2003 following suspected importation on poinsettia (Chu et al. 2006). Recently, MED has displaced MEAM1 and is now the dominant species in most regions of China (Zheng et al. 2017). Insecticides such as organophosphates, pyrethroids, carbamates and insect growth regulator (IGR) used to be applied as the primary means of controlling B. tabaci. However, a strong reduction in the ef ficacy of these insecticides has been reported in some populations of B. tabaci due to rapid evolution of insecticide resistance (Ahmad et al. 2010; Wang et al. 2010; Basit et al.2012; Zheng et al. 2017). Additionally, a relatively high toxicity of these insecticides has been observed in non-target organisms, including arthropods and even humans (David et al. 2007; Andrione et al. 2016; Cook et al. 2016). As a result, it is not an appropriate choice to extensively employ above insecticides with low pesticide residues for producing agricultural products.
In the 1990s, a number of neonicotinoids such as imidacloprid, nitenpyram, acetamiprid, thiamethoxam,clothianidin and dinotefuran were developed and used to control Hemipteran pests around the globe (Bass et al.2015). Currently, B. tabaci MED is dominant in China and has been threating agricultural production throughout the country (Pan et al. 2011, 2015). More importantly, MED has developed resistance to several different insecticides,especially neonicotinoids (Pan et al. 2015; Zheng et al.2017). Anthranilic diamide insecticides have been shown to effectively control a large number of insect pest species from different orders such as Diptera (Peck et al. 2008),Thysanoptera (Jacobson and Kennedy 2011), Lepidoptera (Wakil et al. 2013), Isoptera (Barwary et al. 2015),Hemiptera (Grávalos et al. 2015) and Coleoptera (Lanka et al. 2015). Following the commercial success of chlorantraniliprole, the first anthranilic diamide, cyantraniliprole, the second systemic anthranilic diamide, has been used to control Hemipteran pests (Sattelle et al. 2008).Cyantraniliprole is a powerful tool for managing adult and immature stages of white fly, and for reducing transmission of plant viruses (Lahm et al. 2007; Grávalos et al. 2015;Wang et al. 2017; Zheng et al. 2017). Cyantraniliprole has provided a promising alternative to existing insecticides,as it exhibits novel modes of action and acute toxicological pro files against insect pests.
Before the initial application of any new insecticide, it is important to determine baseline susceptibility to con firm the current resistance situation in the field. Studies of baseline susceptibility of cyantraniliprole in B. tabaci from the United States and Spain demonstrated no cross-resistance to commonly used insecticides, and proved to be an effective way to mitigate resistance of B. tabaci to other insecticides (Li et al. 2012; Grávalos et al. 2015). In the present study, we determined the baseline susceptibility of cyantraniliprole and assessed the development of resistance to cyantraniliprole in B. tabaci from nine provinces in China.
2. Materials and methods
2.1. B. tabaci strains
B. tabaci MED susceptible strain (MED-S) was originally collected from damaged poinsettia in Beijing, China in 2009(Pan et al. 2012). The insects were fed on cotton plants(Gossypium hirsutum L. var. Shiyuan 321) free of any insecticides under a 16 h L:8 h D photoperiod at (27±1)°C and (60±10)% relative humidity (RH). Nine samples of B. tabaci were collected in 2015 and 2016 from different host plants across nine provinces in China. Sampling site,date and host plant are listed in Table 1. More than 3 000 adults were collected randomly from crop leaves to obtain F1progeny. In addition, at least 200 individuals of each sample were collected at random, and then placed in a 1.5-mL centrifuge tube with 95% ethanol and stored at –20°C for later species identi fication. All samples were identi fied as MED according to nucleotide sequences of a fragment of the mitochondrial COI gene (Luo et al. 2002).
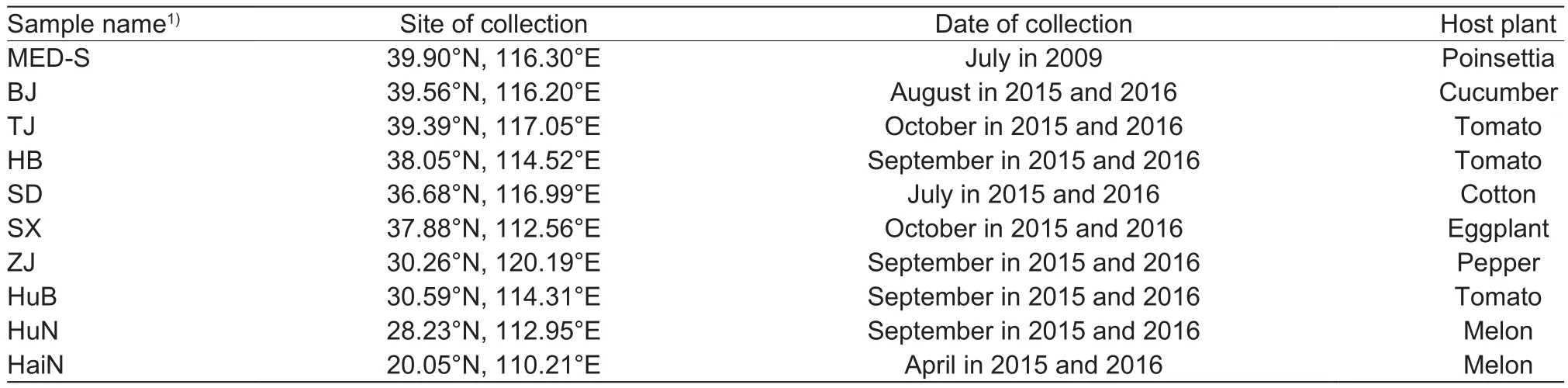
Table 1 Sampling site, date and host plant of Bemisia tabaci Mediterranean (MED) from the fields in China
2.2. Bioassays
Bioassays of cyantraniliprole on adult B. tabaci from test samples were conducted using the leaf-disc dipping bioassay method (Wang et al. 2017). According to the protocol, stock solution (1 000 mg L–1) of the insecticide was prepared in dimethyl sulfoxide (DMSO). Then, five concentrations of insecticide working solutions were prepared by diluting the stock solution in 0.1‰ Triton X-100 in distilled water. Cotton leaf discs (20 mm in diameter)were prepared and dipped for 20 s in one of the five concentrations of insecticide solution with the adaxial surface facing down. Then, the leaf discs were dried by placing them in a flat-bottomed glass tube (76 mm long) containing agar (2 mL of 15 g L–1). Control leaf discs were dipped in 0.1‰ Triton X-100 as described above and four replicates were made. Adult insects reared on insecticide-free cotton plants in the greenhouse were collected in the tubes containing the leaf discs by inverting the tubes over the insects and allowing the adults to fly into the tubes. About 40–60 adults were randomly collected per tube. After sample collection, the open end of each tube was sealed with a cotton plug and maintained at (27±1)°C and (60±10)% RH with a 16 h L:8 h D photoperiod. After 48 h, mortality was monitored under a microscope and immobile adults were considered dead.
2.3. Statistical analysis
Probit analyses were performed to determine statistical signi ficance of the concentration-dependent mortality using PoloPlus (LeOra Software 2002). Resistance factors (RFs)were calculated between the estimated median lethal concentration (LC50) of each field sample and the MED-S strain using the method of Robertson and Preisler (1992).
3. Results
3.1. 2015 resistance monitoring tests
Large variability was observed in the susceptibility of different field samples in 2015, but all samples were signi ficantly more resistant to cyantraniliprole than the MED-S stain.Among the samples from all nine collection sites (Table 1),LC50values ranged from 5.53 to 27.40 mg L–1and slopes ranged from 1.19 (SE=0.11) to 1.01 (SE=0.10), resulting in RFs ranging from 3.30 to 16.36 compared to MED-S strain as the control (Table 2). Among them, one of the field samples, the Zhejiang strain, had higher resistance to cyantraniliprole than the other strains. The LC50value of 27.40 mg L–1for the Zhejiang strain was signi ficantly higher than the LC50values for the other field samples (Table 2).
3.2. 2016 resistance monitoring tests
Results of the 2016 bioassays showed that several tested samples were more resistant to cyantraniliprole compared to 2015 according to the LC50values and RFs (Table 3).The LC50values for three field samples (Shanxi, Hunan and Hubei) ranged from 32.81 to 40.39 mg L–1and the slopes ranged from 1.13 (SE=0.10) to 1.21 (SE=0.11), which were higher than those observed in 2015. Moreover, in these 3 samples, compared with 2015, the fiducial limits of the LC50values and RF were not overlapped, respectively, indicating signi ficant difference in the level of resistance in each sample within two years (Tables 2 and 3). Speci fically, a signi ficantly increased level of resistance was observed in Shanxi (RF from 5.62 to 25.81), Hunan (RF from 3.30 to 20.97) and Hubei (RF from 9.81 to 23.91). The LC50values for the other six samples ranged from 10.98 to 27.20 mg L–1and the slopes ranged from 1.13 (SE=0.10) to 1.16(SE=0.11), corresponding to RF levels of 7.01 to 17.38(Table 3). The overlapping fiducial limits of the sample in 2015 and its counterpart in 2016 suggested no differences in the LC50values. The RF values of field samples ranged from 5.63 to 16.36 in 2015 and from 7.01 to 17.38, also illustrating no signi ficant differences.
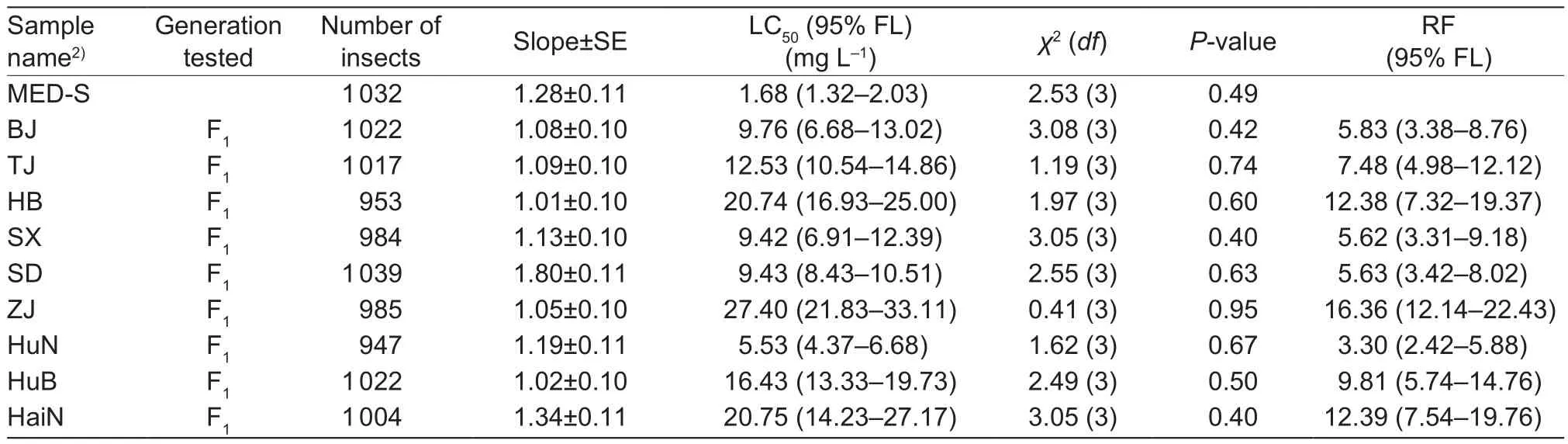
Table 2 Relative resistance to cyantraniliprole on Bemisia tabaci Mediterranean (MED) field samples collected in 20151)
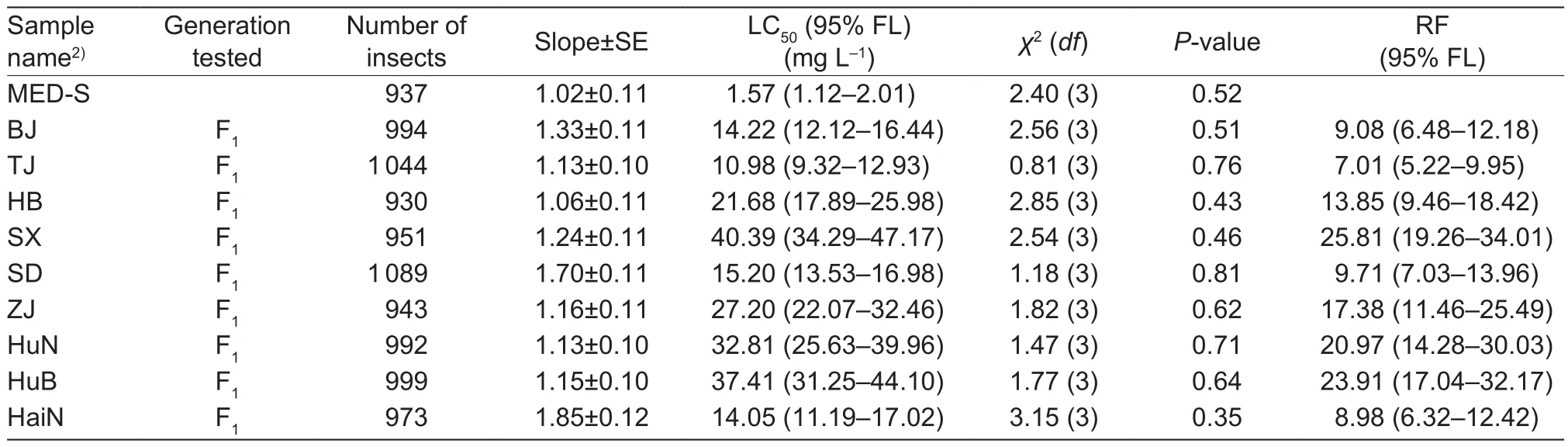
Table 3 Relative resistance to cyantraniliprole on Bemisia tabaci Mediterranean (MED) field samples collected in 20161)
4. Discussion
Cyantraniliprole is the second-generation product in the anthranilic diamide class developed by the DuPont Company,USA, and it provides cross-spectrum control of chewing and sucking pests (Rattan et al. 2015). According to our previous research, cyantraniliprole exhibited highly lethal and sublethal effects on B. tabaci MED (Wang et al. 2017).Application of cyantraniliprole in China helped to deal with the potential threat of resistance to neonicotinoids. Thus, it is necessary to know whether B. tabaci has already evolved,or has the potential to evolve, resistance to cyantraniliprole in the field. In order to address this question, we compared one laboratory susceptible strain with 18 field samples collected from 9 provinces in China in 2015 or 2016. The field samples were then tested to establish the baseline susceptibility of cyantraniliprole against B. tabaci MED adults using a cotton leaf-bioassay. This is the first report to describe geographical differences in susceptibility of B. tabaci to cyantraniliprole in China, and it could be helpful for resistance monitoring and management.
Our results indicate signi ficant variation in susceptibility to cyantraniliprole based on geographically separated samples of B. tabaci MED in China. Similar geographical differences of susceptibility to cyantraniliprole in B. tabaci have been studied in the past. High susceptibility to cyantraniliprole was demonstrated in the United States and Spain, after several years of monitoring resistance (Li et al. 2012; Grávalos et al. 2015). Furthermore, low levels of resistance or toleranlce to cyantraniliprole have been reported in several species of pests such as Frankliniella occidentalis (Bielza and Guillén 2015), Leucinodes orbonalis (Kodandaram et al. 2015),Bactrocera dorsalis (Zhang et al. 2015) and Helicoverpa armigera (Bird 2016), indicating that cyantraniliprole could be an ef ficient alternative for rotation with other insecticides in integrated pest management (IPM) systems.
Our study demonstrated the baseline susceptibility of B. tabaci to cyantraniliprole in China, and showed that resistance levels increased signi ficantly within two years.Compared with varied levels of resistance from 2015 to 2016, the samples from Shanxi, Hunan, and Hubei developed signi ficant resistance to cyantraniliprole after one year of application. In contrast, B. tabaci samples from different regions of the United States and Spain were still highly susceptible to cyantraniliprole after multiple years.In the United States, both B and Q biotypes showed high susceptibility to cyantraniliprole over two years of monitoring resistance (Li et al. 2012). Similarly, the results of seven years of monitoring in Spain indicated B. tabaci MED had very high susceptibility to cyantraniliprole (Grávalos et al.2015). We speculate that, in China, frequent application and overuse of cyantraniliprole could cause rapid development of resistance. The rapid development of resistance to cyantraniliprole has been found in other species of pests from China. Sang et al. (2016) reported obvious regional variation of cyantraniliprole susceptibilities in Spodoptera litura from different locations in South China, and suggested the possibility of resistance to cyantraniliprole in S. litura after long periods of use. In addition, LC50values of three field populations of B. dorsalis, an important pest of fruits and vegetables, were more than 10 μg g–1and LC50values over 120 μg g–1with a 19.44-fold resistant ratio were detected after 14 generations of selection (Zhang et al.2014). Combined with the above results, we speculated that higher levels of resistance against cyantraniliprole could appear in the field in a short period of time owing to the heterogeneity of the field populations and relatively higher selection pressure.
It has been reported that B. tabaci has the potential ability to evolve resistance to many insecticides after widespread application in the field (Horowitz et al. 2005; Horowitz and Ishaaya 2014; Bass et al. 2015). Considering the strong evidence of insecticide resistance in B. tabaci, the risk of developing resistance to cyantraniliprole is concerning.Managing the development of resistance should begin when novel pesticides are first applied (Jutsum et al. 1998), meaning that it is essential to monitor resistance of B. tabaci to cyantraniliprole before it is widely used. The data reported in the current study would provide a useful foundation for monitoring cyantraniliprole resistance in B. tabaci MED populations. However, further studies should be conducted to con firm its detoxi fication mechanisms, and the spectrum of cross-resistance should also be demonstrated. Faced with field-evolved resistance to cyantraniliprole in several areas of China, an urgent and effective resistance management strategy should be launched immediately to delay further development of resistance to cyantraniliprole in B. tabaci.
5. Conclusion
Compared with the MED-S strain, several field samples tested in this research showed higher level of resistance to cyantraniliprole in B. tabaci MED. In Shanxi, Hunan and Hubei, the two-year study of resistance monitoring in field proved that the development of resistance to cyantraniliprole in B. tabaci MED was rapid. Although cyantraniliprole is one novel agent with novel modes of action against B. tabaci, it is urgent to rotate with several other insecticides for delaying the development of resistance.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31601635), the Beijing Natural Science Foundation, China (6174038), and the earmarked fund for Beijing Innovation Consortium of Agriculture Research System, China (BAIC07-2017).
Ahmad M, Arif M I, Naveed M. 2010. Dynamics of resistance to organophosphate and carbamate insecticides in the cotton white fly Bemisia tabaci (Hemiptera: Aleyrodidae) from Pakistan. Journal of Pest Science,83, 409–420.
Andrione M, Vallortigara G, Antolini R, Haase A. 2016.Neonicotinoid-induced impairment of odour coding in the honeybee. Scienti fic Reports,6, 38110.
De Barro P J, Liu S S, Boykin L M, Dinsdale A B. 2011. Bemisia tabaci: A statement of species status. Annual Review of Entomology,56, 1–19.
Barwary Z, Gorzlancyk A, Hu X P. 2015. Effects of concentration, distance, and application methods of Altriset(Chlorantraniliprole) on eastern subterranean termite(Isoptera: Rhinotermitidae). Insect Science,22, 451–460.
Basit M, Saleem M A, Saeed S, Sayyed A H. 2012. Cross resistance, genetic analysis and stability of resistance to buprofezin in cotton white fly, Bemisia tabaci (Homoptera:Aleyrodidae). Crop Protection,40, 16–21.
Bass C, Denholm I, Williamson M S, Nauen R. 2015. The global status of insect resistance to neonicotinoid insecticides.Pesticide Biochemistry and Physiology,121, 78–87.
Bielza P, Guillén J. 2015. Cyantraniliprole: a valuable tool for Frankliniella occidentalis (Pergande) management. Pest Management Science,71, 1068–1074.
Bird L J. 2016. Susceptibility of Helicoverpa armigera(Lepidoptera: Noctuidae) to cyantraniliprole determined from topical and ingestion bioassays. Journal of Economic Entomology,109, 1350–1356.
Chu D, Zhang Y J, Brown J K, Cong B, Xu B Y, Wu Q J, Zhu G R. 2006. The introduction of the exotic Q biotype of Bemisia tabaci from the Mediterranean region into China on ornamental crops. Florida Entomologist,89, 168–174.
Cook N, Green J, Shuker D M, Whitehorn P R. 2016. Exposure to the neonicotinoid imidacloprid disrupts sex allocation cue use during superparasitism in the parasitoid wasp Nasonia vitripennis. Ecological Entomology,41, 693–697.
David D, George I A, Peter J V. 2007. Toxicology of the newer neonicotinoid insecticides: Imidacloprid poisoning in a human. Clinical Toxicology,45, 485–486.
Grávalos C, Fernández E, Belando A, Moreno I, Ros C, Bielza P. 2015. Cross-resistance and baseline susceptibility of Mediterranean strains of Bemisia tabaci to cyantraniliprole.Pest Management Science,71, 1030–1036.
Hogenhout S A, Ammar E D, Whit field A E, Redinbaugh M G.2008. Insect vector interactions with persistently transmitted viruses. Annual Review of Phytopathology,46, 327–359.
Horowitz A R, Ishaaya I. 2014. Dynamics of biotypes B and Q of the white fly Bemisia tabaci and its impact on insecticide resistance. Pest Management Science,70, 1568–1572.
Horowitz A R, Kontsedalov S, Khasdan V, Ishaaya I. 2005.Biotypes B and Q of Bemisia tabaci and their relevance to neonicotinoid and pyriproxyfen resistance. Archives of Insect Biochemistry and Physiology,58, 216–225.
Jacobson A L, Kennedy G G. 2011. The effect of three rates of cyantraniliprole on the transmission of tomato spotted wilt virus by Frankliniella occidentalis and Frankliniella fusca(Thysanoptera: Thripidae) to Capsicum annuum. Crop Protection,30, 512–515.
Jutsum A R, Heaney S P, Perrin B M, Wege P J. 1998. Pesticide resistance: assessment of risk and the development and implementation of effective management strategies.Pesticide Science,54, 435–446.
Kodandaram M H, Rai A B, Sireesha K, Halder J. 2015. Ef ficacy of cyantraniliprole a new anthranilic diamide insecticide against Leucinodes orbonalis (Lepidoptera: Crambidae) of brinjal. Journal of Environmental Biology,36, 1415–1420.
Lahm G P, Stevenson T M, Selby T P, Freudenberger J H,Cordova D, Flexner L, Bellin C A, Dubas C M, Smith B K,Hughes K A, Hollingshaus J G, Clark C E, Benner E A.2007. Rynaxypyr: A new insecticidal anthanilic diamide that actsas a potent and selective ryanodine receptor activator.Bioorganic & Medicinal Chemistry Letters,17, 6274–6279.
Lanka S K, Blouin D C, Stout M J. 2015. Integrating flood depth and plant resistance with chlorantraniliprole seed treatments for management of rice water weevil, Lissorhoptrus oryzophilus (Coleoptera: Curculionidae). Insect Science,22, 679–687.
LeOra Software. 2002. Polo Plus, A User’s Guide to Probit or Logit Analysis. LeOra Software, Berkeley, CA.
Li X C, Degain B A, Harpold V S, Marçon P G, Nichols R L,Fournier A J, Naranjo S E, Palumbo J C, Ellsworth P C.2012. Baseline susceptibilities of B- and Q-biotype Bemisia tabaci to anthranilic diamides in Arizona. Pest Management Science,68, 83–91.
Luo C, Yao Y, Wang R J, Yan F M, Hu D X, Zhang Z L. 2002.The use of mitochondrial cytochrome oxidase mt COI gene sequences for the identi fication of biotypes of Bemisia tabaci (Gennadius) in China. Acta Entomologica Sinica,45, 759–763. (in Chinese)
Pan H P, Chu D, Ge D Q, Wang S L, Wu Q J, Xie W, Jiao X G, Liu B M, Yang X, Yang N N, Su Q, Xu B Y, Zhang Y J.2011. Further spread of and domination by Bemisia tabaci(Hemiptera: Aleyrodidae) biotype Q on field crops in China.Journal of Economic Entomology,104, 978–985.
Pan H P, Chu D, Yan W Q, Su Q, Liu B M, Wang S L, Wu Q J,Xie W, Jiao X G, Li R M, Yang N N, Yang X, Xu B Y, Brown J K, Zhou X G, Zhang Y J. 2012. Rapid spread of Tomato yellow leaf curl virus in China is aided differentially by two invasive white flies. PLOS ONE,7, e34817.
Pan H P, Preisser E L, Chu D, Wang S L, Wu Q J, Zhou X G, Zhang Y J. 2015. Insecticides promote viral outbreaks in China by altering herbivore competition. Ecological Applications,25, 1585–1595.
Peck D C, Olmstead D, Morales A. 2008. Application timing and ef ficacy of alternatives for the insecticidal control of Tipula paludosa Meigen (Diptera: Tipulidae), a new invasive pest of turf in the northeastern United States. Pest Management Science,64, 989–1000.
Rattan R S, Purohit H, Patel C, Suvagia P, Singh V, Portillo H,Annan I B, Alvarez J M. 2015. Effect of cyantraniliprole on feeding cessation of Q biotype Bemisia tabaci (Gennadius)(Hemiptera: Aleyrodidae). Advances in Entomology,3,56–64.
Robertson J L, Preisler H K. 1992. Pesticide Bioassays with Arthropods. CRC Press, Boca Raton, FL, USA.
Sang S, Shu B S, Yi X, Liu J, Hu M Y, Zhong G H. 2016. Crossresistance and baseline susceptibility of Spodoptera litura(Fabricius) (Lepidoptera: Noctuidae) to cyantraniliprole in the south of China. Pest Management Science,72,922–928.
Sattelle D B, Cordova D, Cheek T R. 2008. Insect ryanodine receptors: Molecular targets for novel pest control chemicals. Invertebrate Neuroscience,8, 107–119.
Wakil W, Ghazanfar M U, Riasat T, Qayyum M A, Ahmed S,Yasin M. 2013. Effects of interactions among Metarhizium anisopliae, Bacillus thuringiensis and chlorantraniliprole on the mortality and pupation of six geographically distinct Helicoverpa armigera field populations. Phytoparasitica,41, 221–234.
Wang H L, Yang J, Boykin L M, Zhao Q Y, Wang Y J, Liu S S, Wang X W. 2014. Developing conversed microsatellite markers and their implications in evolutionary analysis of the Bemisia tabaci complex. Scienti fic Reports,4, 6351.
Wang R, Zhang W, Che W N, Qu C, Li F Q, Desneux N, Luo C. 2017. Lethal and sublethal effects of cyantraniliprole,a new anthranilic diamide insecticide, on Bemisia tabaci(Hemiptera: Aleyrodidae) MED. Crop Protection,91,108–113.
Wang Z Y, Yan H F, Yang Y H, Wu Y D. 2010. Biotype and insecticide resistance status of the white fly Bemisia tabaci from China. Pest Management Science,66, 1360–1366.
Zhang R M, He S Y, Chen J H. 2014. Monitoring of Bactrocera dorsalis (Diptera: Tephritidae) resistance to cyantraniliprole in the south of China. Journal of Economic Entomology,107, 1233–1238.
Zhang R M, Jang E B, He S Y, Chen J H. 2015. Lethal and sublethal effects of cyantraniliprole on Bactrocera dorsalis(Hendel) (Diptera: Tephritidae). Pest Management Science,71, 250–256.
Zheng H X, Xie W, Wang S L, Wu Q J, Zhou X M, Zhang Y J. 2017. Dynamic monitoring (B versus Q) and further resistance status of Q type Bemisia tabaci in China. Crop Protection,94, 115–121.
13 February, 2017 Accepted 17 March, 2017
WANG Ran, Tel: +86-10-51503338, E-mail: rwang1105@126.com; Correspondence LUO Chen, Tel: +86-10-51503338, E-mail:luochen1010@126.com
*These authors contributed equally to this study.
© 2018 CAAS. Publishing services by Elsevier B.V. All rights reserved.
10.1016/S2095-3119(16)61613-1
Section editor WAN Fang-hao
Managing editor SUN Lu-juan
 Journal of Integrative Agriculture2018年1期
Journal of Integrative Agriculture2018年1期
- Journal of Integrative Agriculture的其它文章
- Climate change and agriculture: Impacts and adaptive responses in Iran
- Development of elite restoring lines by integrating blast resistance and low amylose content using MAS
- Evaluation of stability and yield potential of upland rice genotypes in North and Northeast Thailand
- ldenti fication of the resistance gene to powdery mildew in Chinese wheat landrace Baiyouyantiao
- New clues concerning pigment biosynthesis in green colored fiber provided by proteomics-based analysis
- Constitutive expression of feedback-insensitive cystathionine γ-synthase increases methionine levels in soybean leaves and seeds
