Construction of Salmonella Pullorum ghost by co-expression of lysis gene E and the antimicrobial peptide SMAP29 and evaluation of its immune ef ficacy in speci fic-pathogen-free chicks
TIAN Qiu-feng, ZHOU Wei, SI Wei YI Fei, HUA Xin YUE Min, CHEN Li-ping LIU Si-guo YU Shen-ye
1 Division of Bacterial Diseases, State Key Laboratory of Veterinary Biotechnology, Harbin Veterinary Research Institute, Chinese Academy of Agricultural Sciences, Harbin 150069, P.R.China
2 Heilongjiang Bayi Agricultural University, Daqing 163319, P.R.China
3 College of Animal Sciences, Zhejiang University, Hangzhou 310058, P.R.China
RESEARCH ARTICLE
Construction of Salmonella Pullorum ghost by co-expression of lysis gene E and the antimicrobial peptide SMAP29 and evaluation of its immune ef ficacy in speci fic-pathogen-free chicks
TIAN Qiu-feng1,2*, ZHOU Wei1,2*, SI Wei1, YI Fei1,2, HUA Xin1, YUE Min3, CHEN Li-ping1, LIU Si-guo1, YU Shen-ye1
1 Division of Bacterial Diseases, State Key Laboratory of Veterinary Biotechnology, Harbin Veterinary Research Institute, Chinese Academy of Agricultural Sciences, Harbin 150069, P.R.China
2 Heilongjiang Bayi Agricultural University, Daqing 163319, P.R.China
3 College of Animal Sciences, Zhejiang University, Hangzhou 310058, P.R.China
In this study, a safety enhanced Salmonella Pullorum (S. Pullorum) ghost was constructed using an antimicrobial peptide gene, and evaluated for its potential as a Pullorum disease (PD) vaccine candidate. The antimicrobial peptide SMAP29 was co-expressed with lysis gene E to generate S. Pullorum ghosts. No viable bacteria were detectable either in the fermentation culture after induction of gene E- and SMAP29-mediated lysis for 24 h or in the lyophilized ghost products. Speci fic-pathogenfree (SPF) chicks were intraperitoneally immunized with ghosts at day 7 of age and no mortality, clinical symptoms or signs of PD such as anorexia, depression and diarrhea were observed. On challenge with a virulent S. Pullorum strain at 4 wk post-immunization, a comparatively higher level of protection was observed in the S. Pullorum ghost immunized chickens with a minimum of pathological lesions and bacterial loads compared to the birds in inactivated vaccine groups. In addition,immunization with the S. Pullorum ghosts induced a potent systemic IgG response and was associated with signi ficantly increased levels of cytokine IFN-γ and IL-4 and relative percentages of CD4+and CD8+T lymphocytes. Our results indicate that SMAP29 can be employed as a new secondary lethal protein to enhance the safety of bacterial ghosts, and to prepare a non-living bacterial vaccine candidate that can prevent PD in chickens.
Salmonella Pullorum, bacterial ghost, antimicrobial peptide, immune response, immune protection
1. Introduction
Pullorum disease (PD), caused by Salmonella Pullorum(S. Pullorum), is one of the most serious diseases in the poultry industry (Barrow and Freitas Neto 2011). S. Pullorum is transmitted both vertically and horizontally by a variety of means which include egg transmission, shell penetration,feed contamination, contact transmission either in the hatcher, brooder, cages or floor, cannibalism of infected birds, egg eating, and through wounds on the skin(Shivaprasad 2000). It can infect chicks at various ages resulting in systemic infection with symtoms including depression, anorexia, dehydration, diarrhoea and adherence of faeces to the vent. Mortality ranges from 0 to 100%,especially in chicks and poults. The greatest mortality usually occurs in birds of 2 to 3 wk of age, with a rapid decline between the 3rd and 4th wk (Shivaprasad 2000).The survivors usually become chronic carriers and may not mature into well developed laying or breeding birds. The production performance of laying hens is severely affected by the bacterium which can enter the egg through the reproductive tract, causing decreased laying, loss of weight,diarrhea, and vertical transmission.
Elimination of infected or disease carriers is the key to controlling S. Pullorum. PD is rare in North America due to a breeding flock testing program, but still common in the rest of the world, especially in some developing regions and countries such as Central and South America, Africa,Eastern Europe, South-East Asia, India and China. The major problem of direct elimination in highly endemic areas is the high economic losses. However, on the other hand, appropriate antibiotic therapy is not enough to treat PD (Barrow and Freitas Neto 2011). In addition, the longterm use of antibiotics has resulted in many public health problems such as the development of multi-drug resistant strains and drug residues in tissues and eggs. Therefore,in many countries there is an urgent need to explore new measures to control PD including immune protection.
Vaccination is considered as an effective way for prevention of PD. Live attenuated vaccines have been used against other Salmonella serovars but have several limitations, such as being associated with lower body weight, residual virulence and tissue persistence (Barrow and Freitas Neto 2011). Inactivated vaccines have been considered as alternatives to live attenuated vaccines, but some surface antigens may be destroyed or deleted in their preparation, rendering such vaccines unable to stimulate the production of high antibody titres (Peng et al. 2011;Jawale et al. 2014).
Compared with the live attenuated and inactivated vaccines, bacterial ghosts (BGs) are an attractive type of non-living vaccine since they maintain the native shape and structure of bacteria, and are capable of inducing both humoral and cellular immune responses, including in mucosal tissues (Mader et al. 1997; Mayr et al. 2005),and without the potential recovery of virulence. The BG system has, therefore, become a novel platform for genetically engineered vaccine technology (Szostak et al. 1996; Jalava et al. 2002; Tabrizi et al. 2004; Jawale et al. 2014). However, the presence of unlysed bacteria remaining in BG preparations are a key obstacle for their further development. To circumvent the problem, and to increase lysis ef ficiency and safety, different approaches,such as the use of a chromosome-plasmid balanced lethal system (Jawale et al. 2014) or a secondary lethal protein(Haidinger et al. 2003; Kwon et al. 2009) have been used.In this study, we have employed the antimicrobial peptides(AMP) SMAP29 as a secondary lethal protein with the aim of constructing a safety-enhanced S. Pullorum ghost vaccine. Its immune ef ficacy (humoral and cellular immune responses, effect on histopathology of tissues and protection studies) was evaluated and compared with inactivated vaccine in SPF chicks.
2. Materials and methods
2.1. Bacterial strains and plasmids
The bacterial strains, plasmids, and primers used in this study are listed in Table 1. S. Pullorum strain 527 was purchased from the China Veterinary Culture Collection Center (CVCC). Escherichia coli DH5α was used as the host for propagating plasmids. Plasmid pBV220-E,described previously (Yu et al. 2011), was used as template for ampli fication of gene E. The high-copy-number plasmid pBVD constructed in this study comprised the prokaryotic λPR-cI857 temperature-sensitive system with a gene for ampicillin resistance, carrying two expression units each controlled by a λPR promoter for high-level protein expression. Each promoter was followed by a ribosomebinding site and multiple-cloning site (MCS) region. A rrnbT1T2 terminator followed the second MCS. This vector provided the option of co-expressing target genes inserted into each MCS at 42°C in the form of a fusion (Fig. 1-A).
2.2. Construction of pBVD vector and lysis plasmids
A 1 219-bp nucleotide fragment consisting of two redesigned MCSs and a λPR promoter containing a mutation (T→C)at the ninth nucleotide of the OR2 in the PR promoter was synthesized and cloned into pUC57 by GenScript Corp(Nanjing, China). The target fragment was digested and inserted into BglII/PstI sites of pBV220 to construct a new vector. After sequencing, the positive vector was designated as pBVD. Subsequently, nucleotide fragments of melittin and DsRed1 were ampli fied and sequentially inserted into NdeI/KpnI and KpnI/XbaI sites of MCS2, resulting in a plasmid named pBVD-meliDsRed for veri fication of expression of melittin. Confocal laser scanning microscopy was performed with slight modi fication to a previous method(Yu et al. 2013). In brief, overnight cultures of S. Pullorum strain 527 harboring pBVD-meliDsRed were transferred into fresh Luria Bertani (LB) broth and inoculated at 35°C. WhenOD600value reached 0.6, production of the fusion fluorescent protein melittin-DsRed was induced by shifting the temperature to 42°C for 2 h. After fixing and resuspension,samples (100 μL) were plated on a glass bottom cell culture dish (Nest Biotech, Wuxi, China). After blocking with bovine serum albumin (BSA) (2%, w/v) and staining nuclear contents with 4´,6´-diamidino-2-phenylindole (DAPI), the dishes were washed 10 times in phosphate buffered saline(PBS, pH 7.0) and examined immediately using a Leica TCS SP5 Laser Confocal Microscope with a 100× oil immersion objective (Leica, Mannheim, Germany).

Table 1 The bacterial strains, plasmids and primers used in this study

Fig. 1 Construction of Salmonella Pullorum (S. Pullorum) ghosts. A, physical map of thermo sensitive non-fusion co-expressing vector pBVD. AmpR, ampicillin resistance gene; ori, replication origin; cIts857, restraining gene of lambda bacteriophage adapted to heat induced expression; PR, tandem promoters of lambda bacteriophage for the high level expression; RBS, ribosome-binding site; MCS1 and MCS2, multiple cloning sites; rrnBT1T2, ribosome rrnB gene providing translation stop signal; NcoI and SalI,restriction sites for insertion of gene E; NdeI and XbaI, restriction sites for insertion of antimicrobial peptide. B, confocal laser scanning microscope images merged with red and blue fluorescence images. Bar=1 μm. Scanning electron microscope of a S. Pullorum after (C) and before (D) lysis. Bars=1 μm.
Primers E-U-NcoI and E-L-SalI in Table 1 were used for ampli fication of the PhiX174 gene E using pBV220-E DNA as the template, in a 50-μL PrimeSTAR Max DNA polymerase (TaKaRa, Dalian, China) reaction with the following ampli fication cycle parameters: 5 min at 95°C,followed by 30 cycles of 30 s at 94°C, 30 s at 59°C, 1 min at 72°C. The PCR product was gel-puri fied and ligated into the pBVD MCS1 and transformed into DH5α-competent cells which were then streaked onto LB plates containing ampicillin (100 μg mL–1). After overnight incubation at 35°C,colonies on the plates were collected by scraping. Plasmid DNA was puri fied by using a plasmid DNA isolation kit. The resulting plasmids were then assessed by PCR, NcoI/SalI digest and sequencing, and the positive clone was named pBVD-E. Subsequently, 8 nucleotide fragments of AMPs from different species listed in Table 1 were ampli fied by splicing overlap extension PCR (SOE-PCR) and inserted into MCS2 of pBVD-E. The recombinant co-expressing plasmids pBVD-E-AMPs were con firmed by sequencing.All the constructed plasmids were transformed individually into S. Pullorum strain 527 for the further use.
2.3. Cell lysis by pBVD-E and pBVD-E-AMPs
S. Pullorum strain 527 containing pBVD-E or pBVD-E-AMPs was inoculated into 10 mL of LB broth containing ampicillin(100 μg mL–1). When the culture reached an OD600=0.4,5 mL of the culture was transferred from 35 to 42°C to induce E & AMP-mediated lysis. To compare the viable cell count of the culture at 35 and 42°C, the remaining 5 mL of culture was then grown at 35°C. Samples were collected and analysed every 30 min before and after induction until the OD600no longer decreased. The cultures were diluted to the same concentration, streaked onto four LB plates containing ampicillin (100 μg mL–1), and incubated at 35°C for 12 h.Viable colonies were counted and the results expressed as CFU mL–1, and lysis ef ficiency was then determined according to the formula: Lysis ef ficiency=(1–CFU after induction/CFU before induction)×100%.
The lysis condition of pBVD-E-SMAP29 in S. Pullorum strain 527 was optimized using a method similar to that described above except that the culture was induced at 43.5°C when the OD600value had reached 0.4, 0.6, 0.8 and 1.0, respectively.
2.4. Production of S. Pullorum ghosts and inactivated vaccine
S. Pullorum strain 527 harboring pBVD-E-SMAP29 was grown at 35°C in LB broth supplemented with ampicillin(100 μg mL–1) in a 15-L bioreactor (BIOSTAT C-15, Sartorius,Göttingen, Germany). When an OD600value of 0.6 was reached, the temperature was shifted from 37 to 43.5°C to promote E- and SMAP29-mediated lysis, cell viability was counted at different time points (1, 2, 3, 6, 12 and 24 h). Cells were harvested by centrifugation at 5 000×g for 20 min after the completion of lysis. Pellets were subsequently washed and diluted with PBS (pH 7.2) to a final concentration of 1.5×109CFU equivalent mL–1. After lyophilization, viable cells were counted as described above. The morphological features of the ghosts were characterized by scanning electron microscopy (SEM) as described previously(Yu et al. 2011). The inactivated S. Pullorum strain 527 vaccine was prepared as described previously (Peng et al.2011). Brie fly, the cultures were grown overnight at 37°C,harvested and inactivated with formalin (0.3%, w/v) for 24 to 48 h, washed three times with PBS (pH 7.2), emulsi fied with equivalent amounts of complete Freund’s adjuvant (for first injection) or incomplete Freund’s adjuvant (for booster injections) to final concentration of 1.5×109CFU mL–1. A 200-μL volume was randomly sampled from both the prepared ghost and inactivated vaccine and cultured on LB plates overnight to check for bacterial growth.
2.5. Vaccination and challenge of SPF chicks
SPF chicks were obtained from the Experimental Animal Center of Harbin Veterinary Research Institute (China).Procedures with chicks were approved by the Animal Ethics Committee of Harbin Veterinary Research Institute of the Chinese Academy of Agricultural Sciences (SYXK (H)2006-032). The 1-d-old SPF chicks were randomly divided into three groups (29 in each group) and housed separately. The chicks in experimental groups were immunized at 7 d of age with S. Pullorum ghosts (1.5×108CFU equivalent/0.1 mL/chick) or inactivated vaccine (1.5×108CFU equivalent/0.1 mL/chick). In the control group, chicks were injected with an equivalent volume of PBS. Each chick was subcutaneously injected at multiple sites (multi-point) twice at 2-wk intervals. Three chicks were euthanized for flow cytometry every 2 wk after the first immunization. Two weeks after the second immunization, all chicks received an intraperitoneal challenge with 1×1010CFU/chick of S. Pullorum strain 527.
2.6. Antibody determination
The levels of anti-S. Pullorum antibodies in sera were determined by enzyme-linked immunosorbent assays(ELISA) as described previously (Peng et al. 2011). Brie fly,96-well microtiter plates were coated by overnight incubation with 100 μL of sonicated S. Pullorum strain 527 cell protein suspension. After blocking with skimmed milk in PBS (5%,w/v), the wells were sequentially washed and incubated with 50 μL of a 1:100 dilution of chicken sera in PBS containing Tween-20 (0.05%, w/v, PBS-T) at 37°C for 1 h and 50 μL of HRP-conjugated sheep anti-chicken IgG (H+L) diluted 1:5 000 in PBS-T. Subsequently wells were incubated with TMB (3,3´,5,5´-tetramethylbenzidine) substrate solution, the reaction stopped by addition of 2 mol L–1sulfuric acid and the OD450readings were recorded on a Microplate Reader(Elx800, Bio-tek, Winooski, VT, USA). Sera were tested in triplicates and the mean OD value was calculated for each serum sample. Chicken sera were tested every week after the first vaccination.
2.7. Analysis of CD4+ and CD8+ T cell subset by flow cytometry
Cell suspensions from the spleens of chickens were prepared as described by Peng et al. (2011). A total of 5×105splenocytes/0.1 mL were incubated with 0.1 mL of 1:500-diluted fluorescein isothiocyanate (FITC)-conjugated mouse anti-chicken CD4 or anti-chicken CD8 monoclonal antibodies (SouthernBiotech, Birmingham, AL, USA) in the dark at 4°C for 30 min. After washing the cells with cold PBS thrice, cells were analyzed on a flow cytometer (FACSAria,BD Biosciences, San Jose, CA, USA). Analysis of 10 000 collected events was performed using the CellQuest software (BD Biosciences). Normal chicken spleen cells were used as the negative control.
2.8. Cytokine analysis
Chicken sera were collected every 2 wk after the first vaccination. The levels of IFN-γ and IL-4 in sera were measured by Sandwich ELISA Test Kits (Cloud-Clone Corp, Houston, TX, USA) according to the manufacturer’s instructions.
2.9. Histopathological examination and bacterial load
Viable chicks from all experimental groups were euthanized on day 14 post-challenge. All birds were subjected to necropsy, and gross lesions were recorded according to the methods described previously (Peng et al. 2011).Liver, spleen, small intestine, thymus, kidney and heart were excised aseptically into samples of 0.5 to 1.0 cm3,then immediately fixed in 10% formol saline for 6 to 24 h,embedded in paraf fin, cut into 5–6 μm thin sections using a microtome (Microm HM355S, Thermo Scienti fic,Kalamazoo, MI, USA), placed onto slides and dried. The slides were washed with xylene and dehydrated with ethanol, subsequently stained with hematoxylin and eosin and examined under 100× optical magni fication in a bright field microscope. Lesion scores were given using a system similar to the one previously reported (Chaudhari et al.2012). The severity of the pathological changes in each bird was evaluated per 10 random microscopic fields using a magni fication of ×100 per slide and assigned a score of 0, 1, 2, or 3. A score of 0 indicated no lesions whereas a score of 1 was given for each case of vascular congestion in various organs, enteritis or necrosis with fibrinous exudation and in filtration of a mixed population of in flammatory cells composed of heterophils, macrophages, lymphocytes and a few plasma cells, resulting in a maximal score of 3 for each bird. The lesion score for dead birds in all groups was 3. The arithmetic mean and standard deviation were determined for each group. The CFUs per g of homogenized tissues of cecum, spleen and liver were determined as described previously (Peng et al. 2011; Sancho et al. 2014). Brie fly,the tissues were aseptically excised, weighed, homogenized and 10-fold serially diluted, and 0.1 mL of 103-, 104-, and 105-fold dilutions were plated on selective plates and incubated at 37°C overnight. The CFUs per g of homogenized tissues were counted. The number of positive samples per total number of samples in each group after bacterial recovery was calculated to examine the percent ef ficacy of protection.
2.10. Statistical analysis
All statistical analyses were performed using GraphPad Prism 5 software (La Jolla, CA, USA). Analysis of variance(ANOVA) was used to determine the signi ficance of differences in the means between multiple experimental groups. Data were expressed as the means±standard deviation (SD).*P<0.05 was considered statistically signi ficant;**P<0.01 and***P<0.001 were considered extremely signi ficant.
3. Results
3.1. Construction of lysis plasmids
A new thermo-sensitive, co-expressing vector pBVD was constructed based on the backbone of plasmid pBV220(Fig. 1-A), in which the mutant PR promoter, that can stably repress the expression of genes (Jechlinger et al. 2005),was inserted into MCS2. To ensure the expression of any gene cloned into MCS2 of pBVD, a non-lysis plasmid named pBVD-meliDsRed was constructed with DsRed1 inserted downstream of the melittin. Fluorescence microscopy results showed that bacteria fluoresced bright red after induction at 42°C for 2 h, indicating that the melittin was successfully expressed in the form of fusion protein with DsRed (Fig. 1-B).
PhiX174 gene E was cloned into MCS1 by digesting the vector with NcoI and SalI enzymes to construct single-gene lysis plasmid pBVD-E. Individual nucleotide fragments of AMPs from eight species, including swine, bovine, bacteria,silkworm and bee, were ampli fied by SOE-PCR and inserted into the MCS2 NdeI/XbaI site of pBVD-E, thereby resulting in a lysis system mediated by protein E and AMP.
3.2. Lysis pattern of pBVD-E and pBVD-E-AMPs
At first, S. Pullorum ghost formation was carried out by shifting the culture from 35 to 42°C with a starting OD600=0.4.The lysis ef ficiency of each constructed lysis plasmid was as follows: cecropin P1 99.374%, melittin 99.381%, indolicidin 99.906%, cathelicidin 98.921%, magainin II 98.454%, nisin Z 99.097%, SMAP-29 99.988%, protegrin-1 99.886%, and pBVD-E 98.355% (Table 2). Therefore, pBVD-E-SMAP29 was selected and optimized for lysis ef ficiency in S. Pullorum at different concentrations and induction durations and shifting the induction temperature to 43.5°C. The results showed that the lysis ef ficiency was as high as 99.999% at the best starting concentration of OD600=0.6 and induction for 3 h (Table 3).
Production of S. Pullorum strain 527 ghosts in a 6-L liquid culture was accomplished under fermentation conditions with optimized parameters. Both of the OD600and the cell viability decreased during 3 h after E- and SMAP29-mediated lysis and remained constant for the next 24 h(Table 4). The concentration of viable cells decreased 107-fold after induction for 3 h, and no viable bacteria weredetected after induction for 24 h (Table 4).
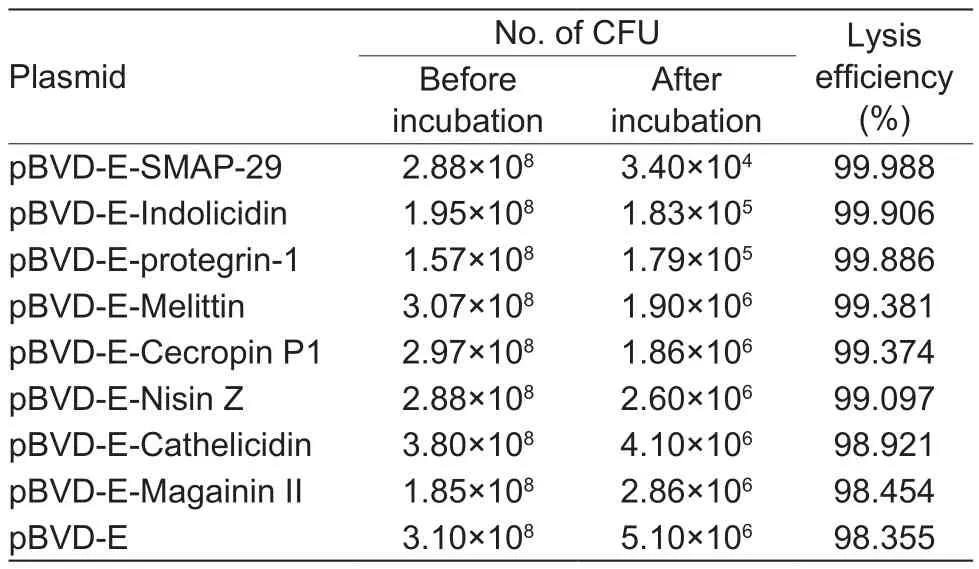
Table 2 Comparison of lysis ef ficiency of pBVD-E and pBVDE-AMPs1)

Table 3 pBVD-E-SMAP29 lysis ef ficiency at different concentrations and induction duration
As shown in the SEM images, S. Pullorum strain 527 ghosts retained the basic cell morphology of bacterial cells(Fig. 1-C and D), although it displayed obvious surfacewrinkles and clear lysis lesions, located mainly at the cell poles. Overall, the BGs produced in our study exhibited relatively complete outer membranes with little change in morphology.

Table 4 Viable cell count after induction of pBVD-E-SMAP29 mediated lysis
3.3. Serological analysis
The systemic IgG antibody to the speci fic antigen of S. Pullorum was evaluated by indirect ELISA at each week post immunization. As determined by matrix titration, the optimal concentration or dilution was 5 μg mL–1for sonicated bacterial protein, 1:100 for chicken sera and 1:5 000 for the secondary antibodies. As shown in Fig. 2-A, the immunized chicks in both the BG and inactivated vaccine groups demonstrated signi ficant elevation in plasma IgG levels. On the first week post immunization (wpi), the vaccinated chicks showed signi ficant differences in antibody levels from those in the control group (P<0.001). The IgG levels of the vaccinated birds were signi ficantly increased on the 4th wpi in comparison with those at the 1st wpi. The differences between the BG and inactivated vaccine groups were not signi ficant over time (P>0.05).

Fig. 2 Detection of immunological parameters. A, IgG levels against the sonicated Salmonella Pullorum protein in chicken sera.B and C, IFN-γ and IL-4 levels in chicken sera. D and E, percentage of CD4+ and CD8+subsets in total T cells. The single peak on the left in each histogram was the negative control. Data are expressed as the means±SD values. *, P<0.05 was considered statistically signi ficant; ** and ***, P<0.01 and P<0.001 were considered extremely signi ficant, respectively; ns, no signi ficant difference.
The concentrations of IFN-γ and IL-4 in the different groups are summarized in Fig. 2-B and C. At 2 wk after the 1st immunization, IFN-γ levels in the sera of vaccinated chicks were signi ficantly different from those in the control group(P<0.001), and the differences of IFN-γ levels between the BG and inactivated vaccine groups were signi ficant (P<0.01). At 4 wk after the 1st immunization,the same reults were obtained except that the P-value between the BG and inactivated vaccine groups was less than 0.05. As to IL-4, the levels in the sera of vaccinated chicks at the two timepoints were signi ficantly different from those in the control group (P<0.001). However, no signi ficant differences of IL-4 between the two vaccinated groups were observed (P>0.05).
3.4. The relative proportion of spleen CD4+ and CD8+ T cell subsets
Splenocytes from the chicks in each group were analyzed by flow cytometry.During the 4 wk after immunization, the number of CD4+and CD8+T cells showed a similar pattern of expansion (Fig. 2-D and E). On the second wpi, the number of CD4+and CD8+T cells in the spleens of the vaccinated group increased,whereas the percentage of splenic CD4+and CD8+T cells in S. Pullorum ghost group was signi ficantly higher than that of the control group (P<0.001), whereas the difference between the two vaccinated groups was not signi ficant (P>0.05).On the fourth wpi, the percentage of CD4+and CD8+T cells in the S. Pullorum ghost group was signi ficantly higher than that of the inactivated vaccine and the control group (P<0.001), with the difference between the latter two groups being signi ficant (P<0.001).
3.5. General conditions in chickens after immunization and the ef ficacy of the ghost vaccine against challenge
No mortality, obvious clinical symptoms, or in fluence on body weight gain were observed in any of the immunized chickens after intraperitoneal injection of S. Pullorum ghosts, and no Salmonella were isolated from the liver, spleen and kidney before challenge (data not shown), indicating the safety of the Salmonella Pullorum ghost vaccine used.
Within 14 d post-challenge with the virulent S. Pullorum strain 527, the percentage of mortality of the ghost immunized group was only 5%, signi ficantly lower than that of the control group (60%, P<0.01, Table 5). There was no signi ficant difference in mortality between the control and inactivated vaccine groups (30%, Table 5). The differences between the survival rate of the two vaccinated groups were signi ficant (P<0.05, Fig. 3), the surviving chicks in each group exhibited different symptom statuses. Only a few cases in the ghost group presented with drowsiness or lassitude, and the remainder presented no abnormal symptoms. In contrast abnormal symptoms of listlessness, drowsiness and anorexia were observed in more chicks in the inactivated vaccine group. In comparison, chicks in the control group presented serious symptoms of anorexia,diarrhea and depression, such as listlessness, twisted necks with drowsiness,a tendency to huddle, fluffed feathers, diminished feed consumption, sticky droppings and staining of the feathers around the anus.


Fig. 3 Comparison of survival rate after challenge with Salmonella Pullorum strain 527. *, P<0.05 was considered statistically signi ficant; ***, P<0.001 were considered extremely signi ficant; ns, no signi ficant difference.
3.6. Histopathologic examination after challenge
To evaluate the protective ef ficacy of the ghost vaccine,the surviving birds were euthanized at day 14 postchallenge for postmortem examination. The lesion severity was scored from 0 to 3 as described in “Materials and methods”. Pathological changes in control chicks occurred systemically including the liver, spleen, kidney and small intestine. Congestion, hemorrhage, necrosis and parenchymal consolidation were observed in conjunction with a substantial in filtration of in flammatory cells. The lesion severity of chicks in the inactivated vaccine group was less than that found in control chicks, which presented moderate lesions, with congestion, hemorrhage, serous and fibrinous exudation in some areas and in flammatory cell in filtration. The chicks in the ghost immunized group were less affected by the challenge infection than those in other two groups (Table 5 and Fig. 4), with no severe and moderate lesions observed, and most close to the normal group in terms of pathology. Congestion and in flammatory cell in filtration exist only mildly in some tissues.
3.7. Bacteriological analysis of tissue homogenates
As shown in Table 5, the number of recovered CFUs in homogenized chicken liver, spleen and cecal tissues was signi ficantly lower in the S. Pullorum ghost group compared to the control group (P<0.001, respectively), and it was also signi ficantly lower than the inactivated vaccine group in cecum and liver (all P<0.05). The differences in bacterial load were signi ficant between the inactivated vaccine group and control group in cecum, liver and spleen (all P<0.01). The number of S. Pullorum-positive chickens was signi ficantly lower in the immunized groups than in the control at day 14 post-challenge, it was also signi ficant between S. Pullorum ghost and inactivated vaccine groups.
4. Discussion
The development of an effective vaccine to protect chicks from Salmonella infection is one of the critical strategies for the control and prevention of PD. BGs represent a new approach in vaccine development and have been applied to a variety of Gram-negative bacterial vaccine candidates.Many efforts have been made to enhance the lysis ef ficiency and safety for large-scale production and industrialization of BGs. A recent report employed a chromosome-plasmid balanced lethal system, which resulted in safety-enhanced Salmonella ghosts (Jawale et al. 2014). Chromosome gene deletions, however, make this technique exceptionally challenging, especially for those bacteria where genetic manipulation is problematic. Additionally, it remains to be seen whether strains engineered in this fashion can maintain the immune determinants of their parental bacteria. The intracellular synthesis of Staphylococcal nuclease A (SNA),as a secondary lethal protein, has been successfully used in combination with the protein E-mediated lysis system(Haidinger et al. 2003; Kwon et al. 2009). Although this approach resulted in the removal of the DNA content in the ghost preparation, the ideal concentration and safety of SNA are uncertain. We therefore decided to focus on AMPs as an alternative secondary lethal protein strategy. AMPs are small peptides with broad-spectrum antibacterial activity and are an integral part of the innate immune system that protects the host from invading pathogenic bacteria (Brogden 2005;Nguyen et al. 2011). Firstly, based on the backbone of plasmid pBV220 (Yu et al. 2011), a thermo-sensitive nonfusion co-expressing vector, pBVD, was constructed, which was approximately 3-fold smaller than a previously reported vector (Kwon et al. 2009), making it more convenient for operation and transformation. Since we had no antiserum against the AMPs, fluorescent protein DsRed was inserted downstream of the gene encoding mellitin to enable expression to be determined. The results showed that the fusion protein Melittin-DsRed was successfully expressed,indicating that the nucleotide fragments inserted into MCS2 can be expressed correctly. Subsequently, the lysis gene E of phiX174 and individual nucleotide fragments of AMPs from eight species (probiotics, silkworm, honeybee, snake,frog, sheep, cow, and swine) were inserted into MCS1 and MCS2. In comparison with the lysis plasmid pBVD-E, all eight pBVD-E-AMPs demonstrated higher lysis ef ficiency,with pBVD-E-SMAP29 being the highest (99.988%). For optimization, different starting concentrations and induction durations were tested using an induction temperature shift to 43.5°C. The lysis ef ficiency was raised to as high as 99.999% with induction for 3 h using a starting OD600=0.6.The transmembrane lesion was clearly present. We noticed that protein E alone could not ef ficiently lyse S. Pullorum cells as it did with S. enteritidis under similar conditions(Peng et al. 2011). The weakened onset of lysis was most likely from either reduced susceptibility of the S. Pullorum cell envelope to protein E, or the reduced power of the λpR-cI857 system in the parent host strain of S. Pullorum.No viable bacteria were detected either in the culture after induction for 24 h or in lyophilized ghost products.

Fig. 4 Histopathological analysis after challenge with Salmonella Pullorum strain 527. The pathological lesions of dissected tissues are listed below. A, mild thrombosis in small liver vessels. B, liver sinusoidal congestion, thrombosis in small liver vessels.C, large-scale congestion. D, normal. E, mild de ficiency of lymphocytes in the splenic corpuscle. F, hyperplasia of red pulp,white intramedullary lymphopenia, lymphopenia around the arterial sheath. G, spleen congestion, signi ficant reduction of white intramedullary lymphocytes. H, normal. I, mild small renal vessel congestion. J, small renal vessel congestion, edema. K, serious kidney congestion. L, normal. M, mild congestion in small blood vessels of intestinal lamina propria. N, intestinal epithelial necrosis and collapse, congestion in small blood vessels of intestinal mucosa, mild neutrophil in filtration in the lamina propria. O, intestinal mucosa necrosis and collapse, signi ficant neutrophil in filtration in intestinal lamina propria. P, normal. Q, mild slurry precipitation of thymic medulla, mild reduction of parenchymal lymphocyte. R, congestion in small thymic vessels, mild reduction in thymic medullary lymphocytes, mild slurry precipitation of thymic medulla. S, mild reduction in thymic medullary lymphocytes and mild neutrophil in filtration. T, normal. U, mild congestion. V, mild edema, congestion in small blood vessels. W, mild congestion in small blood vessels. X, normal. Bars=200 μm.
The humoral immune response plays an important role in preventing Salmonella infection. Many subunit vaccine candidates from components of Salmonella have been reported to induce passive humoral immunity and provide effective protection (Sood et al. 2005; Gómez-Verduzco et al. 2010). BGs retain all morphologic and antigenic features of the viable cell and are therefore potentially superior to inactivated and subunit vaccines. We and others have shown that Salmonella ghost vaccines can evoke a high-level humoral immune response (Peng et al.2011; Jawale et al. 2014). The S. Pullorum ghost vaccine described here stimulated SPF chickens to secrete high systemic IgG titers, with the antibody titer increasing continually with a peak at the fourth wpi. From the first wpi,the antibody titer in the ghost group was higher than that in the control group which was statistically signi ficant and the inactivated vaccine group (although not signi ficantly),indicating that the ghost vaccine was relatively superior to the inactivated vaccine in promoting antibody production.High titers of circulating antibodies is of great value for the elimination of extracellular bacteria (Li et al. 2013), and our previous study showed that maternal antibodies present in eggs ef ficiently protected the newly hatched chickens from S. Enteritidis infection (Si et al. 2014). However, the high titers of antibodies produced by the ghost vaccine will interfere with serological monitoring for S. Pullorum in chickens, in particular breeders. Therefore, further work will focus on the role of maternal antibodies in protection of newly hatched chickens from PD and development of serological markers for differential diagnosis of vaccinated and naturally infected chickens.
It is well known that cell-mediated immune responses play a central role in eliminating intracellular bacterial pathogens,such as Listeria monocytogenes (Harty and Bevan 1992;White and Harty 1998), Mycobacterium tuberculosis(Flynn et al. 1992) and Salmonella (Lo et al. 1999; Lu et al. 1999; Mittrucker and Kaufmann 2000; Zhu et al.2010). Therefore, we investigated the dynamic changes in CD4+and CD8+T-lymphocyte populations and the levels of IL-4 and IFN-γ in immunized chickens to evaluate the cellular immune responses evoked by S. Pullorum ghosts.The results showed that the S. Pullorum ghosts induced higher numbers of CD4+and CD8+T lymphocytes, and the proportions of both CD4+and CD8+T cells in the immunized group increased continually after vaccination, and were signi ficantly higher than that of the control and inactivated vaccine groups. On the other hand, IL-4 and IFN-γ levels in the sera of vaccinated chicks were signi ficantly higher than those in the control group, and the differences of IFN-γ levels between the ghost and inactivated vaccine groups were signi ficant. Previous results showed a similar pattern of T helper 1 (Th1) response and cell-mediated immunity triggered by immunization with S. Enteritidis and S. Gallinarum ghosts (Peng et al. 2011; Won et al. 2016) or attenuated Salmonella vaccines (Sztein et al. 1995; Mader et al. 1997; Lundin et al. 2002; Salerno-Goncalves et al.2002), whereas this type of immune response is rarely induced by inactivated Salmonella (Chong et al. 1996).
It has been previously reported that BG-vaccinated animals exhibit both a robust immune response and resistance to infection by highly virulent strains (Hensel et al.2000; Jalava et al. 2002; Panthel et al. 2003; Mayr et al.2005). Here we have demonstrated that the constructed S. Pullorum ghosts not only stimulate high-level humoral and cellular immune responses but also effectively protected chicks against experimental PD infection. The ghost immunized group showed a mortality of 5% upon challenge with a high dose of a virulent S. Pullorum strain,signi ficantly lower than the control (60%), and comparatively lower than the inactivated vaccine group (30%), while no signi ficant difference was observed between the control and inactivated vaccine groups. A similar trend was also evident in the pathological changes. The lesion severity of chicks in the ghost group was signi ficantly lower than the control. Furthermore, the clearance pattern of the challenge bacteria from the liver, spleen and cecum of the immuninzed chickens was obviously different to that of the control. The surviving vaccinated chickens in the ghost group had signi ficantly lower CFUs in the cecum and liver than those in the control and inactivated vaccine groups. It is known that S. Pullorum infection can persist for more than 40 wk despite the presence of high-titre circulating speci fic antibody, and the clearance of S. Pullorum from splenic macrophages being slow (Wigley et al. 2001). However, in the present study, no challenge strain was detected in any of the internal organs of some of the immunized chickens at day 14 post-challenge (i.e., 7 wk of age) in the ghost vaccined group, indicating that infection had been decreased by the immunizations. Two possible explanations for the immune clearance of S. Pullorum are either that infected macrophages are detectable by T cells within the infected bird, or that the pattern of immune response is modulated during immunization. Previous literature has reported a degree of modulation by S. Pullorum towards a Th2-type response associated with high levels of antibody and poorer cell-mediated immunity (Chappell et al. 2009). However,as described above, immunization with S. Pullorum ghosts induced much higher levels of expression in vivo of the Th1-associated cytokine IFN-γ. Notably, compared to the inactivated vaccine with supplemented adjuvant, no adjuvant was added in the preparation of bacterial ghosts for immunization in this study. The resulting lower cost and simpler production process make the ghost vaccine more attractive than inactivated vaccines. Systemic PD has been controlled in the USA over decades via a series of strict eradication measures, whereas developing countries can not afford the high economic burden inherent in such elimation approaches. S. Pullorum ghosts, such as the one developed in this study, provide a promising auxiliary approach to reduce the economic loss within the poultry industry in highly endemic areas. Further work would focus on extending this study to look at increased survival of adult birds following vaccination.
5. Conclusion
Based on all of the above results, AMPs especially SMAP29 can be employed as new secondary lethal proteins coexpressed with lysis gene E to produce safety-enhanced S. Pullorum ghosts. The resulting ghosts could induce powerful humoral and cell-mediated immune responses in SPF chickens, as well as providing effective protection against high doses of wild-type strains. As an integral part of the innate immune system, the speci fic role of AMPs in ghost immunization requires further investigation.
Acknowledgements
We are grateful to Prof. Paul R. Langford (Imperial College London, London, UK), Dr. Li Xinlei (Harbin Medical University, Harbin, China) and journal referees for important comments and critical reading of the manuscript. This work was supported by grants from the National Key Research and Development Program of China (2016YFD0501608),the National Natural Science Foundation of China(31470893), the Special Fund for Agro-scienti fic Research in the Public Interest, China (201403054) and the National High Technology Research and Development Program of China (2011AA10A210).
Barrow P A, Freitas Neto O C. 2011. Pullorum disease and fowl typhoid - new thoughts on old diseases: A review. Avian Pathology,40, 1–13.
Brogden K A. 2005. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nature Reviews Microbiology,3, 238–250.
Chappell L, Kaiser P, Barrow P, Jones M A, Johnston C, Wigley P.2009. The immunobiology of avian systemic salmonellosis.Veterinary Immunology and Immunopathology,128, 53–59.
Chaudhari A A, Jawale C V, Kim S W, Lee J H. 2012.Construction of a Salmonella Gallinarum ghost as a novel inactivated vaccine candidate and its protective ef ficacy against fowl typhoid in chickens. Veterinary Research,43, 44.
Chong C, Bost K L, Clements J D. 1996. Differential production of interleukin-12 mRNA by murine macrophages in response to viable or killed Salmonella spp. Infection and Immunity,64, 1154–1160.
Flynn J L, Goldstein M M, Triebold K J, Koller B, Bloom B R.1992. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proceedings of the National Academy of Sciences of the United States of America,89,12013–12017.
Gómez-Verduzco G, Tellez G, Quintana A L, Isibasi A, Ortiz-Navarrete V. 2010. Humoral immune response in breeding hens and protective immunity provided by administration of puri fied Salmonella Gallinarum porins. Poultry Science,89, 495–500.
Haidinger W, Mayr U B, Szostak M P, Resch S, Lubitz W.2003. Escherichia coli ghost production by expression of lysis gene E and staphylococcal nuclease. Applied and Environmental Microbiology,69, 6106–6113.
Harty J T, Bevan M J. 1992. CD8+ T cells speci fic for a single nonamer epitope of Listeria monocytogenes are protective in vivo. Journal of Experimental Medicine,175, 1531–1538.
Hensel A, Huter V, Katinger A, Raza P, Strnistschie C, Roesler U, Brand E, Lubitz W. 2000. Intramuscular immunization with genetically inactivated (ghosts) Actinobacillus pleuropneumoniae serotype 9 protects pigs against homologous aerosol challenge and prevents carrier state.Vaccine,18, 2945–2955.
Jalava K, Hensel A, Szostak M P, Resch S, Lubitz W. 2002.Bacterial ghosts as vaccine candidates for veterinary applications. Journal of Controlled Release,85, 17–25.
Jawale C V, Chaudhari A A, Lee J H. 2014. Generation of a safety enhanced Salmonella Gallinarum ghost using antibiotic resistance free plasmid and its potential as an effective inactivated vaccine candidate against fowl typhoid.Vaccine,32, 1093–1099.
Jechlinger W, Glocker J, Haidinger W, Matis A, Szostak M P,Lubitz W. 2005. Modulation of gene expression by promoter mutants of the lambda cI857/pRM/pR system. Journal of Biotechnology,116, 11–20.
Kwon S R, Kang Y J, Lee D J, Lee E H, Nam Y K, Kim S K,Kim K H. 2009. Generation of Vibrio anguillarum ghost by coexpression of PhiX 174 lysis E gene and staphylococcal nuclease A gene. Molecular Biotechnology,42, 154–159.
Li Q, Hu Y, Chen J, Liu Z, Han J, Sun L, Jiao X. 2013.Identi fication of Salmonella enterica serovar Pullorum antigenic determinants expressed in vivo. Infection and Immunity,81, 3119–3127.
Lo W F, Ong H, Metcalf E S, Soloski M J. 1999. T cell responses to Gram-negative intracellular bacterial pathogens: A role for CD8+ T cells in immunity to Salmonella infection and the involvement of MHC class Ib molecules. Journal of Immunology,162, 5398–5406.
Lu S, Manges A R, Xu Y, Fang F C, Riley L W. 1999. Analysis of virulence of clinical isolates of Salmonella enteritidis in vivo and in vitro. Infection and Immunity,67, 5651–5657.
Lundin B S, Johansson C, Svennerholm A M. 2002. Oral immunization with a Salmonella enterica serovar typhi vaccine induces speci fic circulating mucosa-homing CD4+and CD8+T cells in humans. Infection and Immunity,70,5622–5627.
Mader H J, Szostak M P, Hensel A, Lubitz W, Haslberger A G.1997. Endotoxicity does not limit the use of bacterial ghosts as candidate vaccines. Vaccine,15, 195–202.
Mayr U B, Haller C, Haidinger W, Atrasheuskaya A, Bukin E,Lubitz W, Ignatyev G. 2005. Bacterial ghosts as an oral vaccine: A single dose of Escherichia coli O157:H7 bacterial ghosts protects mice against lethal challenge. Infection and Immunity,73, 4810–4817.
Mittrucker H W, Kaufmann S H. 2000. Immune response to infection with Salmonella typhimurium in mice. Journal of Leukocyte Biology,67, 457–463.
Nguyen L T, Haney E F, Vogel H J. 2011. The expanding scope of antimicrobial peptide structures and their modes of action.Trends in Biotechnology,29, 464–472.
Panthel K, Jechlinger W, Matis A, Rohde M, Szostak M P,Lubitz W, Haas R. 2003. Generation of Helicobacter pylori ghosts by PhiX protein E-mediated inactivation and their evaluation as vaccine candidates. Infection and Immunity,71, 109–116.
Peng W, Si W, Yin L, Liu H, Yu S, Liu S, Wang C, Chang Y,Zhang Z, Hu S, Du Y. 2011. Salmonella enteritidis ghost vaccine induces effective protection against lethal challenge in speci fic-pathogen-free chicks. Immunobiology,216,558–565.
Salerno-Goncalves R, Pasetti M F, Sztein M B. 2002.Characterization of CD8+effector T cell responses in volunteers immunized with Salmonella enterica serovar Typhi strain Ty21a typhoid vaccine. Journal of Immunology,169, 2196–2203.
Sancho P, Tejedor C, Sidhu-Munoz R S, Fernandez-Lago L, Vizcaino N. 2014. Evaluation in mice of Brucella ovis attenuated mutants for use as live vaccines against B. ovis infection. Veterinary Research,45, 61.
Shivaprasad H L. 2000. Fowl typhoid and pullorum disease.Revue Scienti fique et Technique - Of fice International des Epizooties,19, 405–424.
Si W, Yu S, Chen L, Wang X, Zhang W, Liu S, Li G. 2014.Passive protection against Salmonella enterica serovar Enteritidis infection from maternally derived antibodies of hens vaccinated with a ghost vaccine. Research in Veterinary Science,97, 191–193.
Sood S, Rishi P, Dhawan V, Sharma S, Ganguly N K. 2005.Protection mediated by antibodies to iron-regulated outermembrane proteins of S. typhi in a mouse peritonitis model.Molecular and Cellular Biochemistry,273, 69–78.
Szostak M P, Hensel A, Eko F O, Klein R, Auer T, Mader H, Haslberger A, Bunka S, Wanner G, Lubitz W. 1996.Bacterial ghosts: Non-living candidate vaccines. Journal of Biotechnology,44, 161–170.
Sztein M B, Tanner M K, Polotsky Y, Orenstein J M, Levine M M.1995. Cytotoxic T lymphocytes after oral immunization with attenuated vaccine strains of Salmonella typhi in humans.Journal of Immunology,155, 3987–3993.
Tabrizi C A, Walcher P, Mayr U B, Stiedl T, Binder M, McGrath J, Lubitz W. 2004. Bacterial ghosts--biological particles as delivery systems for antigens, nucleic acids and drugs.Current Opinion in Biotechnology,15, 530–537.
White D W, Harty J T. 1998. Perforin-de ficient CD8+ T cells provide immunity to Listeria monocytogenes by a mechanism that is independent of CD95 and IFN-γ but requires TNF-α. Journal of Immunology,160, 898–905.
Wigley P, Berchieri A, Page K L, Smith A L, Barrow P A. 2001.Salmonella enterica serovar Pullorum persists in splenic macrophages and in the reproductive tract during persistent,disease-free carriage in chickens. Infection and Immunity,69, 7873–7879.
Won G, Chaudhari A A, Lee J H. 2016. Protective ef ficacy and immune responses by homologous prime-booster immunizations of a novel inactivated Salmonella Gallinarum vaccine candidate. Clinical and Experimental Vaccine Research,5, 148–158.
Yu S, Zhao H, Wang H, Wang X, Shao G, Xu L, Si W, Chen L, Zhang W, Liu S. 2013. Production and characterization of mouse monoclonal antibodies against lysis protein E of phiX174. Journal of Virological Methods,189, 355–361.
Yu S Y, Peng W, Si W, Yin L, Liu S G, Liu H F, Zhao H L, Wang C L, Chang Y H, Lin Y Z. 2011. Enhancement of bacteriolysis of shuf fled phage PhiX174 gene E. Virology Journal,8, doi:10.1186/1743-422X-8-206
Zhu J, Yamane H, Paul W E. 2010. Differentiation of effector CD4 T cell populations. Annual Review of Immunology,28, 445–489.
18 April, 2017 Accepted 4 July, 2017
TIAN Qiu-feng, E-mail: 316308930@qq.com; Correspondence YU Shen-ye, Tel: +86-451-51051733, Fax: +86-451-82733132,E-mail: yushenye@caas.cn; LIU Si-guo, Tel: +86-451-51051737,Fax: +86-451-82733132, E-mail: siguo_liu@hvri.ac.cn
*These authors contributed equally to this study.
© 2018 CAAS. Publishing services by Elsevier B.V. All rights reserved.
10.1016/S2095-3119(17)61696-4
Section editor CHEN Hua-lan
Managing editor ZHANG Juan
 Journal of Integrative Agriculture2018年1期
Journal of Integrative Agriculture2018年1期
- Journal of Integrative Agriculture的其它文章
- Climate change and agriculture: Impacts and adaptive responses in Iran
- Development of elite restoring lines by integrating blast resistance and low amylose content using MAS
- Evaluation of stability and yield potential of upland rice genotypes in North and Northeast Thailand
- ldenti fication of the resistance gene to powdery mildew in Chinese wheat landrace Baiyouyantiao
- New clues concerning pigment biosynthesis in green colored fiber provided by proteomics-based analysis
- Constitutive expression of feedback-insensitive cystathionine γ-synthase increases methionine levels in soybean leaves and seeds
