Effects of Aschersonia aleyrodis on the life table and demographic parameters of Bemisia tabaci
ZHANG Can, SHAO Zhen-fang, HAN Yue-ye, WANG Xing-min, WANG Ze-qing, Peter Dennis Musa,QIU Bao-li, Shaukat Ali
1Key Laboratory of Bio-pesticide Innovation and Application of Guangdong Province, Department of Entomology, South China Agricultural University, Guangzhou 510642, P.R.China
2 Guangdong Engineering Research Centre of Microbial Pesticides, Guangdong New Scene Biological Engineering Co., Ltd.,Yangjiang 529932, P.R.China
3 Huai’an Entry-Exit Inspection & Quarantine Bureau, Huai’an 223001, P.R.China
1. Introduction
The sweet potato whitefly,Bemisia tabaci(Gennadius)(Homoptera: Aleyrodidae) is a worldwide pest of economically important crops (Naranjoet al.2010). Since the 1980s,B.tabaciMiddle East-Asia Minor 1 (MEAM1) cryptic species(formerly ‘B biotype’) has caused significant damage to host plants through defoliation, stunting and yield losses(Toscanoet al.1994; Cahillet al.1995).B.tabacifeeds on the phloem sap of plants and produces honeydew,which can lead to sooty mould production. Moreover, it is a competent vector for more than 150 different plant viruses(Stansly and Naranjo 2010). In China, MEAM1B.tabaciis well distributed across 31 provinces or municipalities where it causes huge economic crop losses (Liu and Liu 2012).Management of heavy whitefly infestations is a difficult task, with synthetic pesticides still being the main control option available to suppress populations (Lianget al.2012).However, injudicious use of chemical pesticides has resulted in the development of insecticide resistance byB.tabaci(Heet al.2013). In addition, the harmful effects of these chemicals on non-target organisms and increasing public environmental awareness has promoted the need to replace chemical insecticides with sustainable and environmentally safer pest control strategies, which includes biological control (Cuthbertson and Murchie 2005; Huanget al.2010).Biological control using bacteria, viruses, fungi, nematodes and protozoa has been suggested to offer alternative means for control ofB.tabaci(Huanget al.2010; Cuthbertsonet al.2011).
Entomopathogenic fungi are widely distributed throughout the fungal kingdom. Some insect-pathogenic fungi have restricted host ranges, others have wide host ranges,while some individual isolates are very specific (Maiaet al.2001). Several species of fungi are potent biocontrol agents of plant pathogenic fungi and arthropods. Isolates ofZoophthora radicans(Brefeld),Paecilomyces fumosoroseus(Wize) Brown & Smith,Fusariumsolani(Mart.) Sacc, andBeauveria bassianaVuill can infect different insect species in greenhouse or field condition (Ibrahim and Low 1993;Pellet al.1993; Vandenberget al.1998; Maiaet al.2001).The entomopathogenic fungus,Aschersonia aleyrodis(Webber), is well known for its pathogenic potential against whitefly (Meekeset al.2000, 2002). In a previous study,A.aleyrodiswas shown to have great potential for control ofB.tabaciunder laboratory and greenhouse conditions(Zhanget al.2017). Previous investigations have also shown that sub-lethal effects of fungal infection are important indicators in evaluating the potential effects of fungal application on population dynamics of a target pest (Blanford and Thomas 2001). Quesada-Moragaet al.(2004) found that egg production, hatching, and the number of nymphs of the German cockroach,Blatella germanica(Linnaeus),declined when exposed toMetarhizium anisopliae(Metschnikoff). Therefore, knowledge concerning the sublethal effect ofA.aleyrodisagainstB.tabacipopulations is required before the application ofA.aleyrodisin the field.
The general aim of this study was to assess the sublethal effects ofA.aleyrodison development and survival ofB.tabaci. Furthermore, the pathogen persistence was assessed by incubation experiments. It is hoped that this research will contribute to a further understanding of the factors influencing the demographic parameters of MEAM1B.tabaci.
2. Materials and methods
2.1. Cultivation of fungal isolate
The research was carried out at the Key Laboratory of Bio-pesticides Innovation and Application, South China Agricultural University (SCAU), Guangzhou, China. The strainA.aleyrodisAa005 was maintained on potato dextrose agar (PDA) plates under laboratory conditions ((26±1)°C,(70±10)% RH). The inoculum for the experiment was produced following the method of Aliet al.(2010).
2.2. Insects and host plants
A stock of MEAM1 whiteflies were collected in Guangzhou from cotton plants and reared at SCAU. The MEAM1B.tabaciwas identified by using the mitochondrial COI sequence as described by Ahmedet al.(2010) and was maintained on eggplants (approximately 8 weeks old) in a greenhouse. The plants were cultured in 18.0-cm pots and kept in isolation cages to avoid any premature infestation from whiteflies or other insects until required. The rearing conditions were set at (26±1)°C, (70±10)% RH, and a photoperiod of 12 h L:12 h D.
2.3. Bioassay on life table and demographic parameters
In the evaluation of the pathogen using life table analysis,the leaves were treated withA.aleyrodisand incubated for different time periods prior to rearing of the cohort eggs.Female fecundity, viability of eggs, and reproductive ability were used for life table analysis to evaluate the potential ofA.aleyrodisto be an effective natural enemy ofB.tabaci.The essence of incubation was to test the persistence and survival of spores on the plant leaves.
Survivorship of B.tabaci in response to A.aleyrodis fungal spores incubated on plant leaves for different time periodsThe objective of incubating the fungal spores was to ascertain the persistence and potency of spores on the plant after prolonged confinement under greenhouse conditions. In this trial the effect of fungal spores incubated at three different time periods (3, 6 and 9 days) was investigated on the development ofB.tabaci. The eggplants bearing 5 leaves per plant were sprayed with 20 mL of fungal suspension (1×107conidia mL–1) to run off and were then allowed to air dry. The inoculated plants were then confined in bioassay cages (60 cm×60 cm×60 cm) in the greenhouse and incubated for 3, 6 and 9 days, respectively.After every incubation period micro-cages were attached to the undersurface of plants and two pairs of adult whitefly were released into each cage for oviposition. The cages were removed after 24 h and the numbers of eggs were adjusted to 10 eggs per leaf on 5 different leaves making up a total of 50 eggs which formed the cohort in each treatment.In the control, the same number of eggs was reared on eggplant leaves without treatment with fungal spores. The position of newly hatchedB.tabaciimmatures was circled with indelible ink for close monitoring. The development and survival of immatures was again monitored on a daily basis using a compound microscope (Zeiss, Germany) for the different fungal treatments and control. Again, leaves bearing pupae were detached from the main plant and enclosed in individual Petri dishes covered with nylon cloth to observe adult emergence. The whole experiment was replicated six times.
Effect of A.aleyrodis on female adult longevity and fecundityThe number of male and female adults that emerged from the above trials (treatedvs. control and from the different incubation periods) was obtained to calculate the sex ratio. A cohort of 10 females were selected and monitored daily for fecundity and longevity. Each female was confined in a micro-cage, clipped to the undersurface of the leaf having fungal inoculum for different incubation periods. The bioassay cages were then kept in experimental chambers. The number of eggs laid was monitored on a daily basis until the last female died in order to determine daily fecundity and longevity.
Life table and demographic parameter modelsTo estimate the possible effect of the fungal treatment on population levels, data of immature survival, adult survival and female reproduction were used to make a cohort life table and fecundity schedule according to Birch (1948).Life and fertility tables were calculated according to the method of Birch (1948) and Maiaet al.(2000). The intrinsic rate of population increase (rm) was calculated using Birch(1948) and Andrewartha and Birch (1964). The calculation equations were as follows:
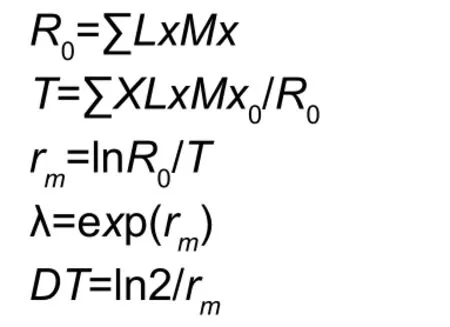
Where,Xis the age in days ofB.tabaci, Lxis the survivorship at the corresponding timex,Mxis the number of female eggs according to sex ratio laid per female per day.The net reproductive rateR0is the mean number of female progeny produced by a single female during its mean life span;rmis the intrinsic rate of increase andλis the finite rate of increase. The doubling time (DT) is defined as the time required for the population to double in size (Krebs 1994).
2.4. Data collection and analysis
Data regarding development, survival, number of eggs laid per female per day, total life span of adult females in treatedvs. control experiments were subjected tot-test atP≤0.05. Development, survival, number of eggs laid per female per day, total life span of adult females in response toA.aleyrodisfungal spores incubated on plant leaves for different time periods were subjected to one-way analysis of variance (ANOVA) atP≤0.05. Treatment means were compared by Tukey’s HSD test. All statistical analysis was performed using SAS ver. 9.1 (SAS Institute 2004).
3. Results
3.1. Effect of fungal pathogen on development time and survivorship of B. tabaci
Developmental periods of differentB.tabaciinstar nymphs were similar in treated and untreated groups (Table 1).The developmental period of eggs observed in the fungal treatment was (6.51±0.64) days while the developmental period of eggs in the control was (6.73±0.47) days. The developmental time for the pupa was (4.61±0.71) and(5.75±0.28) days for treated and untreated groups,respectively. The total developmental period (egg–adult)observed on treated leaves was (27.80±2.09) days while on the controls it was (26.29±1.41) days.
Percentage survivorship varied significantly across the various life stages ofB.tabaciin both treated and untreated groups (Table 2). Egg hatching of treated individuals ((85.36±1.42)%) was lower than the untreated individuals ((91.52±2.10)%). However, no significant difference was observed. The percentage survivorship of 1st instar nymphs varied significantly between the treated and control. The treated 1st instar nymphs had a survivorship of (22.56±1.20)% whereas survival rate of untreated individuals was (89.22±2.80)%. Likewise, the survivorship of 2nd instar nymphs treated withA.aleyrodis((39.30±1.88)%) was significantly lower than the untreated nymphs ((87.00±2.38)%). A similar trend was observed in the 3rd instar nymphs; the survivorship of the treated group being significantly lower than the control (untreated). The results demonstrated a high impact of the pathogen on the survivorship of lower instar nymphs when compared to the untreated group without the pathogen. For 4th instar nymphs and pupa, there was no significant difference in survivorship between the treated group and the control.
肉牛的饲料利用率通常还会直接影响到其养殖效益,因此相关养殖人员还需要通过多种改进措施,来不断提升肉牛的饲料利用率,这样也就能够有效保障肉牛的生长速度、产肉量跟牛肉品质,借此来获得良好的肉牛效益[3]。
3.2. Survivorship of B. tabaci in response to A. aleyrodis fungal spores following different incubation periods on plant leaves
The percentage survivorship of the eggs was relatively high in the three treatments with values of 84.33, 87.24 and 82.30% for incubation periods of 3, 6 and 9 days,respectively (Table 3). There was no significant difference in percentage survivorship of eggs between the three incubation periods. The percentage survivorship for the 1st instar nymphs was significantly lower with values of 26.50,23.28 and 25.60%, respectively, compared to the control of(89.22±2.80)% (Table 2). The 2nd instar nymphs showed a similar trend in survivorship when exposed to incubated spores, with a viability of 64.61–69.35%; lower than the control of (87.00±2.38)%. The survival rate for 3rd and 4th instar nymphs, along with pupa were comparatively high across the treatments with no significant difference in mean values when compared by Tukey’s HSD atP=0.05.These results indicated that pathogenicity of the fungus is persistent after varying incubation periods.
3.3. Effect of A. aleyrodis pathogen on female adult longevity and fecundity
In the treated trials, adult females were confined to leaves that were treated withA.aleyrodispathogen (1×107conidial mL–1). Pre-ovipositional periods of 1 day were observed in the treated and untreated trials. The bulk of eggs were laid in the 1st week of adult emergence followed by a sharp decline in egg production (Fig. 1). The mean number of eggs laid per day per female for the untreated and treated femaleswas 5.01 and 4.89, respectively (Table 4). No significant difference was observed between treatment and control,indicating that the pathogen had no influence on numbers of eggs produced per day. The mean life spans of the females in the untreated and treated trials were (13.25±1.05) and(11.28±1.16) days, respectively, with no significant difference being observed (Table 4).

Table 1 Development time (day) of Bemisia tabaci reared on treated and untreated eggplants (not incubated)

Table 2 Percentage survivorship (%) of Bemisia tabaci reared on treated and untreated leaves of eggplants (not incubated)
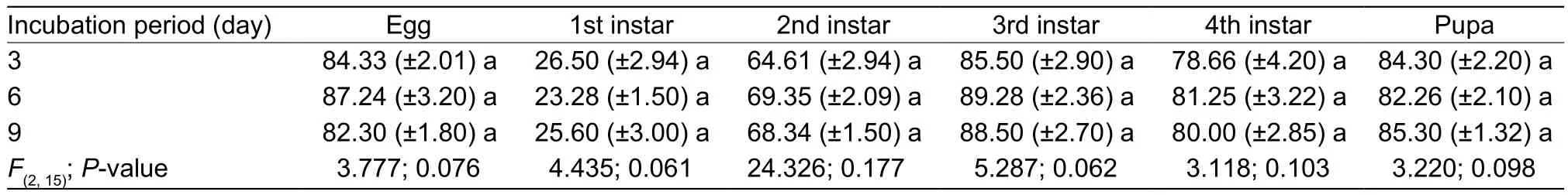
Table 3 Effect of fungal incubation period on percentage survivorship of Bemisia tabaci
The fecundity curves of incubation treatments are presented in Fig. 2. The fecundity curves followed a similar pattern with pre-ovipositional period of one day followed by a sharp increase in the number of eggs laid per day per female. A greater proportion of eggs was recorded during the first week after adult emergence followed by a sharp decline and gradual diminishing of egg production. Thefinal day for egg production was observed on the 9th day for females reared on leaves with pathogen incubated for 3 and 6 days, respectively. The females lived for another 2 days before dying on the 11th day. The females reared on leaves with fungus incubated for 9 days survived for up to 12 days. There was no significant difference detected between incubation and control trials, indicating that the incubation periods had no influence on female whitefly longevity and egg production (Table 5).
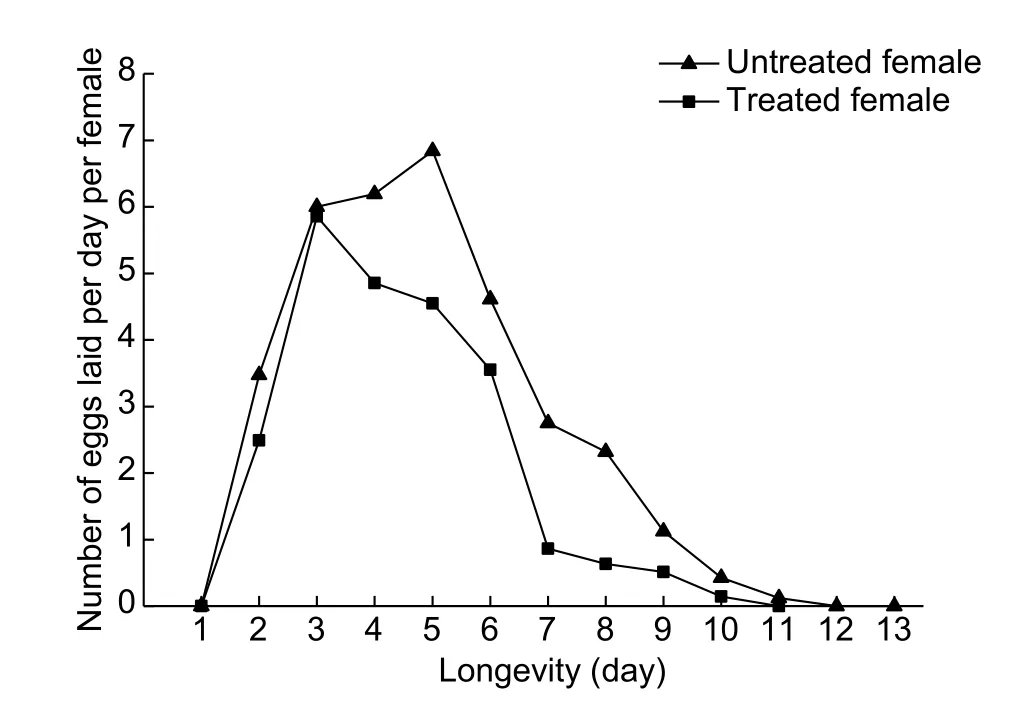
Fig. 1 Comparison of fecundity per day per female of Bemisia tabaci adults treated with Aschersonia aleyrodis pathogen(1×107 conidial mL–1) and untreated controls (without pathogen).

Table 4 Comparison of mean fecundity and longevity of Bemisia tabaci adult females on treated and untreated leaves of eggplants (not incubated)
3.4. Effect of A. aleyrodis pathogen on demographic parameters of B. tabaci
The demographic parameters ofB.tabaci,R0,DT,rmand λ were 2.532 days, 23.153 days, 0.032 and 1.031,respectively. When the cohort eggs were reared on untreated leaves, the demographic parameters were 17.944 days, 6.315 days, 0.110 and 1.123, respectively (Table 6).As noted from these results, the presence of pathogen has a significant effect on the population growth of the pest, with significant reduction of parameters except doubling time.
The effects of incubating the pathogens on the demographic parameters were also investigated. The computed parameters are displayed in Table 7. TheR0values for the three incubation periods (3, 6 and 9 days) were calculated as 5.899, 6.541 and 7.307 days, respectively. Our results indicate that incubation of the pathogen for varying days has a similar effect on theR0values. The values for λ were computed as 1.072, 1.072, 1.083, respectively, for the three incubation periods; interestingly little change in value was observed. This emphasized that incubation of the pathogen had no significant effect on the population growth of the pest. The importance of incubation period indicated that the spores were persistent on the leaves and were able to affectB.tabacigrowth when compared to non-incubated leaves (Table 6). The effect of the pathogen was found viable even when it was exposed for a period of time to external variables; it was still able to alter the demographic parameters in the current study. However, extending the incubation period did not have a significant effect on spore viability in the current study.
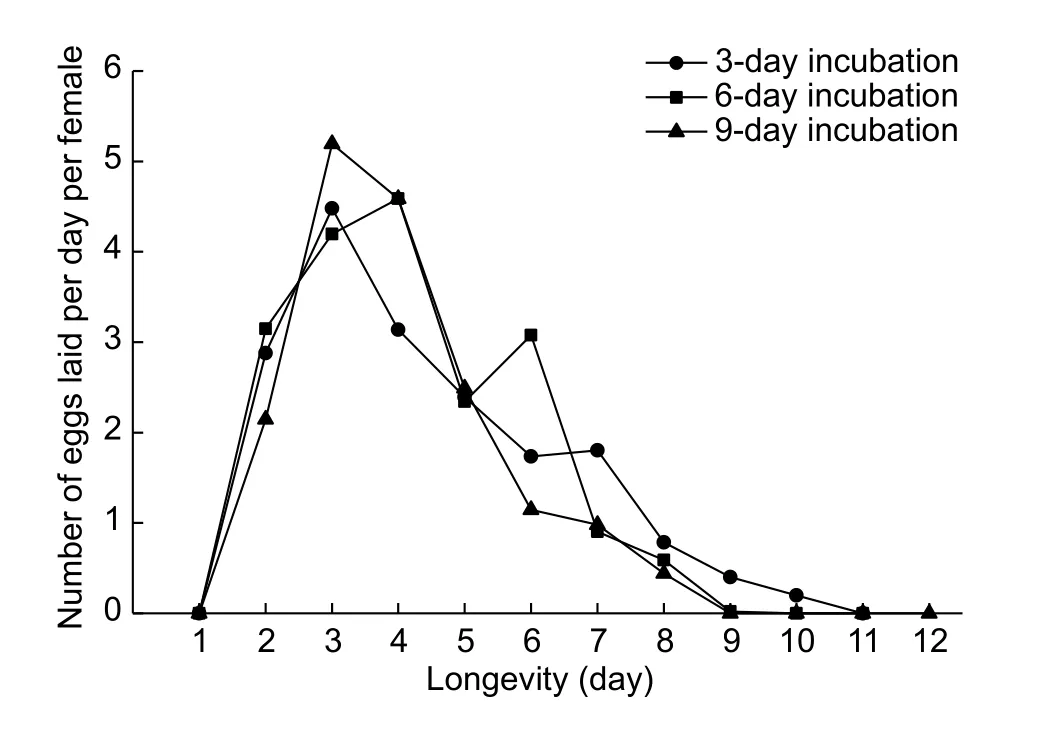
Fig. 2 Comparison of fecundity per day per female of Bemisia tabaci adults reared on leaves treated with different incubation periods of Aschersonia aleyrodis pathogen (1×107 conidial mL–1).
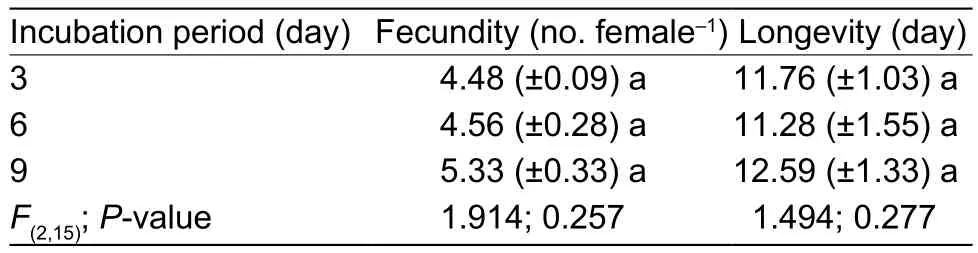
Table 5 Effect of fungal incubational periods on fecundity and longevity of female Bemisia tabaci
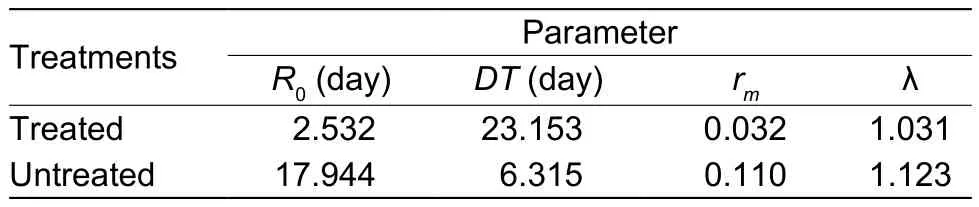
Table 6 Demographic parameters of Bemisia tabaci reared on untreated and treated leaves of eggplants (not incubated)1)
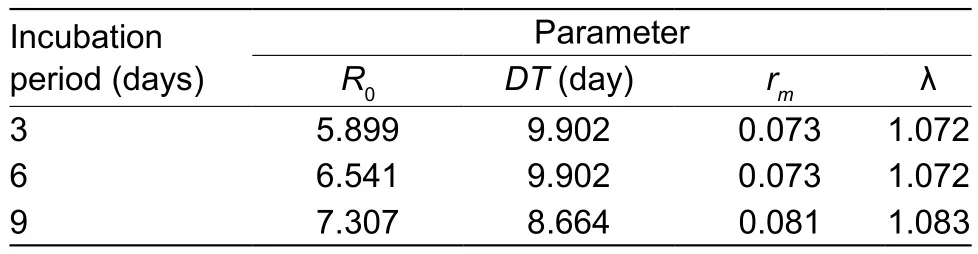
Table 7 Effect of incubation period on demographic parameters of Bemisia tabaci1)
4. Discussion
Entomopathogenic fungi are considered important natural enemies of a broad range of arthropods and are potentially valuable biological control agents of agricultural pests (Qiuet al.2013). In a previous study, the entomopathogenic fungus,A.aleyrodis, was shown to have high pathogenic potential againstB.tabaciunder laboratory and greenhouse conditions (Zhanget al.2017). In this current study, the single conidial concentration (1×107conidial mL–1) was used to assess the sub-lethal effects ofA.aleyrodisonB.tabacipopulations in order to improve our knowledge concerning the development ofA.aleyrodisas a biopesticide againstB.tabaci.
The results showed that fungal application significantly reduced the survivorship of the 1st, 2nd, and 3rd instar nymphs. The findings demonstrated that younger nymphs were more susceptible to fungi, which is similar to thefindings of Cuthbertsonet al.(2005) and Bajwaet al.(2016). However, fungal application had no influence on the developmental time ofB.tabaci. The nymphal mortality has reflected the germination capacity ofA.aleyrodis, which was clearly demonstrated with the high mortality of whiteflies on the treated leaves due to the presence of the pathogen.The result showed thatA.aleyrodisspores were still viable up to 9 days after incubation. Our data were consistent with the study of Meekeset al.(2000) whereA.aleyrodisspores were observed to be viable for even after one month on a leaf surface. These results stressed the existence of a natural enemy as a regulating factor for insect populations, and also gave further evidence for the ability of entomopathogenic fungi to persist on plant leaves.
Life table analysis is considered as one of the most effective analytical tools to evaluate the effectiveness of a regulating factor in insect populations. This method has been used to study population dynamics of a particular species (Tuanet al.2014) and to evaluate the impact of natural enemies on an insect-pest species (Duanet al.2014). The age specific life table was employed to evaluate the pathogenicity after incubation ofA.aleyrodisas a microbial control agent. The premise of this investigation is that the treated leaves will depress the build-up of an insect’s population to below a damaging level when the pathogen is present. Different incubations were implemented to test the persistence of the fungi spores on the leaves after being exposed to external environmental conditions for a period of time. After comparison of the incubated leaves with the controls, theA.aleyrodispathogen has shown no significant impact on the general fecundity and longevity ofB.tabaciadult females.
TheR0values describe the population that would be able to multiply according to the number indicated. From the results obtained in this study, theB.tabacipopulation tends to multiply by 17.944 in the absence of natural enemies(Table 6). When the insect was reared on a leaf treated with fungal spores (not incubated), theR0value was lowered to 2.532; indicating that the population tends to multiply by 2.532 times. In comparison to the control there was drastic depression of the insect population when the eggs were reared on leaves treated with fungal spores. TheR0values were computed as 5.899, 6.541 and 7.307 whenB.tabaciwere reared on leaves with fungal spores incubated for 3,6 and 9 days, respectively. It was indicated that incubation could affect theR0value ofB.tabaci(Huanget al.2010). It was not as high as those treatments that were not incubated.TheR0values for varying incubation periods of spores were not as low as that for treated non-incubated leaves. TheR0value therefore was affected as a result of incubation of spores but was still lower than that of the control.
The most important parameter calculated from the life table is thermwhich includes information regarding age-specific individual fecundity and survivorship in the absence of a natural enemy (Birch 1948; Smith 1979).This parameter facilitates the evaluation of a pathogen concerning its use within a control strategy where biological control is an option. Thus, if a natural enemy is an effective agent in inoculated or seasonal inoculated release, it should be capable of lowering thermvalue of an insect pest (Drostet al.1998). This strategy was clearly demonstrated in this investigation. Thermvalues of the control was 0.110 and was 0.032 when the eggs were reared on treated leaves that were not incubated. Thermvalue (0.032) of treated leaves without incubation indicated high viability of spores on the treated leaves. Thermvalues were 0.073, 0.073 and 0.081 for treated leaves withA.aleyrodisspores incubated for 3, 6 and 9 days respectively. The post inoculation or simple incubation of the fungal spores on the leaves at different days stressed the importance of the fungal spore’s persistence on the leaves. Although thermvalue decreased with incubation period, the viability of spores was still maintained 9 days after treatment (rmvalue 0.081), though it was comparatively lower than thermvalue of the control(0.110). These values indicated that the potential of the spores to act as a microbial control agent when exposed to the environment was maintained for a longer period of time.
TheDTis another demographic parameter that gives insight into the population trend of the insect host in the absence of a natural enemy. It can also be used as an effective parameter for comparative purposes. The doubling time for whiteflies (the time required for the population to double) reared on untreated leaves was significantly lower(6.315 days) than treated leaves. This value suggests that in the absence of the natural enemy, the time for a whitefly population to double was 6.315 days as compared to the treatments with the pathogen present. The values of doubling time for various treatments derived from this study were 23.153 days for treated leaves and 9.902, 9.902 and 8.664 days for treated leaves incubated for 3, 6 and 9 days,respectively, prior to rearing of cohort eggs. These results mean thatA.aleyrodisspores can suppress the increase ofB.tabacipopulations, which is the essence of biological control using entomopathogenic fungi.
In this study, life table and demographic parameters were employed to study the sublethal effects ofA.aleyrodisonB.tabaci. It is apparent thatA.aleyrodisspores can significantly affect the survivorship of youngB.tabacinymphs. The persistence ofA.aleyrodisspores on eggplants for long periods is an important aspect for its application. The impact of the pathogen on the demographic parameters also demonstrates its potential as a bio-control agent. In the quest for alternative control measures to the current dependency on chemicals, consideration should be given to integrated pest management options of whichA.aleyrodiscould be a part of such a strategy for sustainableB.tabacimanagement. It will however be essential to know the synergism between this fungus and other biocontrol agents for more effective control ofB.tabaciwithin integrated pest management strategies.
5. Conclusion
This study indicated thatA.aleyrodiscould impact the survivorship of the 1st, 2nd and 3rd instar nymphs ofB.tabacithough it has no influence in nymphal development time. Following different incubation periods, the spores ofA.aleyrodiscan continue to have a pathogenic effect on survivorship ofB.tabaci. Life table results suggestedA.aleyrodishas no impact in general fecundity and longevity of adult femaleB.tabaci. When the pathogen was exposed to the external environment for up to 9 days, the spores ofA.aleyrodismaintained potency to suppress population levels ofB.tabaci.
Acknowledgements
This research was funded by the Science and Technology Program of Guangzhou of China (201509010023,201604020180), the Science and Technology Program of Guangdong Province of China (2015B090903042) and the National Public Benefit research foundation (201303019).The authors thank Dr. Andrew G S Cuthbertson from South China Agricultural University for assistance with manuscript editing.
Ahmed M Z, Ren S X, Mandour N S, Maruthi M N, Naveed M, Qiu B L. 2010. Phylogenetic analysis ofBemisia tabaci(Hemiptera: Aleyrodidae) populations from cotton plants in Pakistan, China, and Egypt.Journal of Pest Science,83, 135–141.
Ali S, Wu J H, Huang Z, Ren S X. 2010. Production and regulation of extracellular chitinase from the entomopathogenic fungusIsaria fumosorosea.Biocontrol Science and Technology,20, 723–738.
Andrewartha H G, Birch L C. 1964.The Distribution and Abundance of Animals. University of Chicago Press, USA.
Bajwa, G A, Abood F, Ibrahim Y. 2016. Age-specific response ofAtteva sciodoxaMeyrick (Lepidoptera: Yponomeutidae) toBeauveria bassiana(Bals.).Sarhad Journal of Agriculture,32, 1–8.
Birch L C. 1948. The intrinsic rate of natural increase of an insect population.Journal of Animal Ecology, 17, 15–26.
Blanford S, Thomas M B. 2001. Adult survival, maturation, and reproduction of the desert locustSchistocerca gregariainfected with the fungusMetarhizium anisopliaevar acridum.Journal of Invertebrate Pathology, 78, 1–8.
Cahill M, Byrne F J, Gorman K, Denholm I, Devonshire A L.1995. Pyrethroid and organophosphate resistance in the tobacco whiteflyBemisia tabaci(Homoptera: Aleyrodidae).Bulletin of Entomological Research, 85, 181–187.
Cuthbertson A G S, Blackburn L F, Eyre D P, Cannon R J C,Millar J, Northing P. 2011.Bemisia tabaci: The current situation in the UK and the prospect of developing strategies for eradication using entomopathogens.Insect Science,18, 1–10.
Cuthbertson A G S, Murchie A K. 2005. European red spider mite - an environmental consequence of persistent chemical pesticide application.International Journal of Environmental Science and Technology, 2, 287–290.
Cuthbertson A G S, Walters K F A, Northing P. 2005.Susceptibility ofBemisia tabaciimmature stages to the entomopathogenic fungusLecanicillium muscariumon tomato and verbena foliage.Mycopathologia, 159, 23–29.
Drost Y C, Van Lenteren J C, Van Roermund H J W. 1998.Life-history parameters of different biotypes ofBemisia tabaci(Hemiptera: Aleyrodidae) in relation to temperature and host plant: A selective review.Bulletin of Entomological Research, 88, 219–230.
Duan J J, Abell K J, Bauer L S, Gould J, Van Driesche R. 2014.Natural enemies implicated in the regulation of an invasive pest: A life table analysis of the population dynamics of the emerald ash borer.Agricultural and Forest Entomology,16, 406–416.
He S, Wu X Y, Zheng J Z, Han Z Q, Zhao C X, Wang Y Y,Chen H, Chen C, Han J. 2013. A study of control effect ofBemisia tabaci(Gennadius) on greenhouse tomatoes using parasitoidEncarsia formosaGahan.Journal of Anhui Agricultural Sciences, 41, 6244–6245, 6248. (in Chinese)
Huang Z, Ali S, Ren S X, Wu J H. 2010. Effect ofPaecilomyces fumosoroseuson mortality and reproduction ofBemisia tabaciandPlutella xylostella.Insect Science, 17, 140–148.
Ibrahim Y B, Low W. 1993. Potential of mass-production andfield efficacy of isolates of the entomopathogenic fungiBeauveria bassianaandPaecilomyces fumosoroseusagainstPlutella xylostella.International Journal of Pest Management, 39, 288–292.
Krebs C J. 1994.Ecology:The Experimental Analysis of Distribution and Abundance. 4th ed. Harper Collins College Publishers, New York.
Liang P, Tian Y A, Biondi A, Desneux N, Gao X W. 2012. Shortterm and transgenerational effects of the neonicotinoid nitenpyram on susceptibility to insecticides in two whitefly species.Ecotoxicology, 21, 1889–1898.
Liu Y Q, Liu S S. 2012. Species status ofBemisia tabacicomplex and their distributions in China.Journal of Biosafety, 21,247–255.
Maia A H N, Luiz A J B, Campanhola C. 2000. Statistical inference on associated fertility life table parameters using jackknife technique: Computational aspects.Journal of Economic Entomology, 93, 511–518.
Maia M M D, Heasley A, De Morais M M C, Melo E H M, Morais M A, Ledingham, W M, Lima Filho J L. 2001. Effect of culture conditions on lipase production byFusarium solaniin batch fermentation.Bioresource Technology, 76, 23–27.
Meekes E T M, Fransen J J, van Lenteren J C. 2002.Pathogenicity ofAschersoniaspp. against whitefliesBemisia argentifoliiandTrialeurodes vaporariorum.Journal of Invertebrate Pathology, 81, 1–11.
Meekes E T M, van Voorst S, Joosten N N, Fransen J J, van Lenteren J C. 2000. Persistence of the fungal whitefly pathogen,Aschersonia aleyrodis, on three different plant species.Mycological Research, 104, 1234–1240.
Naranjo S E, Castle S J, De Barro P J, Liu S S. 2010. Population dynamics, demography, dispersal and spread ofBemisia tabaci. In: Stansly, P A, Naranjo S, eds.,Bemisia: Bionomics and Management of a Global Pest. Springer, Dordrecht.pp. 185–226.
Pell J K, Wilding N, Player A L, Clark S J. 1993. Selection of an isolate ofZoophthora radicans(Zygomycetes:Entomophthorales) for biocontrol of the diamondback mothPlutella xylostella(Lepidoptera: Yponomeutidae).Journal of Invertebrate Pathology, 61, 75–80.
Qiu J, Song F, MaoL, Tu J, GuanX. 2013. Time-dose-mortality data and modeling for the entomopathogenic fungusAschersonia placentaagainst the whiteflyBemisia tabaci.Canadian Journal of Microbiology, 59, 97–101.
Quesada-Moraga E, Santos-Quirós R, Valverde-Garcia P, Santiago-Alvarez C. 2004. Virulence, horizontal transmission, and sublethal reproductive effects ofMetarhizium anisopliae(Anamorphic fungi) on the German cockroach (Blattodea: Blattellidae).Journal of Invertebrate Pathology, 87, 51–58.
SAS Institute. 2004.SAS/GRAPH 9.1 Reference. SAS Institute,Cary, North Carolina, USA.
Smith P E. 1979. Ecological methods with particular reference to the study of insect populations.Ecology, 60, 1290–1290.
Stansly P A, Naranjo S E. 2010.Bemisia:Bionomics and Management of a Global Pest. Springer, Dordrecht-Heidelberg, London and New York. p. 528.
Toscano N, Henneberry T. Castle S. 1994. Population dynamics and pest status of silverleaf whitefly in the USA.Arab Journal of Plant Protection, 12, 137–148
Tuan S J, Lee C C, Chi H. 2014. Population and damage projection ofSpodoptera litura(F.) on peanuts (Arachis hypogaeaL.) under different conditions using the age-stage,two-sex life table.Journal of Pest Science, 70, 805–813.
Vandenberg J D, Shelton A M, Wilsey W T, Ramos M. 1998.Assessment ofBeauveria bassianasprays for control of diamondback moth (Lepidoptera: Plutellidae) on crucifers.Journal of Economic Entomology, 91, 624–630.
Zhang C, Ali S, Musa P D, Wang X M, Qiu B L. 2017. Evaluation of the pathogenicity ofAschersonia aleyrodisonBemisia tabaciin the laboratory and greenhouse.Biocontrol Science and Technology, 27, 210–221.
 Journal of Integrative Agriculture2018年2期
Journal of Integrative Agriculture2018年2期
- Journal of Integrative Agriculture的其它文章
- Rapid mapping of candidate genes for cold tolerance in Oryza rufipogon Griff. by QTL-seq of seedlings
- A dCAPS marker developed from a stress associated protein gene TaSAP7-B governing grain size and plant height in wheat
- A major quantitative trait locus controlling phosphorus utilization efficiency under different phytate-P conditions at vegetative stage in barley
- Overexpression of IbSnRK1 enhances nitrogen uptake and carbon assimilation in transgenic sweetpotato
- Collision detection of virtual plant based on bounding volume hierarchy: A case study on virtual wheat
- lntegrated management strategy for improving the grain yield and nitrogen-use efficiency of winter wheat
