Host status of Brachypodium distachyon to the cereal cyst nematode
CHEN Chang-long, LIU Shu-sen, LIU Qian NIU Jun-hai, LIU Pei ZHAO Jian-long LIU Zhi-yong,LI Hong-jie, JIAN Heng
1 Department of Plant Pathology, China Agricultural University, Beijing 100193, P.R.China
2 National Key Facility for Crop Gene Resources and Genetic Improvement (NFCRI), Institute of Crop Sciences, Chinese Academy of Agricultural Sciences, Beijing 100081, P.R.China
3 Plant Protection Institute, Hebei Academy of Agricultural and Forestry Sciences/IPM Center of Hebei Province/Key Laboratory of IPM on Crops in Northern Region of North China, Ministry of Agriculture, Baoding 07100, P.R.China
4 Tropical Crops Genetic Resources Institute, Chinese Academy of Tropical Agricultural Sciences, Danzhou 571737, P.R.China
5 Department of Plant Genetics & Breeding, China Agricultural University, Beijing 100193, P.R.China
6 Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, Beijing 100101, P.R.China
1. Introduction
Cereal cyst nematode (Heterodera avenae, CCN), one of the most important plant parasitic nematodes (PPNs),has caused severe damage to cereal crops worldwide.H.avenaeis one of the most important pathogens of wheat(Triticum aestivumL.), which causes substantial yield losses ranging from 30–100% (Bonfilet al. 2004; Nicolet al. 2007; Penget al. 2009). It occurs in 16 provinces in China at high level of prevalence (Penget al. 2015).The development of molecular management strategies will undoubtedly be promoted if we know more about the mechanism of plant-H.avenaeinteraction.Arabidopsis thalianahas been reported as a model host in the study of plant-nematode interactions (Sijmonset al. 1991). This model host facilitates new findings, such as the linkage between cell cycle modifications and the differentiation of syncytia and giant cells, interactions between nematode effectors and plant targets, the role of auxin in feeding-site differentiation and transcriptomics (Joneset al. 2012).Unfortunately, relatively few nematode species can complete their life cycles onArabidopsiswith the exception ofH.avenae(Joneset al. 2012).
As wheat has complex genetics and a lower efficacy for transformation, which limits study of plant-H.avenaeinteraction, an alternative host would be exceptionally useful.Brachypodium distachyon, a new monocot model plant system first proposed by Draperet al. (2001), was taken into consideration. Similar to wheat,B.distachyonbelongs to the Pooideae subfamily of the Poaceae family.The simple growth requirements ofB.distachyon, its small stature (approximately 30 cm at maturity), its short generation time (8–10 weeks), its self-fertility, and its small,fully sequenced diploid genome (approximately 272 Mbp for the Bd21 diploid accession) represent all the desirable features of a powerful plant model (Peraldiet al. 2014).B.distachyonis being developed as a model for grasses.This initiative is comparable to the development ofA.thalianaas a model for dicotyledonous plants. For example, efficient transformation protocols (Păcuraret al.2008; Vainet al. 2008; Vogel and Hill 2008), germplasm collections (Vogelet al. 2006; Filizet al. 2009; Vogelet al.2009), genetic markers (Vogelet al. 2009), mutant collections(http://brachypodium.pw.usda.gov, http://www.brachytag.org), microarrays, and databases (http://www.brachybase.org, http://www.phytozome.net, http://www.modelcrop.org, http://mips.helmholtz-muenchen.de/plant/index.jsp)(IBI 2010) have been developed. These tools make it easy to conduct genetic and molecular experiments onB.distachyon. Furthermore, this species has been reported as a host of a number of pathogens, includingMagnaporthe grisea(Draperet al. 2001; Routledgeet al. 2004),Puccinia striiformis(Draperet al. 2001),P.brachypodii(Barbieriet al. 2011),Fusariumspecies (F.graminearumandF.culmorum) (Peraldiet al. 2011), arbuscular mycorrhizal fungi (Honget al. 2012), andBarley stripe mosaic virus(Cuiet al. 2012), making it a promising pathosystem model for studying plant-pathogen interactions.
In this study, we assessed the host status of 25 lines ofB.distachyontoH.avenaefor their potential as an alternative host.
2. Materials and methods
2.1. Nematodes and inoculation
The 5 different geographical populations ofH.avenaewere sampled from fields in the Daxing District of Beijing City(DX population, Ha3 pathotype group) (Su 2012); Baoding,Hebei Province (BD population, Ha1 pathotype group) (Liet al. 2014); Luannan, Hebei Province (LN population,Ha1 pathotype group) (Liet al. 2014); Xingyang, Henan Province (XY population, Ha3 pathotype group) (Yuanet al.2010); and Xuzhou, Jiangsu Province (XZ population, Ha1 pathotype group) (Liang 2014). They were identified by PCR amplification and sequencing of the internal transcribed spacer region (ITS).
Infective second-stage juveniles (J2s) of CCN were obtained by hatching cysts at 15°C after at least 2 months of incubation at 4°C. The J2 water suspension was inoculated into 2 holes in the soil per plant seedling. The holes were 2 cm deep across and closed to each plant seedling.
2.2. Plant materials and growth conditions
A total of 25 inbred lines ofB.distachyonand their origins are listed in Table 1. Wheat seeds (Triticum aestivum cv.Aikang 58) were purchased from Henan Bainong Seed Co., Ltd., Henan, China. Seeds ofB.distachyonwere embedded in Petri dishes on damp filter paper for 1 day at 25°C followed by incubation for 7 days at 4°C. The wheat seeds were then surface-sterilised for 5 min in 3% NaClO,raised with water and soaked in water for 1 day at room temperature before they were placed in Petri dishes on damp filter paper for 1 day at 25°C. The seeds were then planted in 5.5 cm×5.5 cm×5.5 cm pots filled with sterilised 75% sand mixed with 25% sandy-loam soil. Plants were grown in an artificial environment at 22–25°C with 16 h light/8 h dark photoperiod. When theB.distachyonseedlings were at the 3–4 euphylla stage or the wheat seedlings were approximately 10 cm high, they were inoculated with J2s ofH.avenae, and the inoculated plants were grown at 16°C for 10 days or longer forH.avenaeinfestation. Then plants were grown at 20–25°C to allow the development ofH.avenaefor 3 mon or more until all of the cysts formed and fell into the soil.
2.3. Screening of the B. distachyon lines
A total of 600 J2s ofH.avenaefrom DX per plant were inoculated twice (300 J2s each time) into 25 inbred lines ofB.distachyonat an interval of 10 days, respectively.The nematodes invading into each plant root system were stained with acid fuchsin and counted at 15 and 30 days post the first inoculation (dpi) with 3 plant replicates for each inbred line at each sampling time. The cysts/plant were extracted from the soil and counted for each line with 5–9 plant replicates at 5 mon post the first inoculation (mpi),except for line Adi-3 with 4 plant replicates. Wheat was used as a control with 3 plant replicates. To further evaluate the sensitivity of Koz-1 and TR2A to nematode, 600 J2s/plant were inoculated into each line in a single inoculation with 9 plant replicates. The total number of cysts/plant was counted at 3 mpi.
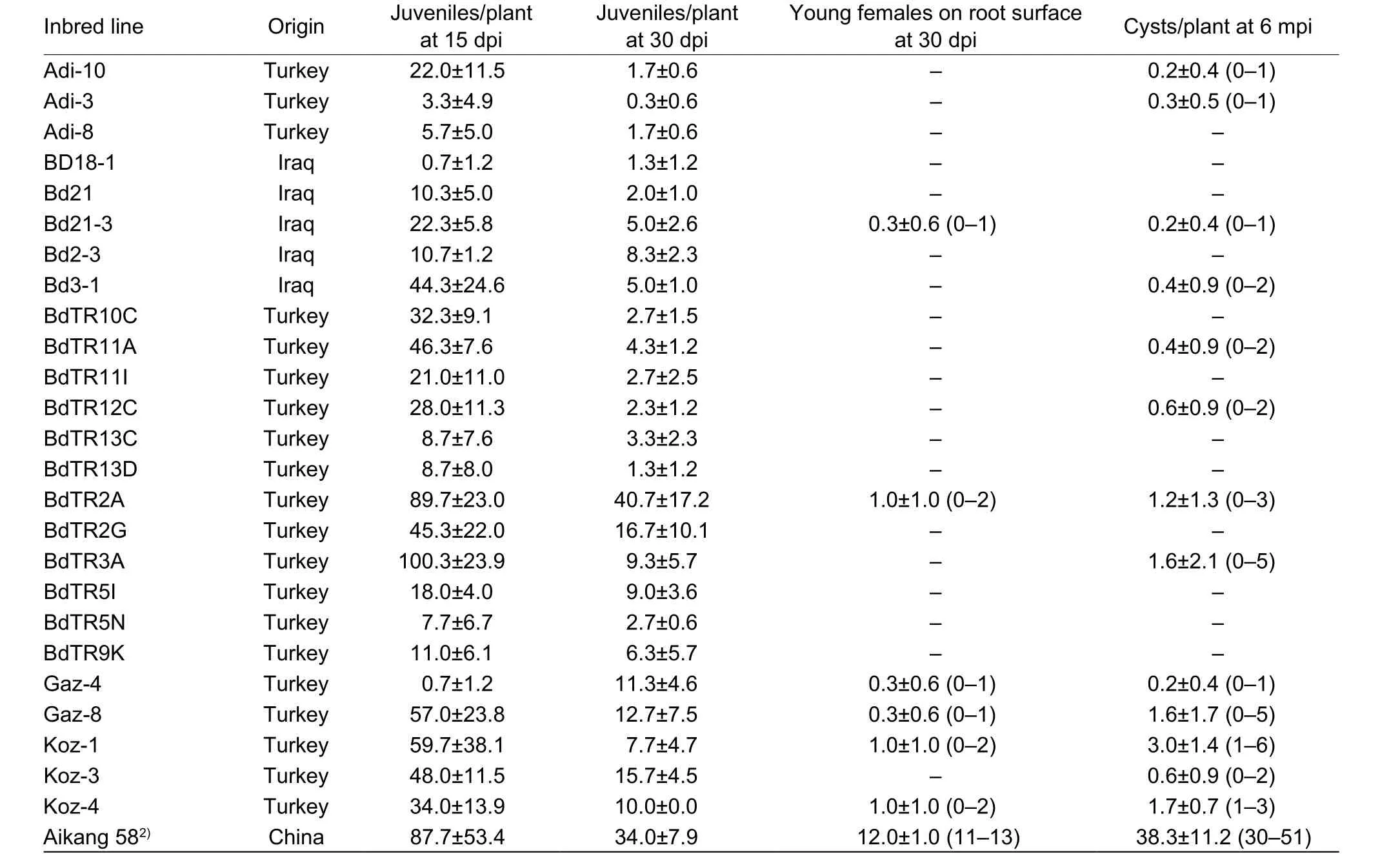
Table 1 Average number of cereal cyst nematode (CCN) on Brachypodium distachyon lines and wheat observed at various times1)
2.4. Phenotype of B. distachyon infested with CCN
A total of 0, 1 000 or 2 000 J2s of the DX population ofH.avenaeper plant were inoculated into Bd21-3 and wheat,respectively. Plant height (10 plant replicates) and root length (5 plant replicates) were assessed at 5 dpi, and plant height was assessed again at 45 dpi (5 plant replicates). The typical root symptoms were photographed. The nematodes inside the roots were stained and counted at 5 dpi (4–5 plant replicates for each nematode dosage).
2.5. Infestation of different geographical populations of H. avenae to B. distachyon
A total of 600 J2s from each of the 5 geographical populations ofH.avenaewere inoculated into line Koz-1,respectively. There were 9 plant replicates for each geographical population. The average numbers of cyst/plant were compared among 5 geographical populations after 3 mon inoculation. Wheat was used as a control with 6–9 plant replicates per geographical population.
2.6. Staining and microscopy
The roots were washed with tap water and stained using the sodium hypochlorite-acid fuchsin method (Bybdet al.1983). The nematodes in or on the roots were observed and photographed under a stereoscopic microscope (OLYMPUS SZ-61, New York, America).
2.7. Statistical analysis
The data were analyzed by a one-way ANOVA or an independent-samplest-test performed in SPSS 13.0.
3. Results
3.1. Half of the lines of B. distachyon produced cysts of H. avenae
Each of the 25 lines ofB.distachyonwas successfully infested by CCN J2s of DX population, while only 13 lines reproduced cysts (0.2–3 cysts/plant) (Table 1), showing resistance. Representative figures of different life stages of CCN on Bd21-3 are shown in Fig. 1, including J2 (Fig. 1-A),third-stage juvenile (J3) (Fig. 1-B), fourth-stage juvenile (J4)(Fig. 1-C), a mature female cyst full of eggs (Fig. 1-D), and J2s hatched from newly formed cyst (Fig. 1-E). Of these 13 lines ofB.distachyon, young nematode females were observed on the root surface of lines Gaz-4, Bd21-3, Koz-4,Gaz-8, Koz-1, and BdTR2A at 30 dpi (0.3–1 cysts/plant)(Table 1), indicating that CCN developed faster on these lines than on other lines.
3.2. Phenotypes of B. distachyon infested by CCN

Fig. 1 Representative figures of the life cycle of Heterodera avenae on line Bd21-3 of Brachypodium distachyon. The arrows indicate second-stage juvenile (J2) (A), third-stage juvenile (J3) (B), fourth-stage juvenile (J4) (C), and a mature female cyst full of eggs (D) and J2s hatched from the cyst (E).Scale bar=200 μm.
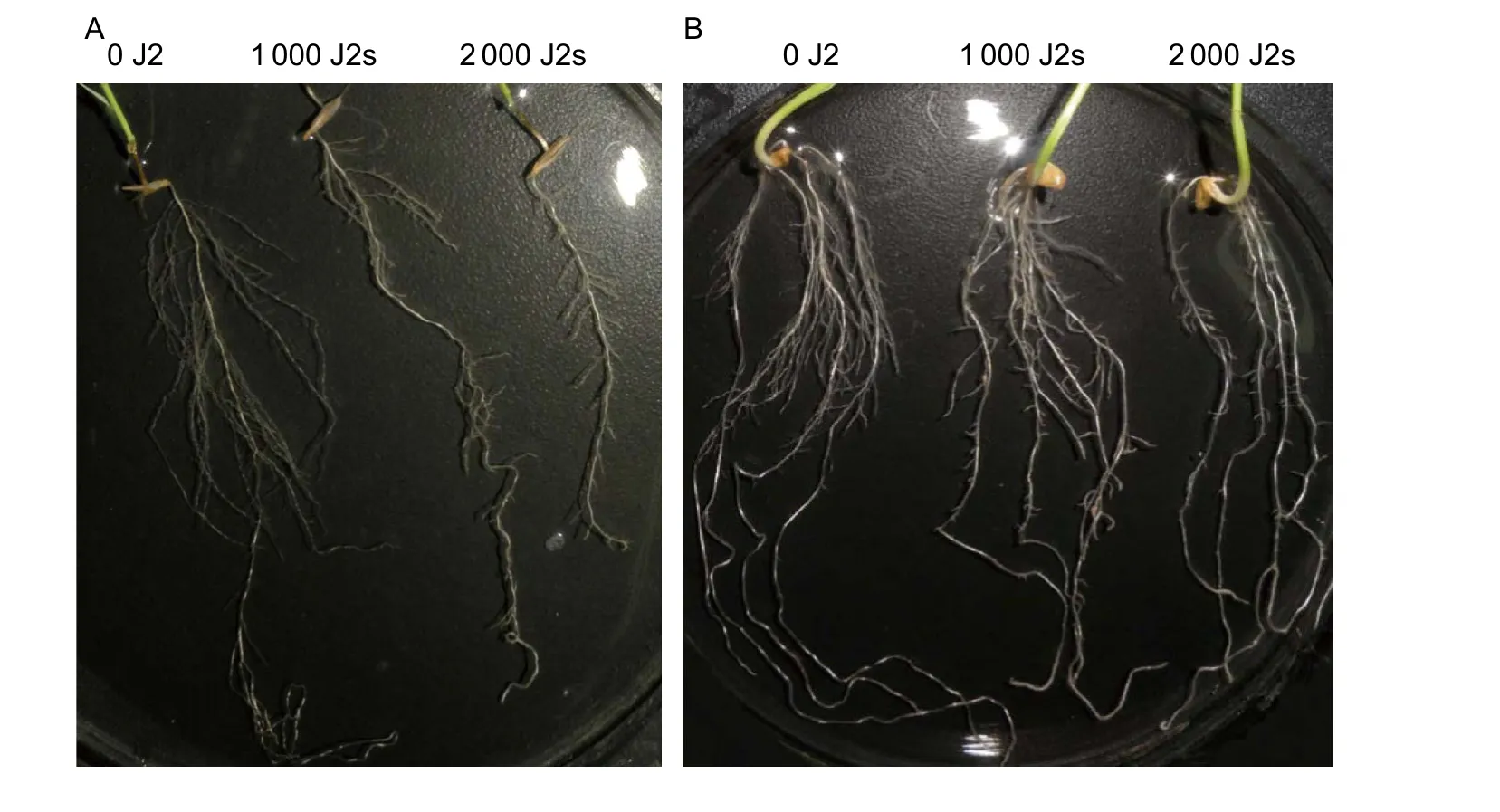
Fig. 2 Phenotypes of line Bd21-3 of Brachypodium distachyon and wheat infected by Heterodera avenae. A total of 0, 1 000 or 2 000 second-stage juveniles (J2s) of H. avenae were used to inoculate each plant, and representative root symptom photographs of Bd21-3 (A) and wheat cultivar Aikang 58 (B) were taken at 5 days post the first inoculation (dpi). Consistent with wheat (B),the fibrous roots of Bd21-3 (A) infected by H. avenae also became shorter and fewer compared to the uninfected roots, and the severity of symptom was enhanced with the increase of nematodes inoculated (1 000 J2s vs. 2 000 J2s).
We qualitatively observed the phenotype of the aerial and underground parts ofB.distachyoninfested by CCN using Bd21-3 line as a representative. Similar to wheat (Fig. 2-B),the entire root system of Bd21-3 infested by CCN appeared to be smaller, and the fibrous roots were also shorter and fewer compared to those of non-inoculated roots at 5 dpi(Fig. 2-A). In addition, the symptom was more obvious in the plants inoculated with 2 000 J2s than those inoculated with 1 000 J2s (Fig. 2-A, B). However, the root length of Bd21-3 (15.0, 14.2, and 12.0 cm/plant for 0, 1 000, and 2 000 J2s, respectively) was not affected by nematode infestation at 5 dpi, whereas that of the wheat cultivar Aikang 58 was affected (19.4, 15.5, and 15.9 cm/plant for 0, 1 000, and 2 000 J2s, respectively) (Fig. 3-A). Plant heights of Bd21-3 and wheat were not affected by CCN infestation evaluating at 5 dpi (5.6–5.9 and 17.5–18.9 cm/plant for Bd21-3 and wheat,respectively) (P>0.05, Fig. 3-B) and 45 dpi (21.3–24.4 and 33.0–35.4 cm/plant for Bd21-3 and wheat, respectively)(P>0.05, Fig. 3-C).
3.3. Comparison of nematode dosages for B. distachyon
We used 1 000 and 2 000 J2s/plant to assess the upper limit of the dosage of the DX population of CCN onB.distachyon.There were no differences between the average numbers of nematodes invading into the roots at 5 dpi either for Bd21-3(43.5 and 33.3 J2s/plant for 1 000 and 2 000 J2s dosage,respectively) or for wheat (400.8 and 467.0 J2s/plant for 1 000 and 2 000 J2s dosage, respectively) (P>0.05, Fig. 4).Accordingly, 1 000 J2s/plant would represent the upper limit of the inoculation dosage of the nematode.
3.4. The most suitable B. distachyon line for CCN infestation
For further study, we would like to choose one line that is relatively more suitable for CCN parasitism and reproduction. According to our initial screening results,Koz-1 can reproduce 3 cysts/plant on average and up to 6 cysts individually. In both cases, these values were the maxima for the evaluated plant lines (Table 1). To confirm the initial result, we conducted another infection assay using Koz-1 and TR2A, the latter plant line can also produce 1.2±1.3 (0–3) cysts/plant in our initial screening (Table 1).The results indicated that Koz-1 reproduced more cysts(4.6±2.2 cysts/plant) than did TR2A (1.7±1.4 cysts/plant)(P<0.01, Fig. 5), indicating that Koz-1 is more suitable as a host, at least for the DX population of CCN.
3.5. Five different geographical populations of CCN can complete their life cycle on line Koz-1
Line Koz-1 can support the life cycle of each of the 5 different geographical populations ofH.avenae(belonging to the Ha1 or Ha3 pathotype group) with only moderate variations(3.6–10.3 cysts/plant) (Fig. 6), suggesting the general compatibility of Koz-1. TheH.avenaepopulation from XZ developed the greatest number of cysts in both Koz-1 (10.3 cysts/plant) and wheat (248.7 cysts/plant) (P<0.05) (Fig. 6).
4. Discussion
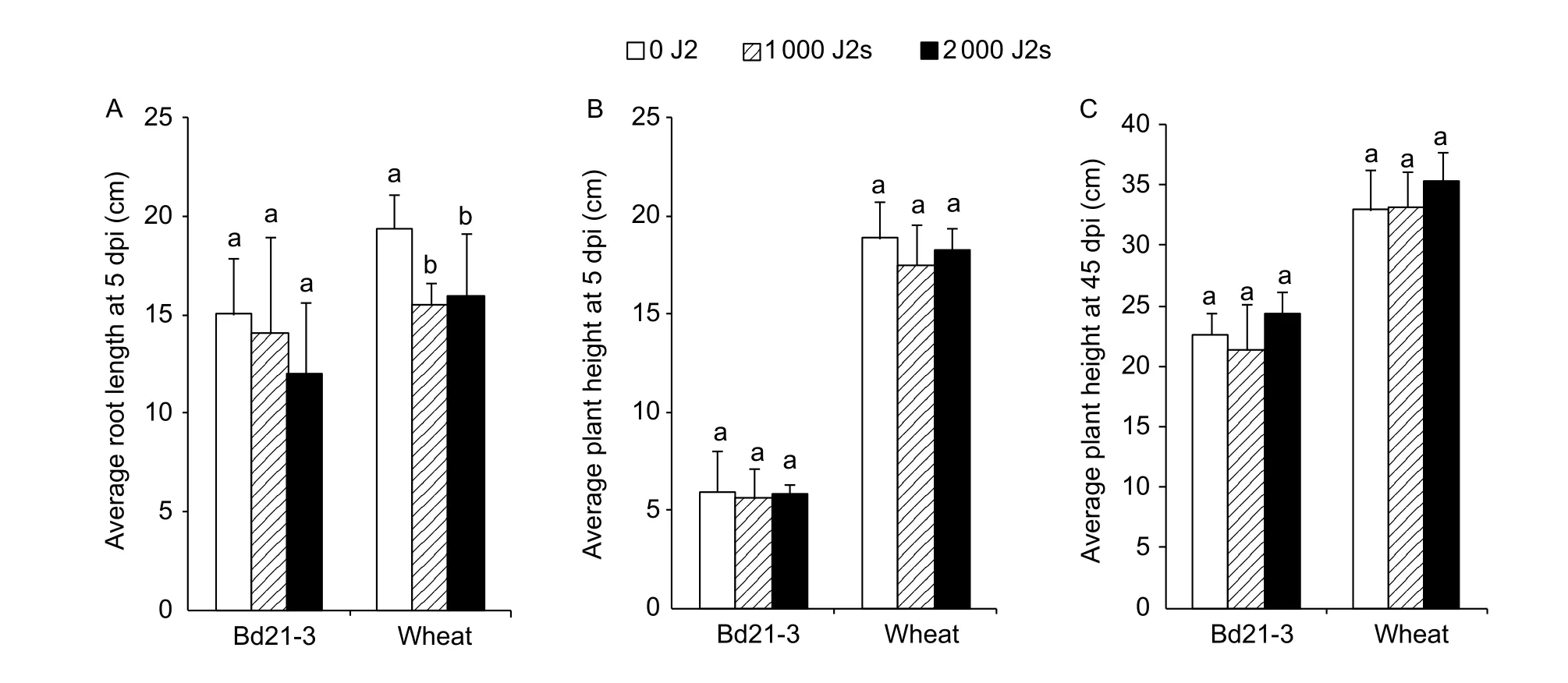
Fig. 3 Comparison of the average root length at 5 days post the first inoculation (dpi) (n=5) (A), the average plant height at 5 dpi(n=10) (B) or at 45 dpi (n=5) (C) among 0, 1 000, and 2 000 second-stage juveniles (J2s) for line Bd21-3 of Brachypodium distachyon and for wheat cultivar Aikang 58. Each column shows the mean and standard deviation. Statistical analyses were conducted for Bd21-3 and wheat independently. Columns labelled with the same letter for Bd21-3 or Aikang 58 do not differ significantly (P>0.05).
Currently,B.distachyonis being developed as a model for grasses similar toA.thalianafor dicotyledonous plants.Previous results showedB.distachyonis a promising pathosystem model for the study of plant-fungi or virus interactions (Draperet al. 2001; Routledgeet al. 2004;Barbieriet al. 2011; Peraldiet al. 2011; Honget al. 2012; Cuiet al. 2012). In this research, we evaluated the sensitivity ofB.distachyontoH.avenae. Our results showed that certain lines ofB.distachyoncould support the complete life cycle ofH.avenae. Similar to wheat, the fibrous roots of the infestedB.distachyonby nematode were obviously fewer and shorter. This indicatesB.distachyonis a host of CCN though not very susceptible. It was reported that Bd21-3 was not a host for CCN, as nematodes penetrated roots but failed to develop to the later developmental stages or form cysts (Konget al. 2016). While in this research,Bd21-3 formed 0.2±0.4 (0–1) cysts/plant, which was not consistent with previous report. These two contrary results might be due to different CCN populations inoculated, that is, we inoculated DX CCN while Konget al. (2016) used population they maintained in a greenhouse by themselves.
In the evaluation assay on wheat resistance toH.avenae,500–800 J2s/plant were always inoculated (Liet al. 2012).In theB.distachyoninoculation, this study showed the upper limit of theH.avenaenematode dosage was 1 000 J2s/plant. AsB.distachyonhas smaller root systems than wheat does, we recommend to innoculateB.distachyon600 J2s/plant for saving nematode. There are more than 13 pathotypes belonging to 3 pathotype groups (Ha1, Ha2 and Ha3) forH.avenae(Yuanet al. 2010). We assessed susceptibility ofB.distachyonto 5 different geographical populations ofH.avenaewhich belong to 2 pathotype groups.B.distachyon(line Koz-1) can support the complete life cycles of 5 different geographical populations ofH.avenae. These results indicate the general compatibility of Koz-1 for the infection and development ofH.avenae.However, different geographical populations ofH.avenaedeveloped different numbers of cysts onB.distachyon. In addition, on wheat, Ha1 pathotype group (XZ, BD and LN)ofH.avenaedeveloped more cysts than Ha3 pathotype group (DX and XY), while there isn’t the same case onB.distachyon(line Koz-1).
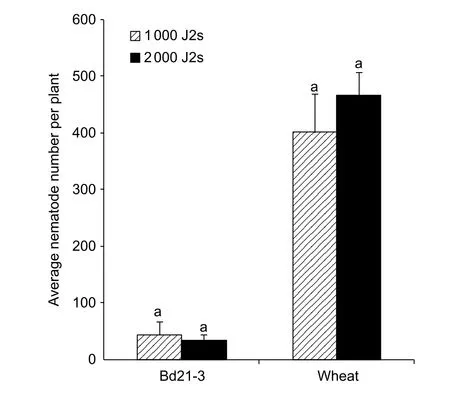
Fig. 4 Average number of nematode in roots of line Bd21-3 of Brachypodium distachyon and wheat cultivar Aikang 58 inoculated with 1 000 and 2 000 second-stage juveniles(J2s)/plant at 5 days post the first inoculation (dpi) (n=4–5).Each column shows the mean and standard deviation.Statistical analyses were conducted for Bd21-3 and Aikang 58 independently. Columns labelled with the same letter for Bd21-3 or wheat did not differ significantly (P>0.05).
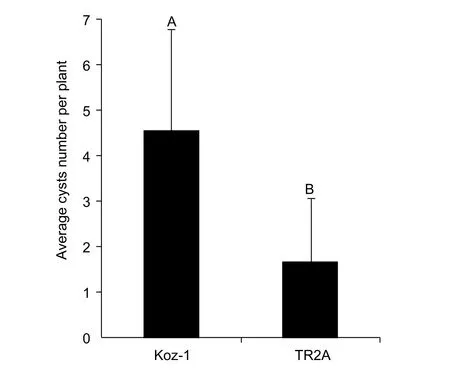
Fig. 5 Comparison of the average cyst number of DX population(from the Daxing District of Beijing City, China) of cereal cyst nematode (CCN) reproduced on Koz-1 and TR2A. Each column shows the average cyst number of DX population of CCN/plant and the standard deviation of 9 plant replicates of each line inoculated with 600 J2s/plant. Columns labelled with different letters indicate significant difference between the average cyst numbers of DX population of CCN obtained from Koz-1 and TR2A (P<0.01).
However, this study also raises several questions.Firstly, the cysts that reproduced onB.distachyonroots were not as abundant as those on wheat. The cysts from Koz-1 ofB.distachyoninfected by the 5 different geographical populations ofH.avenaeexamined ranged from 3.6 to 10.3/plant, while those on wheat ranged from 121.8 to 248.7/plant. One reason might be the smaller root system ofB.distachyoncompared to that of wheat.Another reason might be the tested plant lines are not as susceptible as wheat to the 5 geographical populations ofH.avenaeexamined. As there are large numbers of collections ofB.distachyon(Brkljacicet al. 2011), additional screening experiments should be performed to identify whether there are more susceptible lines toH.avenaein the future. Another confusion in our preliminary screening research was that the average number of nematodes per plant of line BdTR2A (up to 40.7±17.2) was the greatest when observed at 30 dpi, but only few cysts were ultimately formed (1.2±1.3). The reason for the incapacity of these later-stage nematodes to complete their life cycles is not clear, but it is likely associated with the late induction of plant resistance. As Joneset al. (2012) have stated,plant resistance to nematodes can occur not only at early infection stage but also after the successful establishment of a feeding site. For example, during the interactions between resistant cowpea containing theR-geneRkandMeloidogyne incognita, normal nematode feeding occurred,and nematodes were able to develop to later juvenile stages,but giant cell deterioration was observed, and the female nematodes showed arrested development and deterioration and never reached maturity (Daset al. 2008). The same may be the case for the interaction of DX population of CCN and BdTR2A, and observations of syncytium are needed in the future to confirm this hypothesis.
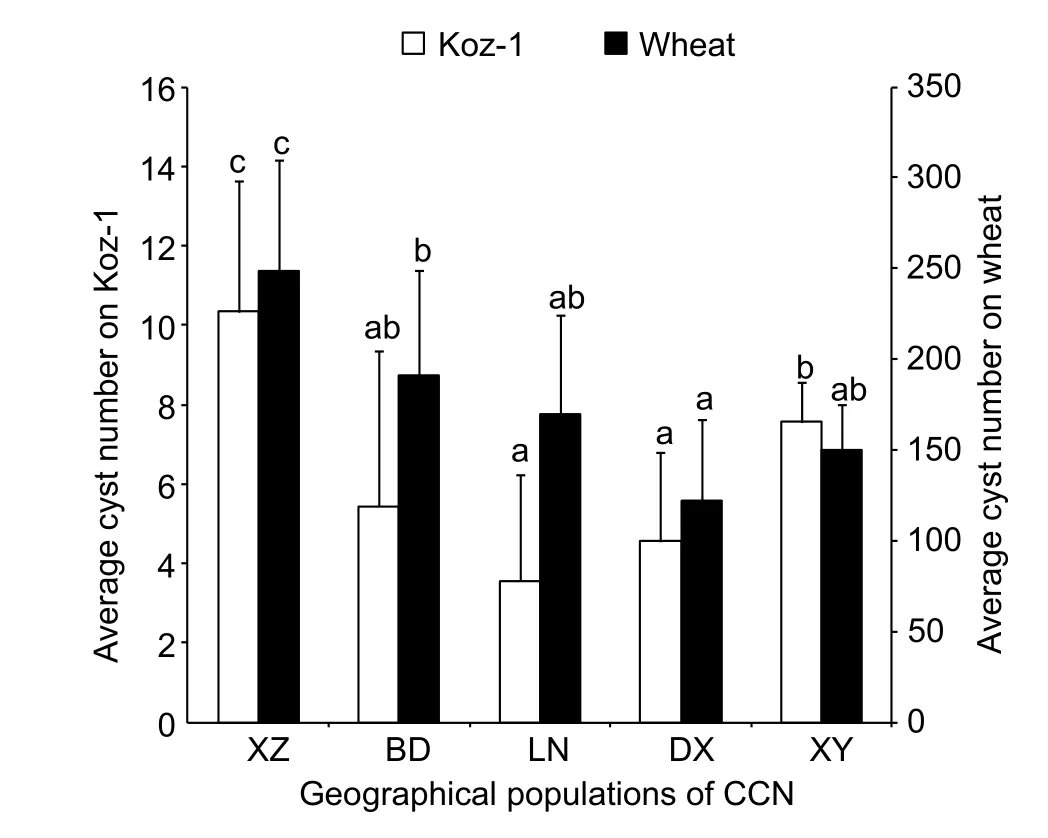
Fig. 6 Average cyst numbers of Koz-1 or wheat inoculated with 600 second-stage juveniles (J2s) of different geographical populations of cereal cyst nematode (CCN) per plant. Each column shows the mean cyst numbers in 6–9 plant replicates and the standard deviation. Statistical analyses were conducted for Koz-1 and wheat cultivar Aikang 58 independently, and columns labelled with the same letter do not differ significantly(P>0.05). XZ, Xuzhou of Jiangsu Province, China; BD, Baoding of Hebei Province, China; LN, Luannan of Hebei Province,China; DX, Daxing District of Beijing City, China; XY, Xingyang of Henan Province, China.
5. Conclusion
Our results suggested that the monocotyledonous model plantB.distachyoncan be infected by the cereal cyst nematodeH.avenaeand a certain lines ofB.distachyoncould support the whole life cycle ofH.avenae. The infection symptoms of the whole root ofB.distachyonwere nearly as same as that of wheat. Furthermore, we found that Koz-1 was the most suitable line for CCN infestation in our study,and Koz-1 can support different geographical populations of CCN which indicates the universality of the line. More lines ofB.distachyonshould be assessed for their susceptibility to CCN in the future and the mechanisms of susceptibility variation can be studied further.
Acknowledgements
We are grateful to Profs. Chen Shulong and Li Xiuhua (Plant Protection Institute, Hebei Academy of Agricultural and Forestry Sciences, Baoding, China), Li Honglian (Henan Agricultural University, Zhengzhou, China), and Li Hongmei(Nanjing Agricultural University, Nanjing, China) for helping with collections or for providing different geographical populations ofHeterodera avenae. This study was funded by the National Key Basic Research Program of China(2013CB127501), the Special Fund for Agro-scientific Research in the Public Interest in China (201503114 and 200903040) and the National Key Research and Development Program of China (SQ2017ZY060063-01).
Barbieri M, Marcel T C, Niks R E. 2011. Host status of false brome grass to the leaf rust fungusPuccinia brachypodiiand the stripe rust fungusP.striiformis.Plant Disease, 95,1339–1345.
Bonfil D J, Dolgin B, Mufradi I, Asido S. 2004. Bioassay to forecast cereal cyst nematode damage to wheat in fields.Precision Agriculture, 5, 329–344.
Brkljacic J, Grotewold E, Scholl R, Mockler T, Garvin D F, Vain P, Caicedo A L. 2011.Brachypodiumas a model for the grasses: Today and the future.Plant Physiology, 157, 3–13.
Bybd D W, Kirkpatrick Jr T, Barker K R. 1983. An improved technique for clearing and staining plant tissues for detection of nematodes.Journal of Nematology, 15, 142–143.
Cui Y, Lee M Y, Huo N, Bragg J, Yan L, Yuan C, Li C, Holditch S J, Xie J, Luo M C, Li D, Yu J, Martin J, Schackwitz W, Gu Y Q, Vogel J P, Jackson A O, Liu Z, Garvin D F. 2012. Fine mapping of theBsr1barley stripe mosaic virus resistance gene in the model grassBrachypodium distachyon.PLoS ONE, 7, e38333.
Das S, DeMason D A, Ehlers J D, Close T J, Roberts P A.2008. Histological characterization of root-knot nematode resistance in cowpea and its relation to reactive oxygen species modulation.Journal of Experimental Botany, 59,1305–1313.
Draper J, Mur L A, Jenkins G, Ghosh-Biswas G C, Bablak P, Hasterok R, Routledge A P. 2001.Brachypodium distachyon. A new model system for functional genomics in grasses.Plant Physiology, 127, 1539–1555.
Filiz E, Ozdemir B S, Budak F, Vogel J P, Tuna M, Budak H.2009. Molecular, morphological, and cytological analysis of diverseBrachypodium distachyoninbred lines.Genome,52, 876–890.
Hong J J, Park Y S, Bravo A, Bhattarai K K, Daniels D A,Harrison M J. 2012. Diversity of morphology and function in arbuscular mycorrhizal symbioses inBrachypodium distachyon.Planta, 236, 851–865.
IBI (The International Brachypodium Initiative). 2010. Genome sequencing and analysis of the model grassBrachypodium distachyon.Nature, 463, 763–768.
Jones J, Gheysen G, Fenoll C. 2012.Genomics and Molecular Genetics of Plant-Nematode Interactions. Springer, London& New York.
Kong L A, Wu D Q, Cui J K, Huang W K, Peng H, Peng D L.2016. Testing and modelling the potential of three diploid plants in Poaceae as a new pathosystem to investigate the interactions between cereal hosts and cereal cyst nematode(Heterodera avenae).Plant Pathology, 65, 682–688.
Li X H, Gu S J, Wang W L, Chen S L. 2012. Effect of different factors on number ofHeterodera avenaecysts produced on the wheat.Journal of Agricultural University of Hebei,35, 56–61. (in Chinese)
Li X H, Ma J, Gao B, Wang R Y, Chen S L. 2014. Characterization of the pathotypes of the cereal cyst nematode,Heterodera avenae, in Hebei Province.Plant Protection, 40, 127–131.(in Chinese)
Liang X D. 2014. Pathotype identification ofHeterodera avenae,resistant evaluation of wheat materials and determing of damage threshold. MSc thesis, Nanjing Agricultural University, China. (in Chinese)
Nicol J M, Elekçioğlu I H, Bolat N, Rivoal R. 2007. The global importance of the cereal cyst nematode (Heteroderaspp.)on wheat and international approaches to its control.Communications in Agricultural and Applied Biological Sciences, 72, 677–686.
Păcurar D I, Thordal-Christensen H, Nielsen K K, Lenk I. 2008.A high-throughputAgrobacterium-mediated transformation system for the grass model speciesBrachypodium distachyonL.Transgenic Research, 17, 965–975.
Peng D L, Nicol J M, Li H M, Hou S Y, Li H X, Chen S L, Ma P, Li H L, Riley I T. 2009. Current knowledge of cereal cyst nematode (Heterodera avenae) on wheat in China. In: Riley I T, Nicol J M, Dababat A A, eds.,Cereal Cyst Nematodes:Status,Research and Outlook.Proceedings of the First Workshop of the International Cereal Cyst Nematode Initiative. 21–23 Oct., 2009. International Maize and Wheat Improvement Centre (CIMMYT), Antalya. pp. 29–34.
Peng D L, Peng H, Huang W K. 2015. Occurrence, distribution and integrated management of the cereal cyst nematodes(Heterodera avenae&H.filipjevi) in China. In: Dababat A A, Muminjanov H, Smiley R W, eds.,Nematodes of Small Grain Cereals: Current Status and Research. FAO, Ankara,Turkey. pp. 17–24.
Peraldi A, Beccari G, Steed A, Nicholson P. 2011.Brachypodium distachyon: A new pathosystem to study Fusarium head blight and otherFusariumdiseases of wheat.BMC Plant Biology, 11, 100.
Peraldi A, Griffe L L, Burt C, McGrann G R D, Nicholson P. 2014.Brachypodium distachyonexhibits compatible interactions withOculimaculaspp. andRamularia collo-cygni, providing the first pathosystem model to study eyespot and ramularia leaf spot diseases.Plant Pathology, 63, 554–562.
Routledge A P, Shelley G, Smith J V, Talbot N J, Draper J, Mur L A. 2004.Magnaporthe griseainteractions with the model grassBrachypodium distachyonclosely resemble those with rice (Oryza sativa).Molecular Plant Pathology, 5, 253–265.
Sijmons P C, Grundler F M W, Mende N, Burrows P R, Wyss U. 1991.Arabidopsis thalianaas a new model host for plant-parasitic nematodes.The Plant Journal, 1, 245–254.
Su Z. 2012. Investigation on the major specie, pathotype of cereal cyst nematode in Beijing and evaluation of wheat cultivars resistance toHeterodera avenae.MSc thesis, Jilin Agricultural University, China. (in Chinese)
Vain P, Worland B, Thole V, McKenzie N, Alves S C,Opanowicz M, Fish L J, Bevan M W, Snape J W. 2008.Agrobacterium-mediated transformation of the temperate grassBrachypodium distachyon(genotype Bd21) for T-DNA insertional mutagenesis.Plant Biotechnology Journal, 6,236–245.
Vogel J, Hill T. 2008. High-efficiencyAgrobacterium-mediated transformation ofBrachypodium distachyoninbred line Bd21-3.Plant Cell Reports, 27, 471–478.
Vogel J P, Garvin D F, Leong O M, Hayden D M. 2006.Agrobacterium-mediated transformation and inbred line development in the model grassBrachypodium distachyon.Plant Cell,Tissue and Organ Culture, 84, 199–211.
Vogel J P, Tuna M, Budak H, Huo N, Gu Y Q, Steinwand M A. 2009. Development of SSR markers and analysis of diversity in Turkish populations ofBrachypodium distachyon.BMC Plant Biology, 9, 88.
Yuan H, Sun J, Yang W, Xing X, Wang Z, Riley I T, Li H.2010. New pathotypes ofHeterodera avenae(cereal cyst nematode) from winter wheat in Zhengzhou, Henan, China.Australasian Plant Pathology, 39, 107–111.
 Journal of Integrative Agriculture2018年2期
Journal of Integrative Agriculture2018年2期
- Journal of Integrative Agriculture的其它文章
- Rapid mapping of candidate genes for cold tolerance in Oryza rufipogon Griff. by QTL-seq of seedlings
- A dCAPS marker developed from a stress associated protein gene TaSAP7-B governing grain size and plant height in wheat
- A major quantitative trait locus controlling phosphorus utilization efficiency under different phytate-P conditions at vegetative stage in barley
- Overexpression of IbSnRK1 enhances nitrogen uptake and carbon assimilation in transgenic sweetpotato
- Collision detection of virtual plant based on bounding volume hierarchy: A case study on virtual wheat
- lntegrated management strategy for improving the grain yield and nitrogen-use efficiency of winter wheat
