Experimental infectivity of Theilerialuwenshuni and Theileria uilenbergi in Chinese Kunming mice
LI You-quan, GUO Peng-fei LIU Jun-long LIU Zhi-jie HAN Yuan LI Xuan LIU Ai-hong GUAN Gui-quan, LIU Guang-yuan, LUO Jian-xun, YIN Hong
1 State Key Laboratory of Veterinary Etiological Biology/Key Laboratory of Veterinary Parasitology of Gansu Province, Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences, Lanzhou 730046, P.R.China
2 Jiangsu Co-Innovation Center for Prevention and Control of Important Animal Infectious Diseases and Zoonoses, Yangzhou 225009, P.R.China
1. Introduction
Theileriaparasites are transmitted by tick species of the familyIxodidaeand cause significant losses to the cattle and sheep industry in endemic areas (Luo and Lu 1997; Aktaset al. 2004; Simuunzaet al. 2011). SomeTheileriaspecies that infect sheep and goats are pathogenic, includingTheileria lestoquardi,Theileria uilenbergi, andTheileria luwenshuni(Luo and Yin 1997; Schnitteret al. 2000; Yinet al. 2007).T.luwenshuniandT.uilenbergiare widely distributed in northern China (Luo and Yin 1997; Yin 2000; Liet al. 2014c) and are transmitted by the ticksHaemaphysalis qinghaiensisandHaemaphysalis longicornisin China(Yin 2000; Yinet al. 2002; Liet al. 2007, 2009, 2014c).T.luwenshuniinfects sheep from Great Britain (Phippset al.2016).T.luwenshuniandT.ulenbergiwere believed to infect only sheep and goats (Luo and Yin 1997; Yin 2000;Yinet al. 2007).
However, a recent study has reported thatT.luwenshuniinfects several animal species, including cervids (Hanet al.2009; Liet al. 2014a, 2015; Leeet al. 2016), Mongolian gazelle (Liet al. 2014b),hedgehog (Chenet al. 2014), cattle, and yak (Qinet al. 2016).T.uilenbergican also infect several cervid species, including sika deer, red deer, and roe deer (Liet al. 2014a, 2015), as well as cattle, yak, and cattle-yak (unpublished data). In the 1990s, some studies reported that mice were used for the isolation ofBabesiaparasites from grazing calves in Japan (Tsujiet al. 1995),Theileria annulataschizonts infected severe combined immunodeficiency (SCID) mice (Fellet al. 1990), andTheileria sergentiinfected SCID mice with bovine erythrocyte transfusion (Tsujiet al. 1992). The Chinese Kunming mouse strain (Mus musculusKunming, KM), an outbreed mouse strain originating from the Swiss Albino mouse,is widely used in pharmacological and gene-related studies throughout China (Yanet al. 2017). Kunming mice are widely used as experimental animals in China because it exhibits many advantages such as high disease resistance, large and frequent litters and rapid growth rate. However, no previous studies have evaluated the infectivity ofT.luwenshuniandT.uilenbergiin mice. Therefore, the present study aimed at determining the experimental infectivity ofT.luwenshuniandT.uilenbergiin Kunming mice by microscopic examination (ME) and PCR analysis.
2. Materials and methods
This study was approved by the Animal Research Ethics Committee of Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences (No.LVRIAEC2012-003). All sheep and mice were handled in accordance with good animal practices found in the Animal Ethics Procedures and Guidelines of the People’s Republic of China.
2.1. Parasites
T.luwenshuniwas isolated from goats from Weiyuan County, Gansu Province,China in 2005 and identified as a singleT.luwenshunispecies based on morphological, transmission and molecular methods.T.uilenbergiwas isolated from sheep from Yongjing County, Gansu Province, China in 2012 and identified as a singleT.uilenbergispecies based on morphological, transmission, and molecular method. The twoTheileriaspp. isolates were cryopreserved in liquid nitrogen at OIE Reference Laboratory for Ovine Theileriosis of Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences.
2.2. Sheep
Two 5-month-old sheep from Jingtai County, Gansu Province were identified to beTheileria-free andBabesia-free using the methods described by Yin (2000),and utilized in this study.
2.3. Inoculation of mice with T. luwenshuni and T. uilenbergi
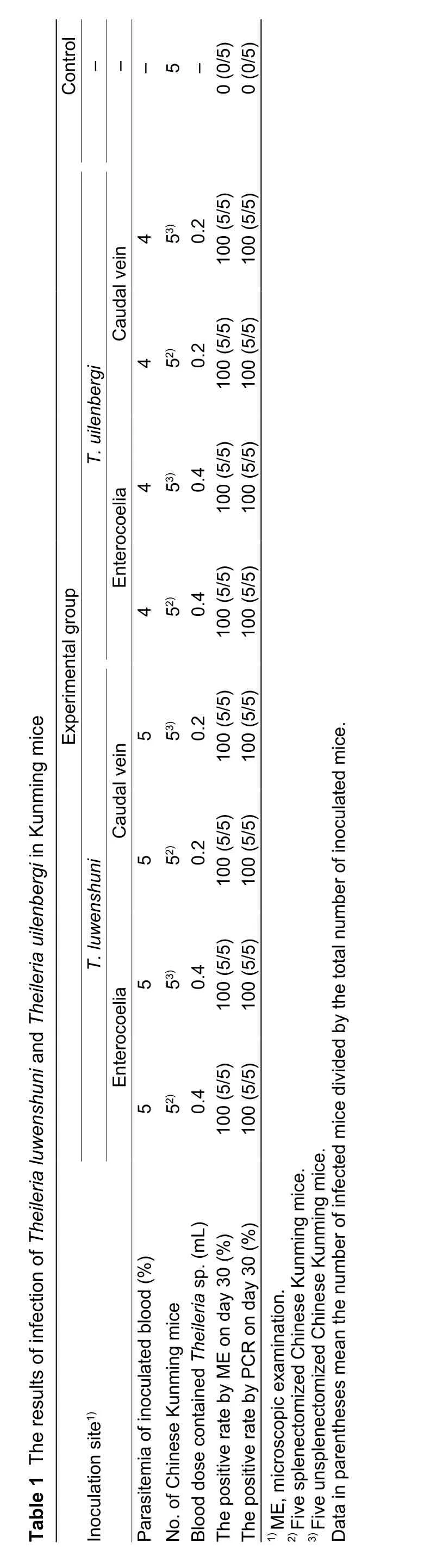
?
Sheep No. 11 was inoculated withT.luwenshuniisolate, and sheep No. 22 was inoculated withT.uilenbergiisolate. When the parasitemia of sheep No. 11 reached 5% and the parasitemia of sheep No. 22 reached 4%, the blood samples of the two sheep were individually collected into anticoagulant tubes and utilized in inoculation. Five mice were used as the control group. In theT.luwenshuniexperimental group, 20 Chinese Kunming mice divided into four groups (Table 1):(1) five splenectomized mice were inoculated intraperitoneally with 0.4 mL ofTheileria-infected blood, (2) five unsplenectomized mice were inoculated intraperitoneally with 0.4 mL ofTheileria-infected blood, (3) five splenectomized mice were inoculated intravenously with 0.2 mL ofTheileria-infected blood, and (4)five unsplenectomized mice were inoculated intravenously with 0.2 mL ofTheileria-infected blood. In theT.uilenbergiexperimental group, the arrangement is similar to theT.luwenshuniexperimental group. Total of 100 μL whole blood was collected into 4 mL blood collection tube containing K3EDTA from the tails of 45 Chinese Kunming mice each 4 days, respectively. Genomic DNA of 45 mice whole blood samples was extracted individually using DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany)according to the manufacturer’s instructions. The concentration of DNA was determined using a NanoDrop ND-2000 Spectrophotometer (Nanodrop Technologies,Wilmington, DE, USA). In addition, the blood smears of 45 mice were prepared using mouse caudal vein blood each 2 days, respectively. Ten days after inoculation, the blood smears were analyzed by microscopic examination every 2 days and genomic DNA was screened by PCR analysis every 4 days. After that, all mice were monitored every 2 days for 3 months by measuring two physiological indexes:red blood cells (RBC) and hemoglobin (Hb).
2.4. Microscopic analysis of blood smears
The blood smears were air-dried, fixed in methanol, stained with a 10% solution of Giemsa in deionized water (pH 7.2),and analyzed microscopically.
2.5. Molecular detection of blood parasites
PCR was used to detectTheileriaspp. in Chinese Kunming mice using genus-specific primers (Allosopet al. 1993) and species-specific primers (Yin 2000;Yinet al. 2008). The specific primers ofT.luwenshuniare Tl-f: GGTAGGGTATTGGCCTACTGA and Tl-r:TCATCCGGATAATACAAGT, with a 340-bp product (Yin 2000; Yinet al. 2008); the specific primers ofT.uilenbergiare Tu-f: GGTAGGGTATTGGCCTACCGG and Tu-r:ACACTCGGAAAATGCAAGCA, with a 340-bp products(Yin 2000; Yinet al. 2008). The specific primers ofTheileriaspp. are 989-f: AGTTTCTGACCTATCAG and 990-r: TTGCCTTAAACTTCCTTG, with a 1 100-bp product(Allosopet al. 1993). The PCRs were performed in an automatic DNA thermocycler (Bio-Rad, Hercules, CA, USA)according to the previously described methods (Allosopet al. 1993; Yin 2000; Yinet al. 2008), and the PCR products were separated by 1.5% agarose gel electrophoresis to assess the presence of specific fragments indicative of the presence ofTheileria. After that, the amplification products were sequenced by GenScript Corporation (Piscataway,NJ, USA), and representative sequences of the 18S rDNA genes ofT.luwenshuniandT.uilenbergiwere analyzed using Basic Local Alignment Search TooL (BLAST) from the National Center for Biotechnology Information (NCBI)(http://www.ncbi.nlm.nih.gov/Blast.cgi). The 18S rRNA gene sequences ofTheileriaspp. obtained from Chinese Kunming mice in this study were deposited in GenBank.
3. Results and discussion
On days 18 to 20,Theileriainfection was detected microscopically in 40 mice from the experimental groups,and the levels of parasitemia in all infected mice were very low, ranging between 0.01 and 0.03% (Fig. 1). NoTheileriaparasites were detected in the control group during the study period. On days 16 to 18,T.luwenshuni-specific DNA fragments were detected in the fourT.luwenshuniinoculated mice groups, andT.uilenbergi-specific DNA fragments were detected in the fourT.ulenbergi-inoculated mice groups. However, noTheileriaparasites were detected in blood samples from the control group by ME and PCR.BLAST analysis indicated that theT.luwenshuni-specific DNA sequences in the experimental groups shared 100%similarity with the sequences fromT.luwenshuniisolate Weiyuan; meanwhile,T.uilenbergi-specific DNA sequences in the experimental groups also shared 100% similarity with the sequences fromT.uilenbergiisolate Yongjing.The 18S rRNA gene sequences ofTheileriaspp. obtained from Chinese Kunming mice in this study were deposited in GenBank under the following accession numbers:KY508396-KY508399 forT.luwenshuni, and KY508400-KY508403 forT.uilenbergi. The RBC count and Hb levels of the 40 infected mice had no obvious changes in the course of infection. In this study, there is not obviously observed changes of the parasitemia rate of the infected Kunming mice between the species ofT. luwenshuniandT. uilenbergi, or injectionviacaodal wein and enterocoelia.Two reasons are for the low parastemia in mice: One is that the mice are not suitable hosts forT.luwenshuniandT.uilenbergi; another is thatT.luwenshuniandT.uilenbergiare not pathogenic to mice so that all inoculated mice have no hemolytic anemia phenomenon.

Fig. 1 Theileria luwenshuni parasite in red blood cells of a Chinese Kunming mouse (see arrow).
Tsujiet al. (1995) used a Bo-RBC-SCID mouse model to isolate theBabesiaparasites from grazing calves in Japan,and Bo-RBC-SCID mice infected withBabesiaparasites developed a high parasitemia and presented with severe clinical symptoms, including hemoglobinuria, jaundice,and hemolytic anemia. Fellet al. (1990) reported that mice injected subcutaneously withT. annulataschizonts developed solid tumors at the injection site, whereas those injected intraperitoneally with these parasites developed ascites. Tsujiet al. (1992) reported the first successful propagation ofT.sergentiin SCID mice supplied periodically with uninfected bovine erythrocytes. Hagiwaraet al. (1993)identified parasite infection in mice inoculated intravenously,intraperitoneally, and subcutaneously. However, the animals inoculated intravenously developed parasitemia earlier than those inoculated intraperitoneally or subcutaneously. Herein we found that the level of parasitemia in the normal Chinese Kunming mice inoculated intravenously was not significantly different from that in mice inoculated intraperitoneally, the possible reason is that the low parasitemia of the infected normal mice influences the difference.
AlthoughT.luwenshuniandT.uilenbergiinfected mice,the infected mice had no obviously pathological changes(e.g., hemolytic anemia). In addition, the parasitemia of these two parasite species was low in mice. Therefore,the Kunming mouse is not a good model of infection with the twoTheileria. However, further studies are needed to evaluate whetherT.luwenshuniandT.uilenbergican efficiently infect SCID mice with jaundice and hemolytic anemia. Furthermore, the elucidation of the role of normal mice as potential reservoir hosts forT.luwenshuniandT.uilenbergiis critical for determining whether they help spread theileriosis to wild ruminants.
4. Conclusion
Our results demonstrated the occurrence of multi-homing parasitism inT.luwenshuniandT.uilenbergiin lab, which were believed to infect only sheep and goats. AlthoughT.luwenshuniandT.uilenbergican infect normal Kunming mice, the Kunming mouse is not suitable for a good model of infection withTheileriaparasites because they did not develop high parasitemia and showed no severe clinical symptoms such as jaundice and hemolytic anemia.
Acknowledgements
This study was financially supported by the National Key Research and Development Program of China(2017YFD0501200, 2016YFC1202000, 2016YFC1202002),the earmarked fund for China Agriculture Research System(CARS-37), the National Natural Science Foundation of China (31272556, 31402189, 31372432), the Agricultural Science and Technology Innovation Program, China(2014ZL010), the National Basic Research Program of China (2015CB150300), the Special Funds for Agroscientific Research in the Public Research, China(201303035), the Gansu International Collaboration Special Project, China (1504WKCA056), the Jiangsu Co-Innovation Center Programme for Prevention and Control of Important Animal Infectious Diseases and Zoonoses, State Key Laboratory of Veterinary Etiological Biology Project, China.We are grateful to International Science Editing (Ireland) for editing the manuscript.
Aktas M, Dumanli N, Angin M. 2004. Cattle infestation byHyalommaticks and prevalence ofTheileriainHyalommaspecies in the east of Turkey.Veterinary Parasitology,119, 1–8.
Allsopp B A, Baylis H A, Allsopp M T, Cavalier-Smith T, Bishop R P, Carrington D M, Sohanpal B, Spooner P. 1993.Discrimination between six species ofTheileriausing oligonucleotide probes which detect small subunit ribosomal RNA sequences.Parasitology, 107, 157–165.
Chen Z, Liu Q, Jiao F C, Xu B L, Zhou X N. 2014. Detection of piroplasms infection in sheep, dogs and hedgehogs in Central China.Infectious Diseases of Poverty, 3, doi:10.1186/2049-9957-3-18
Fell A H, Preston P M, Ansell J D. 1990. Establishment ofTheileria-infected bovine cell lines inscidmice.Parasite Immunology, 12, 335–359.
Hagiwara K, Tsuji M, Ishihara C, Tajima M, Kurosawa T, Iwai H, Takahashi K. 1993.Theileria sergentiinfection in the Bo-RBC-SCID mouse model.Parasitology Research, 79,466–470.
Han J, Jang H, Lee S, Na K. 2009. High prevalence ofTheileriasp. in wild Chinese water deer (Hydropotes inermis argyropus) in South Korea.Veterinary Parasitology, 164,311–314.
Lee S H, Kim K T, Kwon O D, Ock Y, Kim T, Choi D, Kwak D.2016. Novel detection ofCoxiellaspp.,Theileria luwenshuni,andT.ovisendosymbionts in deer keds(Lipoptena fortisetosa).PLoS ONE, 11, e0156727.
Li Y, Chen Z, Liu Z, Liu J, Yang J, Li Q, Li Y, Cen S, Guan G, Ren Q, Luo J, Yin H. 2014a. Molecular identification ofTheileriaparasites of northwestern Chinese Cervidae.Parasites & Vectors, 14, 225.
Li Y, Chen Z, Liu Z, Liu J, Yang J, Li Q, Li Y, Ren Q, Niu Q,Guan G, Luo J, Yin H. 2014b. First report ofTheileriaandAnaplasmain the Mongolian gazelle,Procapra gutturosa.Parasites & Vectors, 7, 614.
Li Y, Liu J, Liu Z, Yang J, Li Y, Li Q, Qin G, Chen Z, Guan G, Luo J, Yin H. 2015. Report ofTheileria luwenshuniandTheileriasp. RSR from cervids in Gansu, China.Parasitology Research, 114, 2023–2029.
Li Y, Luo J, Guan G, Ma M, Liu A, Liu J, Ren Q, Niu Q, Lu B,Gao J, Liu Z, Dang Z, Tian Z, Zhang B, He Z, Bai Q, Yin H. 2009. Experimental transmission ofTheileria uilenbergiinfective for small ruminants byHaemaphysalis longicornisandHaemaphysalis qinghaiensis.Parasitology Research,104, 1227–1231.
Li Y, Luo J, Liu Z, Guan G, Gao J, Ma M, Dang Z, Liu A, Ren Q, Lu B, Liu J, Zhao H, Li J, Liu G, Bai Q, Yin H. 2007.Experimental transmission ofTheileriasp. (China 1)infective for small ruminants byHaemaphysalis longicornisandHaemaphysalis qinghaiensis.Parasitology Research,101, 533–538.
Li Y, Zhang X, Liu Z, Chen Z, Yang J, He H, Guan G, Liu A, Ren Q, Niu Q, Liu J, Luo J, Yin H. 2014c. An epidemiological survey ofTheileriainfections in small ruminants in central China.Veterinary Parasitology, 200, 198–202.
Luo J, Lu W. 1997. Cattle theileriosis in China.Tropical Animal Health and Production, 29(Suppl. 4), 4S–7S.
Luo J, Yin H. 1997. Theileriosis of sheep and goats in China.Tropical Animal Health and Production, 29, 8s–9s.
Phipps L P, Hernández-Triana L M, Goharriz H, Welchman D, Johnson N. 2016. Detection ofTheileria luwenshuniin sheep from Great Britain.Parasites & Vectors, 9, 203.
Qin G, Li Y, Liu J, Liu Z, Yang J, Zhang L, Liu G, Guan G, Luo J, Yin H. 2016. Molecular detection and characterization ofTheileriainfection in cattle and yaks from Tibet Plateau Region, China.Parasitology Research, 115, 2647–2652.
Schnitter L, Yin H, Jianxun L, Ludwig W, Shayan P, Rahbari S, Voss-Holtmann A, Ahmed J S. 2000. Ribosomal small-subunit RNA gene-sequence analysis ofTheileria lestoquardiand aTheileriaspecies highly pathogenic forTheileria buffeli(synT.orientalis) in cattle.Research in Veterinary Science, 86, 352–358.
Simuunza M, Weir W, Courcier E, Tait A, Shiels B. 2011.Epidemiological analysis of tick-borne diseases in Zambia.Veterinary Parasitology, 175, 331–342.
Tsuji M, Hagiwara K, Takahashi K, Ishihara C, Azuma I, Siddiqui W A. 1992.Theileria sergentiproliferates in SCID mice with bovine erythrocyte transfusion.Journal of Parasitology,78, 750–752.
Tsuji M, Terada Y, Arai S, Okada H, Ishihara C. 1995. Use of the Bo-RBC-SCID mouse model for isolation of aBabesiaparasite from grazing calves in Japan.Experimental Parasitology, 81, 512–518.
Yan W, Yao L, Liu W, Sun K, Zhang Z, Zhang L. 2017. A kind of rd1 mouse in C57BL/6J mice from crossing with a mutated Kunming mouse.Gene, 607, 9–15.
Yin H. 2000. Biological and molecular characterization ofTheileriasp. infective to sheep and goats. Ph D thesis,Graduate School of Chinese Academy of Agricultural Sciences, China. pp. 6–122. (in Chinese)
Yin H, Guan G Q, Ma M L, Luo J X, Lu B Y, Yuan G, Bai Q, Lu C, Yuan Z, Preston P. 2002.Haemaphysalis qinghaiensisticks transmit at least two differentTheileriaspecies: One is infective to yaks, one is infective to sheep.Veterinary Parasitology, 107, 29–35.
Yin H, Liu Z, Guan G, Liu A, Ma M, Ren Q, Luo J. 2008. Detection and differentiation ofTheileria luwenshuniandT.uilenbergiinfection in small ruminants by PCR.Transboundary and Emerging Diseases, 55, 233–237.
Yin H, Schnittger L, Luo J X, Ulrike S, Ahmed J. 2007. Ovine theileriosis in China: A new look at an old story.Parasitology Research, 101(Suppl. 2), S191–S195.
 Journal of Integrative Agriculture2018年2期
Journal of Integrative Agriculture2018年2期
- Journal of Integrative Agriculture的其它文章
- Rapid mapping of candidate genes for cold tolerance in Oryza rufipogon Griff. by QTL-seq of seedlings
- A dCAPS marker developed from a stress associated protein gene TaSAP7-B governing grain size and plant height in wheat
- A major quantitative trait locus controlling phosphorus utilization efficiency under different phytate-P conditions at vegetative stage in barley
- Overexpression of IbSnRK1 enhances nitrogen uptake and carbon assimilation in transgenic sweetpotato
- Collision detection of virtual plant based on bounding volume hierarchy: A case study on virtual wheat
- lntegrated management strategy for improving the grain yield and nitrogen-use efficiency of winter wheat
