Molecular cloning and functional characterization of a soybean GmGMP1 gene reveals its involvement in ascorbic acid biosynthesis and multiple abiotic stress tolerance in transgenic plants
XUE Chen-chen, XU Jin-yan, WANG Can, GUO Na, HOU Jin-feng, XUE Dong, ZHAO Jin-ming, XlNG Han
National Center for Soybean Improvement/Key Laboratory of Biology and Genetics and Breeding for Soybean, Ministry of Agriculture/State Key Laboratory of Crop Genetics and Germplasm Enhancement, Nanjing Agricultural University, Nanjing 210095,P.R.China
1.lntroduction
The GDP-D-mannose pyrophosphorylase (GMP) gene encodes a GDP-mannose pyrophosphorylase, which is one of the most important proteins in plant L-ascorbic acid(vitamin C or AsA) biosynthesis (Conklin et al.1999).In plant, AsA is reported to protect against oxidative stress caused by adverse environmental conditions and it functions in various aspects of plant growth (Córdoba and Gonzalez-Reyes 1994).Most notably, AsA plays an important role in maintaining the rate of cell growth and division in the quiescent center of the root (Kerk and Feldman 1995)and conferring tolerance to salt, ozone, and cold stresses(Conklin et al.1996; Veljovic-Jovanovic et al.2001; Chen and Gallie 2005; Eltayeb et al.2007; Zhang et al.2012).Exogenous application of AsA stimulates cell division and growth (Kerk and Feldman 1995), and increases salt tolerance (Zhang et al.2012).
Previous studies showed four pathways of AsA synthesis:D-glucuronic acid (Agius et al.2003), L-galactose (Wheeler et al.1998), l-gulose (Wolucka and Van Montagu 2003), and the myo-inositol pathways (Lorence 2004).The L-galactose pathway is one of the most important pathways in plant AsA biosynthesis (Davey et al.2000; Conklin 2001).The GDP-mannose pyrophosphorylase catalyzes the conversion of D-mannose-1-P to GDP-D-mannose (Hashimoto et al.1997)is important in the L-galactose pathway (Conklin et al.1999).
Previous studies have shown that mutants of GMP cause abnormalities in AsA biosynthesis (Conklin et al.2000; Colville and Smirnoff 2008; Barth et al.2010), protein N-glycosylation (Conklin et al.1999; Lukowitz et al.2001; Qin et al.2008; Barth et al.2010), tolerance to high ammonium(Qin et al.2008; Barth et al.2010; Kempinski et al.2011), cell wall formation (Nickle and Meinke 1998; Conklin et al.1999; Jiang et al.2008), cell expansion and division(Nickle and Meinke 1998; Veljovic-Jovanovic et al.2001; Li et al.2010) and can lead to cell death (Nickle and Meinke 1998; Qin et al.2008; Barth et al.2010).
In contrast to GMP mutation, overexpression of GMP genes is little reported.Overexpression of the GDP-mannose pyrophosphorylase gene in Saccharomyces cerevisiae corrects defects in dolichol-linked saccharide formation and protein glycosylation (Janik et al.2003).In plants, previous studies have only shown that lines overexpressing GMP genes can enhance AsA content (Badejo et al.2007, 2008;Wang et al.2011) and improve tolerance to both low and high temperature stresses (Wang et al.2011, 2012).
In the present study, we first cloned the soybean GmGMP1 gene and investigated its expression pattern in soybean plants.Then we overexpressed this gene in Arabidopsis thaliana and soybean to examine the AsA content levels and other physiological indicator levels in the transgenic lines.The results suggest that GmGMP1 plays a pivotal role in protecting photo systems and enhancing tolerance by displaying the function of GmGMP1 gene or elevating AsA content to enhance antioxidant capacity.
2.Materials and methods
2.1.Plant materials, growth conditions and stress treatment
Soybean (Glycine max L.) cv.Bogao, which was used to isolate GmGMP1, was field-grown under standard agronomic practices at Nanjing Agricultural University.Tissue from roots, stems, leaves, and flowers were collected in the early stage of seed development.In full-bloom stage,flowers were marked, and then immature seeds at 15, 20,25, 30, 40, and 50 days after flowering (DAF) were collected.All materials were immediately frozen in liquid nitrogen and stored at -80°C.
For the experiment on the germination process of soybean cotyledons, seeds were grown in vermiculite at 28/25°C with a 16/8 h (light/dark) photoperiod in an artificial climate box, and cotyledons were collected at 1 to 12 d and then stored at -80°C for RNA extraction.
For controls and osmotic and salt treatments, 3-wk-old seedlings grown in an artificial climate box (as above)were saturated in water, 20% PEG, or 200 mmol L-1NaCl, respectively.For heat and cold stresses, 3-wk-old seedlings were saturated in water and then moved to 42 or 4°C, respectively, with a 16/8 h (light/dark) photoperiod in an artificial climate box.Leaves from all treatments above were harvested at 0, 1, 3, 6, 12, and 24 h, then stored at-80°C for RNA extraction.
Arabidopsis plants used in this study were grown in a greenhouse at 22/20°C with a 16/8 h (light/dark) photoperiod.
2.2.lsolation and sequence analysis of GmGMP1
Semi-quantitative RT-PCR assayTotal RNA was extracted using RNA Prep Pure Plant Kit (Tiangen, Beijing) and isolated RNA was subjected to reverse transcription using the ReverTra Ace®qPCR RT Kit (Toyobo, Japan).The expression levels of GmGMP1 in roots, stems, mature leaves, young leaves, flowers, and immature green bean were determined through semi-quantitative RT-PCR assays(YGGMP1-F 5´-GAGCACAGGGCAGGTTGATA-3´ and YGGMP1-R 5´-GAGCCACTGGCCAACTTAGAA-3´).Genespecific primers were used and soybean actin gene used as a reference gene (actin-F 5´-GTTCTCTCCTTGTATGCA AGTG-3´ and actin-R 5´-CCAGACTCATCATATTCACC TTTAG-3´).PCR products were run on 1% agarose gel and analyzed on a JS-380A Automatic Gel Image Analysis System (Peiqing Science & Technology Co., Ltd., Shanghai,China).
Quantitative real-time PCR analysisQuantitative real-time PCR was performed using the iQ SYBR Green Supermix (Bio-Rad, Singapore) on a Bio-Rad iQ5 Real-Time PCR System.The specificity of the reactions was verified by melting curve analysis.The relative mRNA level for GmGMP1(YGGMP1-F 5´-GAGCACAGGGCAGGTTGATA-3´ and YGGMP1-R 5´-GAGCCACTGGCCAACTTAGAA-3´) gene was calculated as ΔΔCTvalues (Livak and Schmittgen 2001; Schmittgen and Livak 2008) and soybean tubulin genes (tubulin-F 5´-GGAGTTCACAGAGGCAGAG-3´ and tubulin-R 5´-CACTTACGCATCACATAGCA-3´) were used as the internal control for normalization.
Construction of GmGMP1 plant expression vector and plant transformationThe open reading frame(ORF) of GmGMP1 was amplified by PCR and cloned into the pBI121 expression vector.Primers U1 and L1 (U1 5´-GCTCTAGACACATCACAATGAAGGCATTG-3´ and L1 5´-CGGGATCCTGACCTCACTCACATGACAAT-3´)which were designed with a BamHI cloning site at the 5´-end and a XbaI cloning site at the 3´-end Arabidopsis plants were transformed by the floral dip method (Clough and Bent 1998) using Agrobacterium tumefaciens strain EHA105.T3or T4homozygous transgenic lines were used for further study.Soybean plants were transformed by the cotyledonary node method using A.tumefaciens strain EHA105 (Olhoft 2003).PCR analysis of T1plants using GmGMP1 gene-specific primer(5´-ATTGCTGGCCTGAGGCTTTA-3´) and green fluorescent protein (GFP) primer (5´-GCCGTTCTTTTGCTTGTCG-3´).AsA content assay AsA content was determined according to Foyer et al.(1983) with some modifications.Leaves were homogenized with 6% metaphosphoric acid and moved to an ice bath for 30 min.The crude extract was centrifuged at 4°C for 15 min at 13 400×g and 0.75 mL of supernatant was added with 0.5 mL of 10% sodium citrate for neutralization.For every 0.9 mL neutralizer, 0.1 mL of potassium phosphate buffer (pH 5.6) was added.Two samples were prepared as above, one with 30 mmol L-1dithiothreitol (DTT) added and the other with ascorbate oxidase added (AO, Sigma, USA).For the final extract, 150 μL was removed for absorbance measurement at 265 nm in a TECAN M200 Microplate reader (Tecan, Mannedorf, Switzerland).A standard curve was drawn with AsA (Sinopharm Chemical Reagent Co.,Ltd., Shanghai, China).
Malondialdehyde content (MDA)MDA content was measured using the thiobarbituric acid method (Schmedes and Hølmer 1989; Hodges et al.1999) and partly according to GB/T.181-2003 (2003).Arabidopsis rosette leaves were homogenized with 10% trichloroacetic acid (TCA) and centrifuged at 12 000×g for 20 min.For every 0.8 mL of the extract, 2 mL of 0.5% thiobarbituric acid (TBA) containing 20% TCA was added.The mixture was boiled in a water bath for 15 min and then cooled.Of the final extract,150 μL was removed for absorbance measurement at 600,450 and 532 nm in a TECAN M200 Microplate reader.A standard curve was drawn with 1,1,3,3-tetraethoxypropane(Sigma).
Chlorophyll contentTotal chlorophyll was extracted with 80% acetone.After filtering, absorbance of 150 μL of clear chlorophyll solution was measured at 663, 645, and 750 nm in a TECAN M200 Microplate reader.Total chlorophyll was estimated according to Porra et al.(1989).
Superoxide anion radical contentSuperoxide anion radical (O2-.) content was measured using the hydroxylamine oxidation method (Elstner and Heupel 1976).Leaves were homogenized with 2 mL of 50 mmol L-1phosphate buffer(pH 7.8) and centrifuged at 12 000×g for 10 min.Every 0.5 mL of supernatant was added 0.5 mL of 50 mmol L-1phosphate buffer (pH 7.8) and 1 mL of 10 mmol L-1hydroxylamine hydrochloride, and the solution was moved to a 25°C water bath for 30 min.After that, 1 mL of sulfanilic acid was added and thoroughly mixed.Finally, 1 mL of α-naphthylamine was added, the solution was moved to a 25°C water bath for 20 min and then 3 mL N-butanol was added to the extract and it was thoroughly mixed.Of the N-butanol extract,150 μL was removed for absorbance measurement at 530 nm.A standard curve was drawn with sodium nitrite.
2.3.Statistical analysis
Statistical analysis of the experimental data was conducted by analysis of variance with SPSS (Norusis 1990).
3.Results
3.1.Cloning and sequence analysis of GmGMP1, a GDP-D-mannose pyrophosphorylase identified from cDNA of soybean leaf
The primers U and L were designed on the basis of the splicing sequence using in silico cloning.The leaf cDNA was used as a template with the primers (KLGMP1-F 5´-CCTCAGCGAGACGCAAGTG-3´ and KLGMP1-R 5´-GGAAAACCAAATGAAACGGAC-3´) to amplify the sequence.This clone contained a full-length cDNA(1 469 bp) carrying the ORF with both parts of 5´- and 3´-UTR and was named GmGMP1 (accession number:FJ869891) (Fig.1).
The deduced protein sequence of GmGMP1 contained 361 amino acid residues with a calculated molecular mass of 39.63 kDa and a pI (predicted isoelectric point) of 7.11(using online software ExpASy).It appeared that GMP was highly conserved between widely different species.Amino acid alignments and phylogenetic analysis with multiple GMPs found in GenBank showed that GmGMP1 shared similarities with Medicago sativa (MsGMP, AAT58365) and Lycopersicon esculentum (AlGMP, AAT37498) (Fig.1-A).
The GmGMP1 protein sequence had >93.4% identity with the Lycopersicon esculentum GMP protein, and we speculated that they have a similar function.GmGMP1 showed up to 93.1% identity with MsGMP, and only 85.6%with OsGMP (Table 1).Similarly to other GMPs, it also contained an N-terminal catalytic domain and a C-terminal left-handed-beta-helix fold domain, presumably with four turns, each containing three imperfect tandem repeats of a hexapeptide repeat motif (X-[STAV]-X-[LIV]-[GAED]-X) that showed acyltransferase activity (Fig.1-B) (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi).

Fig.1 Phylogeneticanalysis and multiple sequence align ments of GDP-D-mannose pyrophosphorylase (GMP) protein.A, the phylogenetic relationship between GmGMP1 and some other plant GMPs.B, protein sequence alignment of OsGMP (Oryza sativa ssp.japonica AK121972), MsGMP (Medicago sativa AAT58365), AlGMP (Actinidia latifolia ACN38266), MgGMP (Malpighia glabra ABX80393), GmGMP1 (Glycine max FJ869891), StGMP (Solanum tuberosum AAD01737), PpGMP (Prunus persica BAH03298),LeGMP (Solanum lycopersicum AAT37498), NtGMP (Nicotiana tabacum BAB62108), AtGMP (Arabidopsis thaliana AAC78474),PtGMP (Pinus taeda ABR15468), and CpGMP (Carica papaya ACK87007).The black box indicates conserved regions characteristic of GMP proteins.Imperfect tandem repeats of a hexapeptide repeat motif’X-[STAV]-X-[LIV]-[GAED]-X’ (black rectangle drawn) of GmGMP1 were detected by scanning sequences at the website (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi).
3.2.Analysis of GmGMP1 gene expression in different tissues
We determined the expression levels of GmGMP1 in roots,stems, mature leaves, young leaves, flowers, and immature green bean at 25 days after flowering (DAF) from G.max cv.Bogao.The RT-PCR results clearly indicated that GmGMP1 was expressed in all tissues studied but with different transcript levels.The highest expression level of GmGMP1 was in mature leaves and there was also a high level in flowers (Fig.2-A).However, the AsA content did not coincide with the transcript levels of GmGMP1 in different tissues.Mature leaves had the highest content levels of AsA and the highest expression of GmGMP1.Flower showed higher expression of GmGMP1 than other tissues except matured leaves, but the second highest content levels ofAsA was not observed in flower (Fig.2-B).In the immature bean of soybean, GmGMP1 expression increased after flowering and reached its maximum at about 40 DAF and then decreased (Fig.3); however, there were no significant changes in AsA content (data not shown).

Table 1 Amino acid similarity (%) of GDP-D-mannose pyrophosphorylases (GMPs) from different plants1)
The cotyledons of soybean in the germination stage were analyzed to determine the relationship between GmGMP1 and AsA content.The qRT-PCR results clearly indicated that expression of GmGMP1 was significantly upregulated at 3 and 4 d, up to 11-fold, and maintained after 5 d.However, the AsA content of cotyledons increased with the germination process to 3 and 4 d, reaching a maximum of 544% compared with 1 d, and then decreased and was maintained at about 10 mg/100 g (Fig.4-A and B).
3.3.Quantitative RT-PCR analysis revealed that GmGMP1 was induced by abiotic stresses and light
Expression of GmGMP1 was significantly induced by heat, cold, and osmotic stresses (Fig.5).To examine the effect of abiotic stress on the expression level of GmGMP1 mRNA, we performed quantitative RT-PCR analysis of total RNA prepared from leaves of plants subjected to NaCl,cold, osmotic, and heat stresses respectively for 1, 3, 6,12, and 24 h.In particular, expression was significantly elevated in response to heat: After 1 h of heat treatment,GmGMP1 expression reached a maximum above control values and then decreased by 3 h.Similar results were obtained in response to cold and osmotic stresses.Heat,cold, and osmotic stresses induced a similar increase in GmGMP1 expression after 1 h of treatment.However, in NaCl treatment, GmGMP1 expression was different: the first peak appeared 3 h after treatment and then decreased to the control level at 6 h; the second peak appeared 24 h after treatment.In addition, compared with LD (light dark),the expression level of GmGMP1 in soybean cotyledon(5 d after sowing) was 23.3 to 29.3% in DD (constant dark).These results indicated that GmGMP1 was involved in the response to abiotic stresses and light, and with the exception of NaCl stress, the responses to abiotic stresses were rapid.

Fig.2 GmGMP1 relative expression level (A) and L-ascorbic acid (AsA) content (B) in various tissues.R, root; S, stem;ML, mature leaves; YL, young leaves; F, flower; B, immature green bean at 25 days after flowering (DAF).Data represent mean±SD of three technical replicates.

Fig.3 Expression analysis of GmGMP1 in immature green bean at different developmental stages by semi-quantitative RTPCR.Seeds at 15, 20, 25, 30, 40, and 50 days after flowering(DAF), respectively.

Fig.4 GmGMP1 relative expression level (A) and L-ascorbic acid (AsA, B) content during the germination process of cotyledons.Data represent mean±SD of three technical replicates.
3.4.GmGMP1 overexpression enhanced AsA content of Arabidopsis and conferring tolerance to osmotic and high salt stresses during seed germination
In order to improve our understanding about the GmGMP1 gene, we investigated whether Arabidopsis overexpressing GmGMP1, coding for the enzyme synthesizing GDP-mannose pyrophosphorylase, driven by CaMV 35S promoter showed any increase in AsA levels.The AsA contents of four transgenic Arabidopsis lines overexpressing GmGMP1 were significantly higher than those of the vector control(Fig.6).
The increase in the AsA content in GmGMP1 overexpressing transgenic lines may be partly due to the GmGMP1 mRNA transformation effect.We selected three lines for further study to distinguish the phenotypic differences observed in the vector control and transgenic lines.
Previous studies have shown that expression of exogenous genes can affect the normal growth of plants(Wang et al.2012).We basically characterized whole plants of these transgenic lines.There were four aspects of striking phenotypic changes in the transgenic plants.Both the first flowering and first cracking dates after flowering in transgenic lines were significantly earlier than for controls(Fig.7).Interestingly, the rosette leaves were thicker and greener in transgenic lines than in controls.
There was no difference in the number of rosette leaves of early-flowering phenotype of the overexpressing transgenic lines compared with the vector control when grown under long days (16/8 h (light/dark), data not shown).However,flower buds appeared earlier in transgenic lines than in vector control and similarly for first cracking after flowering.There were no differences in the main stem height, the number of stems, number of branches main stem, silique length, and rosette leaf number at the onset of flowering(data not shown).
Low levels of antioxidants promote the formation of(Gao and Zhang 2008) and chlorophyll content is greatly influenced by oxidative stress (Veljovic-Jovanovic et al.2001).Thus we decided to survey, MDA and chlorophyll contents of transgenic lines.Theand MDA levels in transgenic lines were significantly decreased and chlorophyll content obviously increased under untreated circumstances compared with controls (Fig.8-A and B).
Fig.8-C also shows the changes in chlorophyll content on the basis of rosette leaf area at the first flowering date,and the ratio of contents of chlorophyll a to b in the control and transgenic lines under normal growth conditions.Under normal conditions, chlorophyll contents were significantly higher in transgenic lines than in control (P<0.01).Interestingly, chlorophyll a and b contents increased by 39-85% in the transgenic lines compared with controls,but the ratio of chlorophyll a to b did not significantly differ between transgenic lines and controls.

Fig.5 Expression pattern analyses of GmGMP1 in Glycine max under different abiotic stresses (heat, 42°C, A; osmotic stress,PEG 20% w/w, B; cold, 4°C, C; NaCl, 150 mmol L-1, D).The values were normalized against tubulin expression.Data represent mean±SD of three technical replicates.

Fig.6 The L-ascorbic acid (AsA) content (A) and GmGMP1 relative expression level (B) in control and transgenic (At-line-1, 3,and 4) Arabidopsis rosette leaves.Values calculated as mean±SD, n=4.**, significant at P<0.01.
Because the expression level of GmGMP1 was induced by abiotic stress, we speculated that GmGMP1 overexpressing transgenic lines were conferred tolerance to abiotic stresses.This study used GmGMP1 overexpressing transgenic lines to analyze the impact of overexpression on the germination capacity under abiotic stress.

Fig.7 First flowering date (A) and first cracking days after flowering (B) of control plants and GmGMP1 transgenic lines (At-line-1,3, and 4).Values calculated as mean±SD, n=15.**, significant at P<0.01.
After we verified the overexpressing transgenic lines, we investigated whether there was any phenotypic change in the transgenic lines.We compared seed germination with controls under abiotic stress.When treated with water, all seed germination rates were about 98.0% and there were no differences at normal temperature (22/20°C, light/dark).Three transgenic lines (At-line-1, 3 and 4) were used in the experiment, and there were no significant differences between the transgenic and control plants for heat and cold stresses (data not shown).Interestingly, the three transgenic lines showed significantly higher tolerance to salt than controls, as determined by 150 mmol L-1NaCl treatment from 3 to 11 d (Fig.9-A).The most obvious difference was at 5 d, when average germination rates were 39.89,40.36, and 21.75% in the transgenic lines but only 15.4%in the control (Fig.9-A).There were only slight differences between At-line-4 and control before 10 d and there were no significant differences were found after 11 d.After 15 d,the average germination rates were 39.06, 55.67, 68.65, and 38.9% for control, At-line-1, 3, and 4, respectively.Under osmotic stress, the transgenic lines had significantly higher tolerance than control (Fig.9-B), although the At-line-4 was close to the control value.The results suggest that the seeds of transgenic lines were more tolerant of salt and osmotic stress than control, and that overexpression of the GmGMP1 gene resulted in increased tolerance of transgenic Arabidopsis seeds to salt and osmotic stress.
3.5.GmGMP1 overexpression enhanced AsA content of soybean
Soybean overexpressing GmGMP1 was generated by Agrobacterium-mediated transformation using the cotyledonary node method (Olhoft et al.2003) and the transgenic lines were checked by PCR in three successive generations.T3plants of two independent transgenic lines(Gm-line-184 and Gm-line-196) were selected for qRTPCR (Fig.10-A).The qRT-PCR analysis revealed higher expression levels in the leaves of two transgenic lines compare to wild type (Fig.10-A).
4.Discussion
The GMP gene, encoding GDP-D-mannose pyrophosphorylase, plays an important role in the AsA biosynthetic pathway in plants, and down-regulated expression level can lead to decreased AsA content (Conklin et al.1996,1999, 2000; Wheeler et al.1998; Keller et al.1999; Tabata et al.2001; Veljovic-Jovanovic et al.2001; Yabuta et al.2007; Colville and Smirnoff 2008; Barth et al.2010).In contrast, overexpression of the GMP gene can increase AsA content (Badejo et al.2007, 2008; Wang et al.2011).In the present study, the cloning and characterization of a full-length cDNA encoding soybean GMP were described.The GmGMP1, one of the GMP in soybean whose deduced amino acid sequence had 85-93% homology with other known GMPs, and also contains an N-terminal catalytic domain, a C-terminal left-handed-beta-helix fold domain and a hexapeptide repeat motif (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi), shows that plant GMP proteins are highly conserved and may play a similar role to other GMPs(Vuorio et al.1994; Badejo et al.2007; Wang et al.2011).
The GmGMP1 protein sequence had >93.4% identity to the tomato (L.esculentum) GMP protein, and the gene is constitutively expressed in roots, stems, leaves, flowers,and fruits of tomato (Zou et al.2006).Like other GMP genes, the RT-PCR results clearly indicated expression of GmGMP1 in all tissues (Zou et al.2006; Badejo et al.2007;Wang et al.2011), but with different transcript levels.The same to tomato GMP gene (Wang et al.2011), the highest expression levels of GmGMP1 was found in mature leaves(Fig.2-A), no in immature green fruits (Zou et al.2006;Badejo et al.2007); correspondingly, the highest AsA content was also observed in mature leaves.Interestingly,flowers which had higher expression levels of GmGMP1 than other tissues also contained lower AsA contents similar to that of stems (Fig.2-B).A similar phenomenon was foundin the immature bean of soybean-up-regulated expression of GmGMP1 did not enhance the AsA content (Fig.3).Due to the rapid cell expansion and division in flowers and immature bean of soybean, we speculate that the main function of the higher GmGMP1 expression in these areas was not AsA synthesis but cell expansion and division.Other similar reports suggested that the GMP impacted on cell expansion and division immediately or indirectly via AsA (Smirnoff and Pallanca 1996; Nickle and Meinke 1998;Tabata et al.2001; Veljovic-Jovanovic et al.2001; Li et al.2010).We conclude that the GmGMP1 gene was not only connected with AsA content but also may play a vital role in cell expansion and division, which was consistent with other researches showing that higher expression of GMPase for 3-4 d may be required to provide cell wall sugars (Tabata et al.2002).As storage organs, cotyledons have steady rates of cell division in the germination compared to the seed formation stage.The qRT-PCR results clearly showed significant up-regulation of GmGMP1 during the germination process of cotyledons, with higher levels of transcripts than the 1 d and had the same trend as the AsA content (Fig.4-A and B).The same to other GMP genes (Badejo et al.2007;Wang et al.2011), the work described in the present paper suggests that increased AsA content is a determining feature of the properties associated with high GmGMP1 expression in soybean.


Fig.9 Seed germination rates of GmGMP1 overexpressing Arabidopsis plants under different stresses (NaCl, A; osmotic,B).Seeds from control or homozygous transgenic plants (Atline-1, 3, and 4) were sown on quartz sand and filter paper saturated with 150 mmol L-1 NaCl or 10% PEG.Error bars indicate SD, n=4.
AsA is an abundant low molecular weight, nonenzymatic antioxidant that protects plants against abiotic stresses(Foyer et al.2001; Lu et al.2009; Wang et al.2012; Li et al.2013), and studies have shown that salt stress (Shalata and Neumann 2001; Huang et al.2005; Zhang et al.2012),wounding (Badejo et al.2009; Ioannidi et al.2009), light(Tabata et al.2002; Yabuta et al.2007), drought (Yang et al.2004), and heat acclimation (Dat et al.1998) can induce AsA synthesis in plants.The GMP gene, playing a crucial role in the synthesis of AsA, may be induced by light (Tabata et al.2002), darkness (Badejo et al.2009),wounding (Badejo et al.2009), AsA content (Badejo et al.2009), and other genes (Zhang et al.2012).However,little is known about induction of genes by abiotic stress.In the present study, quantitative RT-PCR revealed that GmGMP1 in soybean leaves was induced by heat, cold,and osmotic stresses.After 1 h of heat, cold, or osmotic stress, GmGMP1 expression was up-regulated and was significantly higher than in controls - reaching a maximum at 1 h after treatment and then decreasing, following the same trend as AsA content (unpublished data).This pattern is similar to that reported concerning induction by darkness or wounding (Badejo et al.2009) and we reason that GmGMP1 of soybean was directly induced by heat, cold and osmotic stresses.However, the most notable difference was that GmGMP1 expression differed under NaCl treatment.The first peak appeared not 1 h but 3 h after treatment and then decreased to control levels at 6 h; the second peak appeared 24 h after treatment.What are the possible mechanisms? The first is that GmGMP1 was immediately induced by heat, cold, and osmotic stresses in soybean leaves, but not by salt stress.The second is that another gene, which was induced by salt stress, up-regulated GmGMP1 expression (Zhang et al.2012).We predicted the 2 888-bp GmGMP1 promoter sequence upstream of the translation start codon ATG (http://www.phytozome.com/genePage.php?crown&er=1&method=3251&search=1&searchText=clusterid%3A34526824&detail=1), and analyzed the promoter sequence using PLANTCARE(http://bioinformatics.psb.ugent.be/webtools/plantcare/html/).We found that the GmGMP1 promoter contained HSE core sequences (cis-acting element involved in heat stress responsiveness), LTR core sequences (cis-acting element involved in low-temperature responsiveness),and MBS core sequences (MYB binding site involved in drought-inducibility), but no sequences concerning salt responsiveness (Appendix A).Thus the expression of GmGMP1 might be induced indirectly, not directly, by NaCl treatment.These findings, including the present data,suggest that the GmGMP1 was directly and indirectly induced by abiotic stresses in soybeans.In addition,GmGMP1 may be induced by light, as also found in other reports (Tabata et al.2002; Yabuta et al.2007).
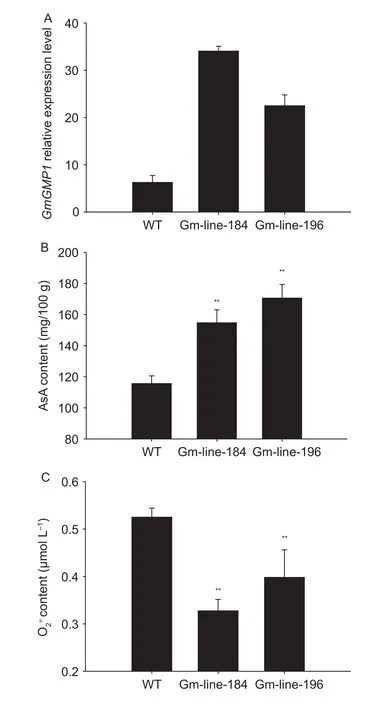
Fig.10 Relative GmGMP1 mRNA expression (A), L-ascorbic (AsA) content (B), and content (C) in transgenic and wild type control soybean.Values calculated as mean±SD, n=4.**, significant at P<0.01.
As reported here, the functional GMPase which generates GDP-mannose for AsA biosynthesis (Conklin et al.1996, 1999, 2000; Wheeler et al.1998; Keller et al.1999; Tabata et al.2001; Veljovic-Jovanovic et al.2001;Yabuta et al.2007; Colville and Smirnoff 2008; Barth et al.2010) and protein N-glycosylation (Conklin et al.1996, 1999;Hashimoto et al.1997; Yoda et al.2000; Warit et al.2000;Agaphonov et al.2001; Lukowitz et al.2001; Qin et al.2008;Barth et al.2010; Kempinski et al.2011) is important for high NH4+tolerance (Qin et al.2008; Barth et al.2010; Kempinski et al.2011), salt tolerance (Koiwa et al.2003; Huang et al.2005), ozone tolerance (Conklin et al.1996; Gao and Zhang 2008; Barth et al.2010), cell wall synthesis (Conklin et al.1996, 1999; Nickle and Meinke 1998; Ning and Elbein 1999; Tomlin et al.2000; Agaphonov et al.2001; Lukowitz et al.2001; Jiang et al.2008; Nairn et al.2008; Kempinski et al.2011), and cell expansion and division (Smirnoff and Pallanca 1996; Nickle and Meinke 1998; Tabata et al.2001;Veljovic-Jovanovic et al.2001; Li et al.2010).Differing to the study of mutants, the GMP gene overexpression has been little studied and major studies have concentrated on enhanced AsA biosynthesis (Badejo et al.2007, 2008;Wang et al.2011) and tolerance to temperature stress by enhancing antioxidation capacity (Wang et al.2011, 2012).We assessed the function of GmGMP1 in Arabidopsis and soybean plants through an overexpressing approach,and three lines of Arabidopsis and two lines of soybean were used.The same to others (Badejo et al.2007, 2008;Wang et al.2011), AsA content was enhanced along with up-regulation of GmGMP1 expression; however, the Atline-3 which had the highest expression level of GmGMP1 showed no higher AsA content than the other lines (Fig.6).There were similar results for the overexpressing transgenic soybean lines (Fig.10).We predicted that GmGMP1 was overexpressed in soybean and Arabidopsis in an attempt to overcome rate-limiting steps in production, but the effect was limited at enhanced AsA biosynthesis.While GMPase may be essential for the L-galactose pathway (Conklin et al.1996; Wheeler et al.1998), this study suggests that increased GMP supply from early steps of the L-galactose pathway improved AsA production in a limited range and that superabundance was useless for AsA biosynthesis.
AsA may protect plant cells from ROS-mediated damage by reducing the accumulation of ROS (such as, singlet oxygen, ozone and hydrogen peroxide) during abiotic stress(Conklin et al.1996, 1997; Noctor and Foyer 1998; Smirnoff 2000; Sanmartin et al.2003; Mahajan and Tuteja 2005; Zou et al.2006; Yang et al.2007; Gao and Zhang 2008; Barth et al.2010).MDA formation is relevant to the damage of plant oxide stress (Del Rio et al.2005).
Under normal conditions, the growth of transgenic plants compared to control showed no differences in main stem height, number of stems, number of branches per main stem, silique length, and rosette leaf number at the onset of flowering (data not shown).Interestingly, the first flowering days and the first cracking days after flowering were significantly earlier than in controls (Fig.7).Other recent publications had reported contrasting results in that early flowering was observed in an AsA-deficient vtc1/cyt1 mutant and down-regulation of GMPase gene expression in plants, and late flowering in plants with artificially elevated AsA levels (Pastori et al.2003; Conklin and Barth 2004;Kotchoni et al.2009).However, there are also reports that the flowering time in the vtc1 mutant was later than in the wild type (Veljovic-Jovanovic et al.2001; Pavet et al.2005).There are few reports concerning flowering of overexpressing GMP gene transgenic lines.In the present study, we reported an interesting phenomenon in overexpressing GmGMP1 transgenic lines in Arabidopsis, that early flowering and early-cracking, but rosette leaf number at the onset of flowering was no different.It is well established that lateflowering plants form more leaves (Koornneef et al.1991),so the present suggests indicated that increased GmGMP1 may be causing fast growth.Relatively, overexpressing GmGMP2 transgenic lines of Arabidopsis also had enhanced AsA content and showed similar rosette leaf numbers at the onset of flowering and similar flowering and cracking times to controls (unpublished data).Therefore, it remains possible that the fast growth in transgenic lines plants was attributable not to the AsA content but to GmGMP1.Promotion of cell division may be a major reason (Smirnoff and Pallanca 1996; Nickle and Meinke 1998; Tabata et al.2001; Veljovic-Jovanovic et al.2001; Li et al.2010).
Previous studies suggested that the GMP gene contacted with the tolerance of abiotic by regulating AsA content (Shalata and Neumann 2001; Pastori et al.2003;Conklin and Barth 2004; Huang et al.2005; Zhang et al.2012), and overexpressing the GMP gene could increase tolerance to heat and cold stresses (Wang et al.2011,2012).In the present study, germination rate of seeds from overexpressing Arabidopsis plants under different stresses showed that GmGMP1 overexpression did not significantly enhance tolerance to heat and cold stresses (data not shown), but tolerance to high salt and osmotic stresses increased dramatically (Fig.9).Increased tolerance to osmotic stress was also found in overexpressing GmGMP2 transgenic lines in Arabidopsis (unpublished data), we presumed that the reason of increased tolerance was due to the increased in AsA content.Huang et al.(2005)reported that AsA deficiency in the vtc-1 mutant seemed to be the main reason for the sensitivity of the vtc-1 mutant to salt stress, but did not pay attention to the function of the GMP gene.Deficient N-glycosylation, a phenomenon independent of AsA deficiency in the vtc-1 mutant, may affect its cellulose biosynthesis, cell wall stability, cell viability, and tolerance of NH4+(Veljovic-Jovanovic et al.2001; Qin et al.2008; Barth et al.2010; Kempinski et al.2011).In other gene mutants, this could lead to temperature sensitivity (Hoeberichts et al.2008), salt sensitivity (Koiwa et al.2003; Kang et al.2008), and depletion of cells(Uccelletti et al.2005).Overexpression of GDP-mannose pyrophosphorylase in Saccharomyces cerevisiae corrects defects in dolichol-linked saccharide formation and protein glycosylation (Janik et al.2003).Overexpression of GmGMP2 in Arabidopsis, which increased the AsA content,did not enhance tolerance to salt stress (unpublished data)but overexpressing two glycosyltransferase genes enhanced tolerance to salt (Hu 2010).Therefore, we speculate that the GmGMP1 in the transgenic lines seemed to be the main reason for the tolerance of salt stress, possibly, the GmGMP1 activity played a unique role in the germinated stage, and the different response to other abiotic stresses may be related to an indirect response to salt stress (Fig.5).
5.Conclusion
Our results show that the GmGMP1 gene had an ORF of 1 083 bp encoding a polypeptide of 361 amino acids and was induced by abiotic stress in soybean.The overexpression of GmGMP1 in Arabidopsis and soybean can increase AsA content and reducecontent.The overexpression of GmGMP1 in Arabidopsis reduced damage from oxidative stress, increased tolerance to osmotic and high salt stresses during the seed germination and earlier flowering.
Acknowledgements
This work was supported by the Genetically Modified Organisms Breeding Major Projects, China (2016ZX08004),the earmarked fund for China Agriculture Research System(CARS-004-PS10), and the Program for Changjiang Scholars and Innovative Research Team in University, China(PCSIRT13073).
Appendixassociated with this paper can be available on http://www.ChinaAgriSci.com/V2/En/appendix.htm
Agaphonov M O, Packeiser A N, Chechenova M B, Choi E S, Ter-Avanesyan M D.2001.Mutation of the homologue of GDP-mannose pyrophosphorylase alters cell wall structure, protein glycosylation and secretion in Hansenula polymorpha.Yeast, 18, 391-402.
Agius F, González-Lamothe R, Caballero J L, Muñoz-Blanco J, Botella M A, Valpuesta V.2003.Engineering increased vitamin C levels in plants by overexpression of a D-galacturonic acid reductase.Nature Biotechnology,21, 177-181.
Badejo A A, Fujikawa Y, Esaka M.2009.Gene expression of ascorbic acid biosynthesis related enzymes of the Smirnoff-Wheeler pathway in acerola (Malpighia glabra).Journal of Plant Physiology, 166, 652-660.
Badejo A A, Jeong S T, Goto-Yamamoto N, Esaka M.2007.Cloning and expression of GDP-D-mannose pyrophosphorylase gene and ascorbic acid content of acerola (Malpighia glabra L.) fruit at ripening stages.Plant Physiology and Biochemistry, 45, 665-672.
Badejo A A, Tanaka N, Esaka M.2008.Analysis of GDP-D-mannose pyrophosphorylase gene promoter from acerola(Malpighia glabra) and increase in ascorbate content of transgenic tobacco expressing the acerola gene.Plant and Cell Physiology, 49, 126-132.
Barth C, Gouzd Z A, Steele H P, Imperio R M.2010.A mutation in GDP-mannose pyrophosphorylase causes conditional hypersensitivity to ammonium, resulting in Arabidopsis root growth inhibition, altered ammonium metabolism, and hormone homeostasis.Journal of Experimental Botany,61, 379-394.
Chen Z, Gallie D R.2005.Increasing tolerance to ozone by elevating foliar ascorbic acid confers greater protection against ozone than increasing avoidance.Plant Physiology,138, 1673-1689.
Clough S J, Bent A F.1998.Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana.The Plant Journal, 16, 735-743.
Colville L, Smirnoff N.2008.Antioxidant status, peroxidase activity, and PR protein transcript levels in ascorbatedeficient Arabidopsis thaliana vtc mutants.Journal of Experimental Botany, 59, 3857-3868.
Conklin P L.2001.Recent advances in the role and biosynthesis of ascorbic acid in plants.Plant, Cell & Environment, 24,383-394.
Conklin P L, Barth C.2004.Ascorbic acid, a familiar small molecule intertwined in the response of plants to ozone,pathogens, and the onset of senescence.Plant, Cell &Environment, 27, 959-970.
Conklin P L, Norris S R, Wheeler G L, Williams E H, Smirnoff N, Last R L.1999.Genetic evidence for the role of GDP-mannose in plant ascorbic acid (vitamin C) biosynthesis.Proceedings of the National Academy of Sciences of the United States of America, 96, 4198-4203.
Conklin P L, Pallanca J E, Last R L, Smirnoff N.1997.L-ascorbic acid metabolism in the ascorbate-deficient Arabidopsis mutant vtc1.Plant Physiology, 115, 1277-1285.
Conklin P L, Saracco S A, Norris S R, Last R L.2000.Identification of ascorbic acid-deficient Arabidopsis thaliana mutants.Genetics, 154, 847-856.
Conklin P L, Williams E H, Last R L.1996.Environmental stress sensitivity of an ascorbic acid-deficient Arabidopsis mutant.Proceedings of the National Academy of Sciences of the United States of America, 93, 9970-9974.
Córdoba F, Gonzalez-Reyes J A.1994.Ascorbate and plant cell growth.Journal of Bioenergetics and Biomembranes,26, 399-405.
Dat J F, Foyer C H, Scott I M.1998.Changes in salicylic acid and antioxidants during induced thermotolerance in mustard seedlings.Plant Physiology, 118, 1455-1461.
Davey M W, Montagu M, Inzé D, Sanmartin M, Kanellis A,Smirnoff N, Benzie I J J, Strain J J, Favell D, Fletcher J.2000.Plant L-ascorbic acid: chemistry, function, metabolism,bioavailability and effects of processing.Journal of the Science of Food and Agriculture, 80, 825-860.
Elstner E F, Heupel A.1976.Inhibition of nitrite formation from hydroxylammoniumchloride: A simple assay for superoxide dismutase.Analytical Biochemistry, 70, 616.
Eltayeb A E, Kawano N, Badawi G H, Kaminaka H, Sanekata T,Shibahara T, Inanaga S, Tanaka K.2007.Overexpression of monodehydroascorbate reductase in transgenic tobacco confers enhanced tolerance to ozone, salt and polyethylene glycol stresses.Planta, 225, 1255-1264.
Foyer C, Rowell J, Walker D.1983.Measurement of the ascorbate content of spinach leaf protoplasts and chloroplasts during illumination.Planta, 157, 239-244.
Foyer C H, Theodoulou F L, Delrot S.2001.The functions of inter- and intracellular glutathione transport systems in plants.Trends in Plant Science, 6, 486-492.
Gao Q, Zhang L.2008.Ultraviolet-B-induced oxidative stress and antioxidant defense system responses in ascorbatedeficient vtc1 mutants of Arabidopsis thaliana.Journal of Plant Physiology, 165, 138-148.
GB/T.181-2003.2003.Determination of Propyldialdehyde in Lard.Ministry of Health of the People’s Republic of China.(in Chinese)
Hashimoto H, Sakakibara A, Yamasaki M, Yoda K.1997.Saccharomyces cerevisiae VIG9 encodes GDP-mannose pyrophosphorylase, which is essential for protein glycosylation.Journal of Biological Chemistry, 272, 16308-16314.
Hodges D M, DeLong J M, Forney C F, Prange R K.1999.Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds.Planta, 207,604-611.
Hoeberichts F A, Vaeck E, Kiddle G, Coppens E, Cotte B,Adamantidis A, Ormenese S, Foyer C H, Zabeau M, Inze D, Perilleux C, Breusegem F V, Vuylsteke M.2008.A temperature-sensitive mutation in the Arabidopsis thaliana phosphomannomutase gene disrupts protein glycosylation and triggers cell death.Journal of Biological Chemistry,283, 5708-5718.
Huang C, He W, Guo J, Chang X, Su P, Zhang L.2005.Increased sensitivity to salt stress in an ascorbate-deficient Arabidopsis mutant.Journal of Experimental Botany, 56,3041-3049.
Hu H Q.2010.Identification of Arabidopsis glucosyltransferase genes involved in plant salt tolerance.Ph D thesis,Shandong University, China.(in Chinese)
Ioannidi E, Kalamaki M S, Engineer C, Pateraki I, Alexandrou D,Mellidou I, Giovannonni J, Kanellis A K.2009.Expression profiling of ascorbic acid-related genes during tomato fruit development and ripening and in response to stress conditions.Journal of Experimental Botany, 60, 663-678.Janik A, Sosnowska M, Kruszewska J, Krotkiewski H, Lehle L,Palamarczyk G.2003.Overexpression of GDP-mannose pyrophosphorylase in Saccharomyces cerevisiae corrects defects in dolichol-linked saccharide formation and protein glycosylation.Biochimica et Biophysica Acta (General Subjects), 1621, 22-30.
Jiang H, Ouyang H, Zhou H, Jin C.2008.GDP-mannose pyrophosphorylase is essential for cell wall integrity,morphogenesis and viability of Aspergillus fumigatus.Microbiology, 154, 2730-2739.
Kang J S, Frank J, Kang C H, Kajiura H, Vikram M, Ueda A, Kim
S, Bahk J D, Triplett B, Fujiyama K, Lee S Y, von Schaewen A, Koiwa H.2008.Salt tolerance of Arabidopsis thaliana requires maturation of N-glycosylated proteins in the Golgi apparatus.Proceedings of the National Academy of Sciences of the United States of America, 105, 5933-5938.
Keller R, Renz F S, Kossmann J.1999.Antisense inhibition of the GDP-mannose pyrophosphorylase reduces the ascorbate content in transgenic plants leading to developmental changes during senescence.The Plant Journal, 19, 131-141.
Kempinski C F, Haffar R, Barth C.2011.Toward the mechanism of NH4
+sensitivity mediated by Arabidopsis GDP-mannose pyrophosphorylase.Plant Cell Environment, 34, 847-858.
Kerk N M, Feldman N J.1995.A biochemical model for the initiation and maintenance of the quiescent center: Implications for organization of root meristems.Development, 121, 2825-2833.
Koiwa H, Li F, McCully M G, Mendoza I, Koizumi N, Manabe Y, Nakagawa Y, Zhu J, Rus A, Pardo J M.2003.The STT3a subunit isoform of the Arabidopsis oligosaccharyl transferase controls adaptive responses to salt/osmotic stress.The Plant Cell, 15, 2273-2284.
Koornneef M, Hanhart C J, Veen J H.1991.A genetic and physiological analysis of late flowering mutants in Arabidopsis thaliana.Molecular and General Genetics,229, 57-66.
Kotchoni S O, Larrimore K E, Mukherjee M, Kempinski C F,Barth C.2009.Alterations in the endogenous ascorbic acid content affect flowering time in Arabidopsis.Plant Physiology, 149, 803-815.
Li J, Li M, Liang D, Cui M, Ma F.2013.Expression patterns and promoter characteristics of the gene encoding Actinidia deliciosa L-galactose-1-phosphate phosphatase involved in the response to light and abiotic stresses.Molecular Biology Reports, 40, 1473-1485.
Li Q, Li B H, Kronzucker H J, Shi W M.2010.Root growth inhibition by NH4+in Arabidopsis is mediated by the root tip and is linked to NH4+efflux and GMPase activity.Plant Cell Environment, 33, 1529-1542.
Livak K J, Schmittgen T D.2001.Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCTmethod.Methods, 25, 402-408.
Lorence A.2004.Myo-inositol oxygenase offers a possible entry point into plant ascorbate biosynthesis.Plant Physiology,134, 1200-1205.
Lu H, Han R L, Jiang X N.2009.Heterologous expression and characterization of a proxidomal ascorbate peroxidase from Populus tomentosa.Molecular Biology Reports, 36, 21-27.
Lukowitz W, Nickle T C, Meinke D W, Last R L, Conklin P L, Somerville C R.2001.Arabidopsis cyt1 mutants are deficient in a mannose-1-phosphate guanylyltransferase and point to a requirement of N-linked glycosylation for cellulose biosynthesis.Proceedings of the National Academy of Sciences of the United States of America, 98,2262-2267.
Mahajan S, Tuteja N.2005.Cold, salinity and drought stresses:An overview.Archives of Biochemistry and Biophysics,444, 139-158.
Nairn C J, Lennon D M, Wood-Jones A, Nairn A V, Dean J F.2008.Carbohydrate-related genes and cell wall biosynthesis in vascular tissues of loblolly pine (Pinus taeda).Tree Physiology, 28, 1099-1110.
Nickle T C, Meinke D W.1998.A cytokinesis-defective mutant of Arabidopsis (cyt1) characterized by embryonic lethality,incomplete cell walls, and excessive callose accumulation.The Plant Journal, 15, 321-332.
Ning B, Elbein A D.1999.Purification and properties of mycobacterial GDP-mannose pyrophosphorylase.Archives of Biochemistry and Biophysics, 362, 339-345.
Noctor G, Foyer C H.1998.Ascorbate and glutathione: Keeping active oxygen under control.Annual Review of Plant Biology, 49, 249-279.
Norusis M J.1990.SPSS Advanced Statistics User’s Guide.SPSS, Chicago.
Olhoft P M, Flagel L E, Donovan C M, Somers D A.2003.Efficient soybean transformation using hygromycin B selection in the cotyledonary-node method.Planta, 216,723-735.
Olmos E, Kiddle G, Pellny T, Kumar S, Foyer C H.2006.Modulation of plant morphology, root architecture, and cell structure by low vitamin C in Arabidopsis thaliana.Journal of Experimental Botany, 57, 1645-1655.
Pastori G M, Kiddle G, Antoniw J, Bernard S, Veljovic-Jovanovic S, Verrier P J, Noctor G, Foyer C H.2003.Leaf vitamin C contents modulate plant defense transcripts and regulate genes that control development through hormone signaling.The Plant Cell, 15, 939-951.
Pavet V, Olmos E, Kiddle G, Mowla S, Kumar S, Antoniw J,Alvarez M E, Foyer C H.2005.Ascorbic acid deficiency activates cell death and disease resistance responses in Arabidopsis.Plant Physiology, 139, 1291-1303.
Porra R J, Thompson W A, Kriedemann P E.1989.Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy.Biochimica et Biophysica Acta (Bioenergetics), 975,384-394.
Qin C, Qian W, Wang W, Wu Y, Yu C, Jiang X, Wang D, Wu P.2008.GDP-mannose pyrophosphorylase is a genetic determinant of ammonium sensitivity in Arabidopsis thaliana.Proceedings of the National Academy of Sciences of the United States of America, 105, 18308-18313.
Del Rio D, Stewart A J, Pellegrini N.2005.A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress.Nutrition, Metabolism and Cardiovascular Diseases, 15, 316-328.
Sanmartin M, Drogoudi P D, Lyons T, Pateraki I, Barnes J,Kanellis A K.2003.Over-expression of ascorbate oxidase in the apoplast of transgenic tobacco results in altered ascorbate and glutathione redox states and increased sensitivity to ozone.Planta, 216, 918-928.
Schmedes A, Hølmer G.1989.A new thiobarbituric acid(TBA) method for determining free malondialdehyde(MDA) and hydroperoxides selectively as a measure of lipid peroxidation.Journal of the American Oil Chemists’Society, 66, 813-817.
Schmittgen T D, Livak K J 2008.Analyzing real-time PCR data by the comparative CTmethod.Nature Protocols, 3,1101-1108.
Shalata A, Neumann P M.2001.Exogenous ascorbic acid(vitamin C) increases resistance to salt stress and reduces lipid peroxidation.Journal of Experimental Botany, 52,2207-2211.
Smirnoff N.2000.Ascorbic acid: Metabolism and functions of a multi-facetted molecule.Current Opinion in Plant Biology 3, 229-235.
Smirnoff N, Pallanca J E.1996.Ascorbate metabolism in relation to oxidative stress.Biochemical Society Transactions, 24,472-478.
Tabata K, Oba K, Suzuki K, Esaka M.2001.Generation and properties of ascorbic acid-deficient transgenic tobacco cells expressing antisense RNA for L-galactono-1,4-lactone dehydrogenase.The Plant Journal, 27, 139-148.
Tabata K, Takaoka T, Esaka M.2002.Gene expression of ascorbic acid-related enzymes in tobacco.Phytochemistry,61, 631-635.
Tomlin G C, Hamilton G E, Gardner D C, Walmsley R M, Stateva I, Oliver S G.2000.Suppression of sorbitol dependence in a strain bearing a mutation in the SRB1/PSA1/VIG9 gene encoding GDP-mannose pyrophosphorylase by PDE2 overexpression suggests a role for the Ras/cAMP signal-transduction pathway in the control of yeast cell-wall biogenesis.Microbiology, 146, 2133-2146.
Uccelletti D, Staneva D, Rufini S, Venkov P, Palleschi C.2005.Enhanced secretion of heterologous proteins in Kluyveromyces lactis by overexpression of the GDP-mannose pyrophosphorylase, KlPsa1p.FEMS Yeast Research, 5, 735-746.
Veljovic-Jovanovic S D, Pignocchi C, Noctor G, Foyer C H.2001.Low ascorbic acid in the vtc-1 mutant of Arabidopsis is associated with decreased growth and intracellular redistribution of the antioxidant system.Plant Physiology,127, 426-435.
Vuorio R, Härkönen T, Tolvanen M, Vaara M.1994.The novel hexapeptide motif found in the acyltransferases LpxA and LpxD of lipid A biosynthesis is conserved in various bacteria.FEBS Letters, 337, 289-292.
Wang H S, Yu C, Zhu Z J, Yu X C.2011.Overexpression in tobacco of a tomato GMPase gene improves tolerance to both low and high temperature stress by enhancing antioxidation capacity.Plant Cell Reports, 30, 1029-1040.
Wang H S, Zhu Z J, Feng Z, Zhang S G, Yu C.2012.Antisensemediated depletion of GMPase gene expression in tobacco decreases plant tolerance to temperature stresses and alters plant development.Molecular Biology Reports, 39,10413-10420.
Warit S, Zhang N, Short A, Walmsley R M, Oliver S G, Stateva L I.2000.Glycosylation deficiency phenotypes resulting from depletion of GDP-mannose pyrophosphorylase in two yeast species.Molecular Microbiology, 36, 1156-1166.
Wheeler G L, Jones M A, Smirnoff N.1998.The biosynthetic pathway of vitamin C in higher plants.Nature, 393, 365-369.
Wolucka B A, Van Montagu M.2003.GDP-mannose 3´,5´-epimerase forms GDP-L-gulose, a putative intermediate for the de novo biosynthesis of vitamin C in plants.Journal of Biological Chemistry, 278, 47483-47490.
Yabuta Y, Mieda T, Rapolu M, Nakamura A, Motoki T, Maruta T, Yoshimura K, Ishikawa T, Shigeoka S.2007.Light regulation of ascorbate biosynthesis is dependent on the photosynthetic electron transport chain but independent of sugars in Arabidopsis.Journal of Experimental Botany,58, 2661-2671.
Yang L, Zheng B, Mao C, Qi X, Liu F, Wu P.2004.Analysis of transcripts that are differentially expressed in three sectors of the rice root system under water deficit.Molecular Genetics and Genomics, 272, 433-442.
Yang X, Wen X, Gong H, Lu Q, Yang Z, Tang Y, Liang Z,Lu C.2007.Genetic engineering of the biosynthesis of glycinebetaine enhances thermotolerance of photosystem II in tobacco plants.Planta, 225, 719-733.
Yoda K, Kawada T, Kaibara C, Fujie A, Abe M, Hashimoto H, Shimizu J, Tomishige N, Noda Y, Yamasaki M.2000.Defect in cell wall integrity of the yeast saccharomyces cerevisiae caused by a mutation of the GDP-mannose pyrophosphorylase gene VIG9.Bioscience, Biotechnology,and Biochemistry, 64, 1937-1941.
Zhang Z, Wang J, Zhang R, Huang R.2012.The ethylene response factor AtERF98 enhances tolerance to salt through the transcriptional activation of ascorbic acid synthesis in Arabidopsis.The Plant Journal, 71, 273-287.
Zou L P, Li H X, Ouyang B, Zhang J H, Ye Z B.2006.Cloning, expression, and mapping of GDP-D-mannose pyrophosphorylase cDNA from tomato (Lycopersicon esculentum).Acta Genetica Sinica, 33, 757-764.(in Chinese)
 Journal of Integrative Agriculture2018年3期
Journal of Integrative Agriculture2018年3期
- Journal of Integrative Agriculture的其它文章
- Characteristic analysis of tetra-resistant genetically modified rice
- A wheat gene TaSAP17-D encoding an AN1/AN1 zinc finger protein improves salt stress tolerance in transgenic Arabidopsis
- Characterization of GhSERK2 and its expression associated with somatic embryogenesis and hormones level in Upland cotton
- GmNAC15 overexpression in hairy roots enhances salt tolerance in soybean
- Responses of the antioxidant system to fluroxypyr in foxtail millet(Setaria italica L.) at the seedling stage
- Light interception and radiation use efficiency response to tridimensional uniform sowing in winter wheat
