Effects of different drying methods on quality, bacterial viability and storage stability of probiotic enriched apple snacks
CUI Li, NIU Li-ying, LI Da-jing, LIU Chun-quan, LIU Ying-ping, LIU Chun-ju, SONG Jiang-feng
1 Institute of Farm Product Processing, Jiangsu Academy of Agricultural Sciences, Nanjing 210014, P.R.China
2 College of Food Science and Engineering, Yangzhou University, Yangzhou 225127, P.R.China
RESEARCH ARTICLE
Effects of different drying methods on quality, bacterial viability and storage stability of probiotic enriched apple snacks
CUI Li1, NIU Li-ying1, LI Da-jing1, LIU Chun-quan1, LIU Ying-ping2, LIU Chun-ju1, SONG Jiang-feng1
1 Institute of Farm Product Processing, Jiangsu Academy of Agricultural Sciences, Nanjing 210014, P.R.China
2 College of Food Science and Engineering, Yangzhou University, Yangzhou 225127, P.R.China
Effects of four different drying methods on the colour, texture, sensory quality, microstructure, bacterial viability and storage stability of probiotic-enriched apple snacks were assessed. The drying methods were air drying (AD), freeze drying (FD),freeze drying followed by microwave vacuum drying (FD+MVD) and air drying followed by explosion puf fing drying (AD+EPD).Overall, FD+MVD can be used as a suitable drying method for the development of probiotic enriched apple snacks in consideration of colour, texture, sensory quality, bacterial viability and storage stability. Probiotic bacteria in FD+MVD-dried samples remained above 1×106CFU g–1for 120 days at 25°C. Interestingly, bacterial viability in FD+MVD-dried samples turned out to be signi ficantly higher than FD-dried samples during storage for 120 days.
drying, apple snacks, probiotic viability, microwave vacuum
1. Introduction
Probiotics are de fined as live microorganisms which when administered in adequate amounts confer a health bene fit on the host (FAO and WHO 2006).
Regular consumption of viable probiotics can confer a number of health bene fits such as a reduction of cholesterol(Nguyen et al. 2007), anti-diabetic properties (Yadav et al.2007), improvement of lactose tolerance (Shah 2007), and immune system stimulation (De Moreno de LeBlanc et al.2008).
Currently, industrial probiotic foods mainly belong to dairy products, they have the disadvantages and side effects.With the increased awareness of the relationship between fruit and health, healthier fruit snacks with good taste and probiotics will be in high demand globally.
Many drying methods are used in the food industry to remove moisture from fresh fruits and vegetables in the production of shelf-stable products. Freeze drying (FD)has been widely used to obtain high quality and high-value dehydrated fruit and vegetables. But expensive and very slow dehydration process is its major drawback (Huang et al. 2009). Therefore, it is desirable to evaluate new drying technologies to achieve better product quality and more reasonable cost. Microwave drying is a rapid drying method resulting in products with unique characteristics while retaining fine quality (Wang et al. 2004, 2007;Zhang et al. 2006; Clary et al. 2007). Several different combination drying methods of freeze drying and microwave drying were used to save energy and consumption of FD method. For example, freeze drying followed by microwave vacuum drying (FD+MVD) and microwave vacuum drying followed by freeze drying (MVD+FD) (Hang et al. 2012).FD+MVD was consisted of freeze drying food particulates to a moisture content of about 35%, and following a short microwave treatment. This method can plasticize crunchy and fragile freeze dried fruits and vegetables (Litvin et al.1998). So, FD+MVD has been proved to be an alternative method of drying to obtain high-quality dehydrated fruits and vegetables, which were comparable with freeze dried ones in chemical properties (carotene and vitamin C retention)and physical properties (colour, texture, and rehydration ratio) (Cui et al. 2008).
Pei et al. (2014) used freeze drying (FD) and three different combinations of drying methods: freeze drying combined with hot air drying (FD+AD), freeze drying combined with vacuum drying (FD+VD) and freeze drying combined with microwave vacuum drying (FD+MVD) to dry button mushroom slices. They concluded that FD+MVD method has advantages in terms of ef ficiency and energy saving and could be preferred to produce high-quality products.
Noorbakhsh et al. (2013) evaluated the changes of survival of bacteria, total titratable acidity and sensory evaluation of apple slices dried with FD, air drying followed by radiant energy under vacuum drying (AD+REV), and AD during or after storage at 25°C for 30 days. Radiant energy under vacuum (REV) is a form of vacuum microwave dehydration. They concluded that AD+REV(same as AD+MVD) is a suitable candidate to produce probiotic-enriched dried apple. Hot air drying (AD) is the simplest and popular drying method, but it leads to a large deformation of the products and also thermally-induced deterioration (Ratti 2001; Mujumdar 2004). In our previous research, we also found that AD+MVD apple snacks had large deformation, which gave an unacceptably poor quality product. This result is in agreement with that of Wang et al. (2017), who reported that FD+MVD okra crisp bar showed more crispness and less shrinkage than AD+MVD ones.
This study aimed at developing protocols for producing probiotic-enriched apple snacks with quality comparable or superior to those of AD+MVD ones.
2. Materials and methods
2.1. Cultivation of microorganisms
Lactobacillus plantarum SICC 1.376 was obtained from the Southwest Center of Industrial Culture Collection in China (SICC), and used in the experiments. The stock culture was maintained on MRS agar (Beijing Land Bridge Technology Co., Ltd., Beijing, China) slants at 4°C. The lactic acid bacterium was cultivated in 100 mL (200 mL in some cases) of MRS broth (Beijing Land Bridge Technology Co., Ltd., Beijing, China) in a 250-mL (or 500-mL) flask at 37°C for 12 h, and the final culture growth generally ranged from 3.43×109to 4.23×1010CFU mL–1. The cultures used in all experiments were freshly prepared using the same procedure.
2.2. Sample preparation
Apples (cultivar Fuji) were bought from a local market and stored at 4°C for 24 h before use. Apples were washed,peeled, de-cored and cut into square-shaped samples with(10±0.2) mm length following their vertical axis. Apple cubes were steam blanched for 2 min and were cooled.
2.3. Vacuum impregnation of probiotic
Apple samples’ vacuum impregnation were performed according to Noorbakhsh et al. (2013) and Cui et al. (2017)with some modi fications.
Lyophilized cultures were grown on MRS broth. The cells were harvested by centrifugation at 4 800 r min–1at 4°C for 10 min and were washed twice with sterile 0.85% saline solution. Washed cells were transferred into impregnation liquid, which was sucrose solution.
The vacuum impregnation with the impregnation liquid obtained from the above procedure was performed at 35°C in the glass vacuum-pressure desiccato. Apple cubes were submerged into the impregnation solution in a ratio of 1:2 (w/w)placed in desiccato exposing to vacuum pressure of 0.09 MPa for 15 min. Then atmospheric pressure was restored leaving samples under the liquid for an additional 20 min.
2.4. Drying of probiotic-enriched apple snacks
Probiotic impregnated apple cubes were dried with AD, FD,FD+MVD and AD+EPD in triplicate.
Air drying (AD)Apple cubes (100 g) were dried at 40°C for 12 h in the cabinet air drier. The moisture content of apple cubes at the end of air drying was (2.96±0.2)%.
Freeze drying (FD)Apple cubes (100 g) were first frozen at (-28±3)°C, and FD was performed in a lab-scale freeze dryer (FD-1A-50, Beijing Boyikang Laboratory Instruments Co., Ltd., China) at -50°C with a chamber pressure of 15 Pa for 30 h until constant weight was obtained with (3.83±0.2)%water content.
Air drying followed by explosion puf fing drying(AD+EPD)Apple cubes (100 g) were pre-dried at 40°C for 3 h in the cabinet air drier. The moisture content of apple cubes at the end of air drying was (17.32±0.2)%. The drying process was finished using the electric heating air flow expanding equipment (QDPH-5L; Tanjing Qinde New Material Technology Co., Ltd., Tanjing, China)
The puf fing processes were as follows. First, in the puf fing chamber, the samples were placed on a stainless steel grid and the decompression valve was closed. The samples were indirectly heated to 80°C by the steam generator and maintained at this temperature for 15 min. During sample heating, the puf fing chamber was in flated to an absolute pressure of 0.1 MPa by the air compressor, and the vacuum chamber was evacuated to –0.09 MPa by the vacuum pump.In the depressurizing treatments, the absolute pressure was decreased from 0.1 to –0.09 MPa in the puf fing chamber by opening the decompression valve. The temperature of the puf fing chamber decreased from 80 to 65°C. The puffed samples were vacuum-dried for 90 min under these conditions, yielding puffed apple cubes. All of the parameters were determined by preliminary studies.
Freeze drying followed by microwave vacuum drying(FD+MVD)Apple cubes (100 g) were first frozen at(-28±2)°C, and FD was performed in a lab-scale freeze dryerat -50°C with a chamber pressure of 15 Pa for 10 h until constant weight was obtained with (24.83±0.2)% water content. A microwave vacuum oven (WZD1S; Nanjing Sanle Microwave Technology Development Co., Ltd., Nanjing,China) was used for the microwave vacuum drying process;6 min at 400 W for three times while absolute pressure was at 78.8 kPa.
2.5. Enumeration of L. plantarum in dried apple snacks
Dried apple cubes were powdered and homogenized in sterile 0.85% saline using laboratory Lab-Blender(Stomacher 400, Seward Laboratory, London, UK) at medium speed, for 2 min. Homogenized samples were serially diluted in 0.85% saline solution and spread over a standard agar plate (MRS agar). The plates were incubated at 37°C for 48 h and colonies were counted. The viability of L. plantarum was expressed as the log10of the number of the colony forming units (CFU g–1(dry basis)).
2.6. L. plantarum location and microstruction of dried apple snacks
L. plantarum location and microstruction of dried apple cubes were examined using Scanning Electron Microscopy with a Zeiss EVO-LS10 SEM (Carl Zeiss Microscopy Limited,UK). The samples were analyzed to investigate whether probiotic bacteria had penetrated the apple tissue. The samples were coated with gold (70 s at 20 mA at 25°C) prior to imaging. The microscopic images were taken at random locations within the apple samples and only representative images are shown.
2.7. Sensory evaluation
Triple packages of dried probiotic-enriched apple cubes were taken for sensory evaluation. The affective test methodology was used, in which 20 trained panelists described the texture, flavour, colour, taste and overall acceptability of the samples, based on the 10 points hedonic scale with 10 being extremely good and one being extremely poor. Each panelist was asked to read and sign an informed consent form. A score equal to five was used as a minimum threshold for satisfactory acceptability.
2.8. Determination of physicochemical characteristics
Physical and chemical properties were measured using five wedges per replicate of treated and control samples.Colour and hardness were measured first as described below. Samples were then homogenized for subsequent measurements.
Measurement of hardnessThe hardness of dried apple cubes was measured as the stress at the maximum force using a puncture test. The stress at the maximum force is related to the hardness of the apple cubes. The measurements were performed in a texture analyzer CT3(Brook field Ltd., MA, USA) at a constant speed of 1.0 mm s–1using a cylindrical puncture probe 4 mm in diameter (TA-44). The texture was determined by punching the center of each cube. The maximum force values were recorded in gram. During compression experiments, cross-head speed was 2.5 mm min–1. Before each experiment, a randomly chosen single apple cube dried under the given conditions was placed on the bottom parallel plate and compressed. Compression experiments were performed in 15 replications. The averaged force and energy required to cause 3.0 and 5.5 mm deformation, respectively, were determined on the basis of force-deformation curves.
Measurement of colourThe Hunter colour L*, a*, and b*values were determined by grinding samples into powder using a colour difference meter (WSCS; SHENGUANG,Shanghai Precision Scienti fic Instrument Co., Ltd.,Shanghai, China). The information given by L*, a* and b*is generally expressed as the total colour of apple samples,with positive L* values representing the brightness and negative values representing dullness, a* for redness to greenness, and b* for yellowness to blueness.
2.9. Storage of dried probiotic-enriched apple snacks
Dried apple cubes ((2±0.2) g) were placed in individual aluminum foil bags, flushed with nitrogen prior to sealing.Packages were divided into two groups and stored at 25°C for up to 120 days. Duplicate packages from each dried samples were taken at time intervals and the number of surviving bacteria was enumerated.
2.10. Statistical analysis
Results were reported as means±SD for three replications.In all experiments, one-way analysis of variance in combination with Tukey’s test for individual comparisons was performed. All statistical calculations were made at the P<0.05 level using 2005 SAS (version 9.1; SAS Institute Inc., Cary, NC, USA).
3. Results and discussion
3.1. Survival and location of L. plantarum in dried apple snacks
Bacterial counts of dried apple samples showed that the population of survived L. plantarum was greatly affected by drying methods (Fig. 1). The reduction of the bacterial population in FD-dried samples was 0.79-log, while the reduction of FD+MVD, AD, and AD+EPD was 1.21-, 1.83-and 5.38-log, respectively. Above results showed that FD+MVD was competitive with FD in protecting bacteria during dehydration. FD has been widely used to obtain high-quality and high-value dehydrated fruit and vegetables(Huang et al. 2009). However, it is an expensive and very slow dehydration process. To achieving same moisture content, the time of FD needed (36 h) was almost twice than that of FD+MVD (18.5 h).

Fig. 1 The number of Lactobacillus plantarum in probioticenriched apple cubes after four dehydration methods. AD,air drying; AD+EPD, air drying followed by explosion puf fing drying; FD, freeze drying; FD+MVD, freeze drying followed by microwave vacuum drying. Data are means±SD.
It is recommended that probiotic cultures must be present at suf ficiently high numbers in the products.Although the minimum therapeutic dose remains unclear,the concentration of 1×106–7CFU g–1or mL of products was typically proposed (Shah et al. 2000). Since bacterial counts of AD+EPD-dried samples are below 1×106–7CFU g–1, it is obvious that AD+EPD is not suitable for drying probiotic rich apple cubes.
Results of scanning electron microscopy (SEM)con firmed the presence of rod-shaped bacteria being embedded in the apple cells spaces of all samples(Fig. 2-A–D). Similar observations were reported. In these cases, fruit (apple, guava and papaya) pieces were vacuum impregnated with Lactobacillus (Lactobacillus rhamnosus CECT 245, Lactobacillus casei 01, Lactobacillus rhamnosus CECT 275 and L. rhamnosus ATCC 7469)solution, the cells imbibed in the intercellular spaces of fruit (Betoret et al. 2003; Krasaekoopt and Suthanwong 2008; Puente et al. 2009; Noorbakhsh et al. 2013). If apple samples were just dipped in probiotic solution, cells can only be observed on the surface of apple samples. No bacteria were seen neither within cells nor at intercellular junctions (Rößle et al. 2010).
3.2. Measurement of appearance
Drying methods signi ficantly effected the appearance of probiotic-enriched apple snacks (Fig. 3-A–D). FD+MVD-dried samples have natural yellow colour of dried apple.Their appearance showed no brown, no shrinkage and little puf fing. Compared with FD+MVD-dried samples, the appearance of FD-dried samples showed no difference,unless their colour were white, like the colour of fresh apple.It can be seen from Fig. 4-C and D that there are marked sag and brown in the middle of the AD- and AD+EPD-dried samples. It is obvious, AD and AD+EPD couldn’t achieve the acceptable level of snack products. So, both of them were not suitable for drying probiotic-enriched apple cubes.
3.3. Measurement of microstructure
Fig. 4 showed SEM images which were obtained after the four drying treatment. After AD, apple cubes exhibited severe tissue shrinkage and collapse. There were almost no open structures (Fig. 4-C and G). The structure of the AD+EPD-dried apple cubes were similar to that of the AD dried ones,except that a small amount of open structure could still be observed (Fig. 4-D and H). Honeycomb network structure of closely connected, and puffed cells were observed in FD- and FD+MVD-dried samples (Fig. 4-A and E; B and F). The tissues of FD+MVD-dried samples were observed to be more porous than FD-dried samples, possibly as a result of microwave puf fing. Because of the rapid drying rate of the MVD-dried samples, the water inside the cells rapidly evaporated during microwave treatment and the vapor pressure increased, causing cell wall rupture. Thus,the porous tissues of the FD+MVD-dried samples were more obvious than those in FD-dried samples. The cell walls of FD-dried samples looked comparatively smoother and thinner than those of the FD+MVD-dried samples.
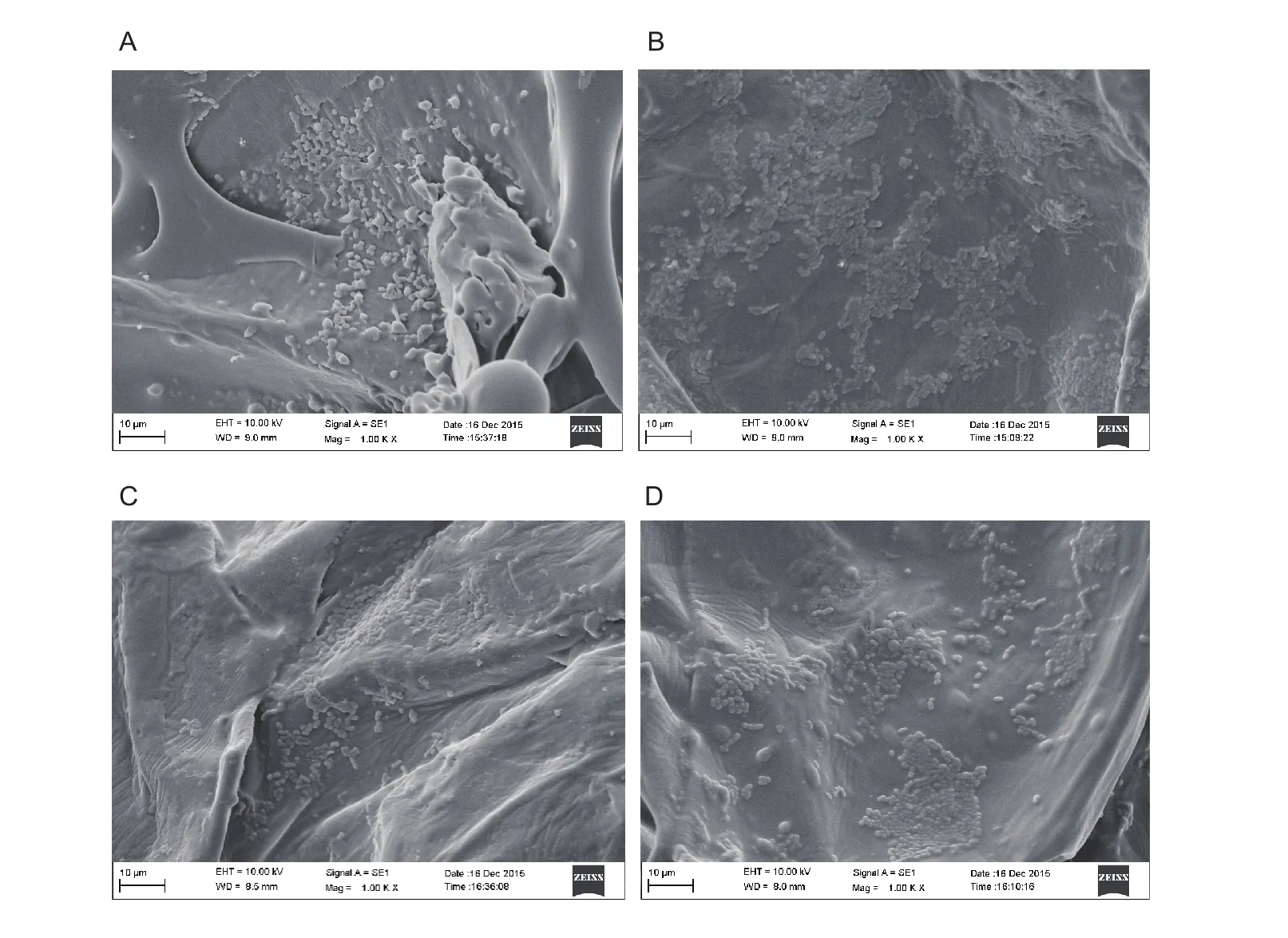
Fig. 2 The location of Lactobacillus plantarum in apple cubes dried by four dehydration methods using scanning electron microscopy(SEM). A, freeze drying (FD). B, freeze drying followed by microwave vacuum drying (FD+MVD). C, air drying (AD). D, air drying followed by explosion puf fing drying (AD+EPD).
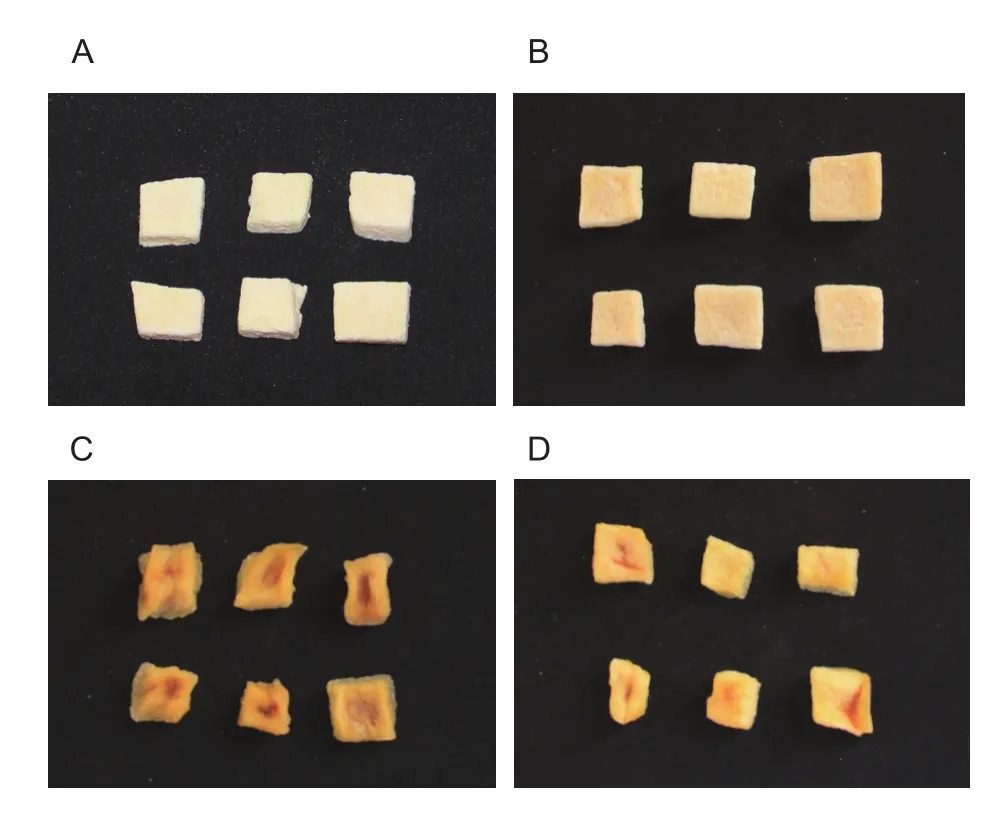
Fig. 3 Appearance of apple cubes dried by four dehydration methods. A, freeze drying (FD). B, freeze drying followed by microwave vacuum drying (FD+MVD). C, air drying (AD). D,air drying followed by explosion puf fing drying (AD+EPD).
3.4. Sensory evaluation
The quality attributes of snacks would have a desirable appearance, texture, taste, colour and enough probiotic.Considering the appearance evaluation and probiotic concentration, AD and AD+EPD were not suitable for drying probiotic-enriched apple cubes. In following, we focused on FD and FD+MVD methods.
The results of sensory evaluation of products at time zero demonstrated that the average acceptance of the two samples was above the average value set of five. The value of FD+MVD-dried samples is better than FD-dried samples in texture, colour, flavour and overall.
Most of panelists stated that FD+MVD-dried samples were more crisp than FD-dried samples (Fig. 5-A). The results of microstructure were in accordance with the sensory evaluation of texture. The cell walls of freezedried samples looked smoother and thinner than those of the FD+MVD-dried samples, explaining the noncrisp and spongy texture obtained by the freeze-dried apple chips (Sham et al. 2001). Panelists also described that the colour of FD+MVD-dried samples showed yellow and FD-dried samples showed white. So, the yellow colour makes FD+MVD-dried samples looked more natural and attractive than FD-dried samples. Sensory properties of the FD+MVD- and FD-dried samples remained above the acceptable level at 25°C after 120 days (Fig. 5-B).
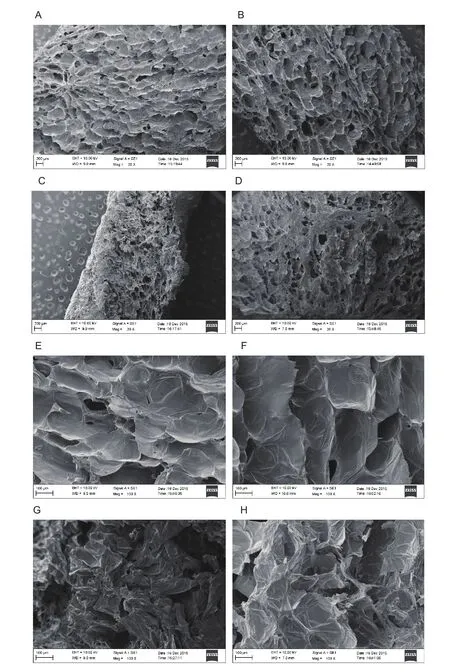
Fig. 4 The microstrcture of apple cubes dried by four dehydration methods (magni fication of the microscope is 20 in A–D and 100 in E–H) using scanning electron microscopy (SEM). A and E, freeze drying (FD). B and F, freeze drying followed by microwave vacuum drying (FD+MVD). C and G, air drying (AD). D and H, air drying followed by explosion puf fing drying (AD+EPD).
3.5. Measurement of hardness
Among the quality attributes of snacks, texture are important quality attributes. For snack chips, crispness is identi fied as very important driver of consumers’ choices. Hardness and porous structure is often used for measure crispness. The hardness of the FD+MVD-dried samples was signi ficantly higher compared with FD-dried samples (Table 1), possibly because little damage was induced in apple by FD, resulting in an integrated but very thin cell wall. This thin cell wall had a poor ability to resist external forces, leading to reduced hardness compared with samples dried using FD+MVD(Jiang et al. 2015).

Fig. 5 Sensory evaluation of FD (freeze drying) and FD+MVD (freeze drying followed by microwave vacuum drying) samples. A,time zero. B, after 120 days of storage at 25°C.

Table 1 Effect of FD (freeze drying) and FD+MVD (freeze drying followed by microwave vacuum drying) on the colour and texture of probiotic enriched apple cubes
3.6. Measurement of colour
The results of sensory evaluation and instrumental assessment of FD+MVD- and FD-dried samples’ colour were not consistent. FD+MVD-dried samples have yellow colour and FD-dried samples showed white. We expected that FD-dried samples had the higher lightness and lower yellow. But the L* of FD-dried samples is lower and the b* is not lower than FD+MVD-dried samples. This phenomenon should be because of the microwave process of FD+MVD form a tough, leathery skin on the surface of the samples(Hanson 1976). The skin makes the external and internal colour of FD+MVD-dried samples different. So, it is possible that after grinding samples into powder, the difference of colour between FD+MVD- and FD-dried samples was not signi ficant.
3.7. Survival and vitality of the embedded probiotic bacterias during storage at 25°C
FD- and FD+MVD-dried samples were stored at 25°C for 120 days. Microbial content of these samples were evaluated during storage. Results were shown in Fig. 6.

Fig. 6 The storage stability of Lactobacillus plantarum in apple cubes dried by FD (freeze drying) and FD+MVD (freeze drying followed by microwave vacuum drying) at 25°C. Data are means±SD.
The bacterial population of storage at 0 day was 8.05 log10CFU g–1for FD+MVD-dried samples and 7.99 log10CFU g–1for FD-dried samples. After 120 days storage at 25°C, 7.03 log10CFU g–1bacterial population was detected in FD+MVD-dried samples while in freeze dried samples survival it was 6.35 log10CFU g–1. The log reduction in the bacterial population was 1.02 and 1.64, for FD+MVD and FD-dried samples, respectively.
In other word, one log reduction in the bacterial population in FD+MVD-dried samples at 25°C occurred after 120 days of storage. The bacterial population of these results are signi ficant higher than that reported by Noorbakhsh et al.(2013), who performed a shelf life study on AD+MVD-dried apples, enriched with Lactobacillus rhamnosus and reported one log reduction after 23 days of storage at 25°C.
The higher survival of bacteria in FD+MVD-dried apple samples compared to FD-dried samples during the storage at 25°C may be related to following factors, such as heat stress of probiotic bacteria, apple matrix and titrable acidity.
Factors in fluencing the stability of probiotics in apple samples can be categorized into three areas including formulation factors (strains of probiotic bacteria and microbial interactions, pH and titrable acidity, oxygen content, moisture content, food ingredients and additives),process factors (incubation temperature, heat treatment,types of inoculation, and storage temperature), and packaging materials and systems (Tripathi and Giri 2014).
Heat stress is known to produce heat-shock proteins(HSPs) in many bacterial strains (Anekella and Orsat et al. 2013; Barbosa et al. 2015). Thus, HSPs inducing by microwave vacuum drying can potentially work a role on survival of probiotic during storage.
Survival of probiotics during storage is considerably affected by pH and titratable acidity of the products(Mortazavian et al. 2010). Lactobacilli are capable of growing and surviving in fermented products with pH values between 3.7 and 4.3 (Boylston et al. 2004). The total titratable acidity in the freeze dried samples was signi ficant higher than that in the AD+REV-dried samples after 180 days of storage at 25°C (Noorbakhsh et al. 2013). The higher survival of bacteria in AD+REV-dried apple samples compared to FD-dried samples during the storage at 25°C may be related to the total titratable acidity. Therefore,further studies should be addressed to assessing the titratable acidity in both FD+MVD- and FD-dried apple samples during the storage at 25°C.
Microwave pretreatment leads to destructive changes in the plant tissue (Sham et al. 2001; Huang et al. 2012).Powers higher than 900 W produced greater tissue microstructural damage due to alteration of the cell wall network (Latorre et al. 2013). The intermolecular friction produced by microwave heating may cause internal cell pressure leading to rupture resulting in a loss of cell contents and organization. The rupture maybe has the effect of microencapsulation of probiotic cells. The knowledge of the structure and chemical composition may help to highlight the modi fications that occur during microwave processing.
4. Conclusion
It becomes clear from our findings that FD+MVD is a better candidate to produce probiotic-enriched dried apple snacks with more bacterial stability at room temperature and better sensory acceptation, such as crispness and colour, compared with FD. FD+MVD surely has economic advantages over freeze drying due to shorter process time and reduced energy demand. FD+MVD was superior to AD+MVD in protecting bacteria during storage at 25°C for 120 days.
Acknowledgements
This research was financially supported by the Key Projects in the Jiangsu Province Key Research & Development Program, China (BE 2016363).
Anekella K, Orsat V. 2013. Optimization of microencapsulation of probiotics in raspberry juice by spray drying. LWT-Food Science and Technology,50, 17–24.
Barbosa J, Borges S, Teixeira P. 2015. In fluence of sub-lethal stresses on the survival of lactic acid bacteria after spraydrying in orange juice. Food Microbiology,52, 77–83.
Betoret N, Puente L, Díaz M J, Pagán M J, García M J, Gras M L,Martínez-Monzó J, Fito P. 2003. Development of probioticenriched dried fruits by vacuum impregnation. Journal of Food Engineering,56, 273–277.
Boylston T D, Vinderola C G, Ghoddusi H B, Reinheimer J A. 2004. Incorporation of bi fidobacteria into cheeses:Challenges and rewards. International Dairy Journal,14,375–387.
Clary C D, Meijia-meza E, Wang S, Petrucci V E. 2007.Improving grape quality using microwave vacuum drying associated with temperature control. Journal of Food Science,72, E23–E28.
Cui L, Niu L Y, Li D J, Liu C Q. 2017. Optimization of probiotics enrichment process in carrot pieces. Food Sciences,[2017-4-28]. http://kns.cnki.net/kcms/detail/11.2206.TS.20170428.1243.030.html (in Chinese)
Cui Z W, Li C Y, Song C F, Song Y. 2008. Combined microwavevacuum and freeze drying of carrot and apple chips. Drying Technology,26, 1517–1523.
FAO, WHO. 2006. Health and Nutritional Properties of Probiotics in Food Including Powder Milk With Live Lactic Acid Bacteria. Report of A Joint FAO/WHO Expert Consultation on Probiotics in Food: Health and Nutritional Properties and Guidelines for Evaluation. pp. 1–33.
Hanson L P. 1976. General dehydration processes. In:Commercial Processing of Fruits. Noyes Data Corporation.Park Ridge, New Jersey. pp. 266–332.
Huang L L, Zhang M, Wang L P, Mujumdar A S, Sun D F. 2012.In fluence of combination drying methods on composition,texture, aroma and microstructure of apple slices. LWTFood Science and Technology,47, 183–188.
Huang L L, Zhang M, Yan W Q, Mujumdar A S, Sun D F. 2009.Effect of coating on post-drying of freeze-dried strawberry pieces. Journal of Food Engineering,92, 107–111.
Jiang N, Liu C, Li D, Zhou Y. 2015. Effect of blanching on the dielectric properties and microwave vacuum drying behavior of Agaricus bisporus slices. Innovative Food Science &Emerging Technologies,30, 89–97.
Krasaekoopt W, Suthanwong B. 2008. Vacuum impregnation of probiotics in fruit pieces and their survival during refrigerated storage. Kasetsart Journal,42, 723–731.
Latorre M E, de Escalada Pla M F, Rojas A M, Gerschenson L N.2013. Blanching of red beet (Beta vulgaris L. var. conditiva)root. Effect of hot water or microwave radiation on cell wall characteristics. LWT-Food Science and Technology,50,193–203.
Litvin S, Mannheim C H, Miltz J. 1998. Dehydration of carrots by a combination of freeze drying, microwave heating and air or vacuum drying. Journal of Food Engineering,36, 103–111.
De Moreno de LeBlanc A, Chaves S, Carmuega E, Weill R,Antóine J, Perdigón G. 2008. Effect of long-term continuous consumption of fermented milk containing probiotic bacteria on mucosal immunity and the activity of peritoneal macrophages. Immunobiology,213, 97–108.
Mortazavian A M, Khosrokhvar R, Rastegar H, Mortazaei G R. 2010. Effects of dry matter standardization order on biochemical and microbiological characteristics of freshly made probiotic doogh (Iranian fermented milk drink). Italian Journal of Food Science,22, 98–102.
Mujumdar A S. 2004. Guide to Industrial Drying. Colour Publications, Mumbai, India. pp. 28–46.
Nguyen T D T, Kang J H, Lee M S. 2007. Characterization of Lactobacillus plantarum PH04, a potential probiotic bacterium with cholesterol-lowering effects. International Journal of Food Microbiology,113, 358–361.
Noorbakhsh R, Yaghmaee P, Durance T. 2013. Radiant energy under vacuum (REV) technology: A novel approach for producing probiotic enriched apple snacks. Journal of Functional Foods,5, 1049–1056.
Pei F, Yang W J, Shi Y, Sun Y, Mariga A M, Zhao L Y, Fang Y, Ma N, An X X, Hu Q H. 2014. Comparison of freezedrying with three different combinations of drying methods and their in fluence on colour, texture, microstructure and nutrient retention of button mushroom (Agaricus bisporus)slices. Food and Bioprocess Technology,7, 702–710.
Puente D L, Betoret V N, Cortés R M. 2009. Evolution of probiotic content and color of apples impregnated with lactic acid bacteria. Vitae,16, 297–303.
Ratti C. 2001. Hot air and freeze-drying of high-value foods: A review. Journal of Food Engineering,49, 311–319.
Rößle C, Auty M A, Brunton N, Gormley R T, Butler F.2010. Evaluation of fresh-cut apple slices enriched with probiotic bacteria. Innovative Food Science & Emerging Technologies,11, 203–209.
Shah N P. 2007. Functional cultures and health bene fits.International Dairy Journal,17, 1262–1277.
Shah N P, Ali J F, Ravula R R. 2000. Populations of Lactobacillus acidophilus, Bi fidobacterium spp., and Lactobacillus casei in commercial fermented milk products. Bioscience and Micro flora,19, 35–39.
Sham P W Y, Scaman C H, Durance T D. 2001. Texture of vacuum microwave dehydrated apple chips as affected by calcium pretreatment, vacuum level, and apple variety. Journal of Food Science,66, 1341–1347.
Tripathi M K, Giri S K. 2014. Probiotic functional foods: Survival of probiotics during processing and storage. Journal of Functional Foods,9, 225–241.
Wang D, Li D J, Jiang N, Zhang Z Y, Niu L Y, Cui L, Song J F,Liu C J, Liu C Q. 2017. Effect of drying methods on quality and energy consumption of okra crisp bar. Science and Technology of Food Industry,38, 101–105. (in Chinese)
Wang J, Wang J S, Yu Y. 2007. Microwave drying characteristics and dried quality of pumpkin. International Journal of Food Science and Technology,42, 148–156.
Wang J, Xi Y S, Yu Y. 2004. Microwave drying characteristics of potato and the effect of different microwave powers on the dried quality of potato. European Food Research and Technology,219, 500–506.
Yadav H, Jain S, Sinha P R. 2007. Antidiabetic effect of probiotic dahi containing Lactobacillus acidophilus and Lactobacillus casei in high fructose fed rats. Nutrition,23, 62–68.
Zhang M, Tang J, Mujumdar A S, Wang S. 2006. Trends in microwave related drying of fruits and vegetables. Trends in Food Science & Technology,17, 524–534.
18 January, 2017 Accepted 30 June, 2017
CUI Li, E-mail: clisu1@163.com; Correspondence LI Da-jing, Tel/Fax: +86-25-84391255, E-mail: lidajing@163.com
© 2018 CAAS. Publishing services by Elsevier B.V. All rights reserved.
10.1016/S2095-3119(17)61742-8
Section editor WANG Qiang
Managing editor WENG Ling-yun
 Journal of Integrative Agriculture2018年1期
Journal of Integrative Agriculture2018年1期
- Journal of Integrative Agriculture的其它文章
- Climate change and agriculture: Impacts and adaptive responses in Iran
- Development of elite restoring lines by integrating blast resistance and low amylose content using MAS
- Evaluation of stability and yield potential of upland rice genotypes in North and Northeast Thailand
- ldenti fication of the resistance gene to powdery mildew in Chinese wheat landrace Baiyouyantiao
- New clues concerning pigment biosynthesis in green colored fiber provided by proteomics-based analysis
- Constitutive expression of feedback-insensitive cystathionine γ-synthase increases methionine levels in soybean leaves and seeds
