N concentration of old leaves and twigs is more sensitive to stand density than that of young ones in Chinese fir plantations:a case study in subtropical China
Yuebao Di·Xiaoli Fu·Huimin Wang·Wenhua Li·Silong Wang
Introduction
Nutrient diagnosis is a useful method for evaluating plant nutrient status(Lemaire et al.2008).Various types of plant tissues have been tested as indicators of the nutrient status of trees,and have attracted more attention with the development of ecological stoichiometry,which mainly focuses on the nutrient status and ratios in plant tissues and their responses to environmental changes(Luo et al.2013;Sterner and Elser 2002;Schreeg et al.2014).Foliage is usually preferred,as it is considered to be the tissue most sensitive to change in the nutrient status of plants(Moore et al.2004;Bussotti and Pollastrini 2015).Foliar nitrogen(N)concentration,because of its high correlation with leaf photosynthesis and soil N status(Ollinger et al.2002;Turner et al.2009),has been used in forest management ranging from forest health concerns to designing fertilization plans.Twigs can also be used in N diagnosis for some tree species(Van den Driessche 1974;Grove 1990).
In general,newly mature leaves are used in nutrient analysis(Jones and Case 1990).This is suitable for deciduous species,but might be questionable for evergreen species due to the nutrient translocation among leaves of different ages(Luyssaert et al.2002;Bussotti and Pollastrini 2015).Generally,evergreen trees tend to maintain a relatively favorable nutrient status in active young leaves for positive carbon(C)gain and high N use ef ficiency(Escudero and Mediavilla 2003;Marschner 2011).As a consequence,when nutrient de ficiency is slight and latent,old leaves might be a better indicator of nutrient status of plants due to the nutrient translocation from the old to the young leaves(Reuter 1997;Schreeg et al.2014).
Foliar N concentration of trees can be affected by soil N availability,moisture stress,and light competition.(Hobbie and Gough 2002;Townsend et al.2007;Van den Driessche 1974).Foliar N concentration can decline in response to increasing stand density,especially under the condition of N limitation(Litton et al.2004;Will et al.2005).Foliar N concentration increases in trees remaining after thinning due to the reduced competition for N and increased N availability(Carlyle 1995;Thibodeau et al.2000;Inagaki et al.2008).But in some situations,foliar N concentration is not responsive to change in stand density(Brix 1991;Mitchell et al.1996).Turner et al.(2009)reported decreasing N concentration in composite(all age)lodgepole pine (Pinus contorta)foliage with increasing tree density,while the N concentration in new foliage remained at a favorable N level.These studies reported the effects of leaf age on foliar N concentration and its sensitivity to stand density.Yet foliar nutrient analysis conventionally focuses on young leaves without considering the potential role of old leaves in indicating nutrient status and environmental changes caused by thinning.
We hypothesized that N concentrations would be more sensitive to stand density in old leaves than in young leaves.To test this hypothesis,we analyzed the dynamics of C,N concentrations and C:N ratio in the leaves and twigs of different age classes in two stand types in 31-year-old Chinese fir (Cunninghamia lanceolata)plantations with similar soil conditions but different stand densities to address the following questions:(1)Are foliar C and N concentrations,and C:N ratios similar regardless of leaf ageing and stand density in Chinese fir plantations?;(2)Are N concentrations of old and young leaves equally sensitive to stand density in Chinese fir plantations?;(3)Do twigs and leaves respond similarly to stand density?
Materials and methods
Site description
This study was conducted in Huitong County,Hunan Province in subtropical China(26°52′N,109°42′E).This site lies at the transition zone from the Yunnan-Guizhou plateau to the low mountains and hills on the south side of the Yangtze River.The elevation ranges from 300 to 1000 m a.s.l.A humid mid-subtropical monsoon climate characterizes this area with mean annual temperature and precipitation of 16.5°C and 1200 mm,respectively(Wang et al.2013).The soil is classi fied as red soil in Chinese soil classi fication,equivalent to Ultisol in USDA Soil Taxonomy.The zonal vegetation was formerly an evergreen broadleaved forest,which has been nearly removed by human activities.Plantations of Chinese fir,a fast-growing,evergreen coniferous tree with high yield and excellent wood quality,are now the major forest component in this area(Chen et al.2000).
Large areas of pure Chinese fir plantations were planted in 1983 at 480–560 m a.s.l.The initial planting density was about 2000 trees per hectare.By thinning at two levels of intensity,two stand densities,low(LD)and high density(HD),were formed in the tenth year(1993).The soil characteristics,including pro file,texture,and mineral composition,were similar between stand types in 1983(Chen et al.2000).At 30 years of age in 2013,the plantations were mature,and a survey of the stand structural characteristics,soil chemical properties,and tree fine root(diameter ≤2 mm)biomass at 0–40 cm depth was conducted in May(Fu et al.2015).HD had higher tree density and fine root biomass,and a lower basal area than did LD.Soil nutrient availability was similar in LD and HD(Table 1).The understory layers in these stands were dominated by shrubs(mainly Maesa japonica(Thb.)Moritzi,Clerodendrum cyrtophyllum Turcz,Eurya japonica Thunb.)and a few herbs(mainly Lophatherum gracile Brongn.,Arthraxon hispidus(Thunb.)Makino,Stenoloma chusanum Ching,and Woodwardia japonica(L.F.)Sm.).
Sampling design and data collection
Within each stand type, five independent patches were randomly selected on different hills separated by more than 100 m.In each patch,one 200-m2circular sampling plot was demarcated.All sampling plots were situated at midslope with slopes ranging from 25°to 30°.In May 2013,three representative trees were selected for leaf sampling in each plot on the basis of average height and diameter at breast height(DBH).Because branches with suf ficient sunlight exposure produce more shoots and leaves,leaves and twigs from first-order branches exposed to sunlight were collected from each sampled tree.Leaves and twigs were divided into three age classes,one year old(Age-1),two years old(Age-2)and three years old(Age-3).New shoots produced in 2013 were not collected because they were not fully developed at the time of the sampling period.There were very few leaves and twigs older than 3 years,thus they were included into three-year-old tissues.Generally,the apex of a long shoot produces a yearly growth increment that bears a single age class of leaves in evergreen conifers.Thus,we can count age classes from shoot tips by the order of branching and this can be validated by counting tree rings at the base of the shoots(Ewers and Schmid 1981;Luo et al.2005).The two-and three-year-old leaves and twigs were classi fied as old tissues,while one-year-old leaves and twigs were classi fied as young tissues.Samples from three trees of each plot were pooled according to the tissue type and age class.A total of 60 samples(2 stand types×5 plots×3 age classes×2 tissue types)were obtained.
The leaves and twigs of Chinese fir generally grow and senesce at the same time,and fall together in the form of whole branches,but the dead leaves and twigs usually stay on the stem for several years before falling(Zhang and Sheng 2001;Chen et al.2015).Thus,three dead hanging branches from different trees were collected in each plot and were separated into leaf and twig litters.The age classes of leaf and twig litters were not considered because it was dif ficult finding intact dead branches to collect enough samples of different age-class leaf and twig litters in this 31-year-old Chinese fir plantation.Leaf litter and twig litter were mixed separately for each plot,and then 20 composite litter samples were obtained(2 stand types×5 plots×2 tissue types).All samples were oven-dried to constant weight at 75°C and ground to pass through no.100 meshes.C and N concentrations were determined with a Vario Max CN-element analyzer(Elementar Analysensysteme GmbH,Hanau,Germany).
N resorption ef ficiency was calculated using Eq.(1)(Aerts 1996):
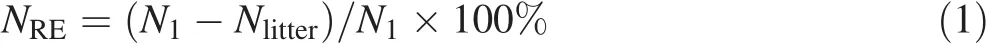
where,NREis N resorption ef ficiency;N1is N concentration of a one-year-old leaf or twig,and Nlitteris N concentration of the leaf or twig litters.
To understand the detailed N resorption process from litter/old tissues to one-year-old tissues,annual N resorption rate was de fined by Eq.(2):

where,ANRRis annual N resorption rate,x is the age class of a leaf or twig,and N1is N concentration of a one-yearold leaf or twig.Here,x ranges from one to three,and litter tissues are regarded as the last age-class tissues.Thus,NREshould be the cumulative ANRR.
Statistical analysis
All statistical analyses were carried out using SPSS 16.0 and the signi ficance level for all analyses was α=0.05.The effects of stand density,tissue age and the interactions between them were determined by two-way analysis of variance with repeated measures.One-way analysis of variance was used to compare means of leaf and twig NREbetween LD and HD;the differences in the C,N concentrations,C:N ratios and ANRRof leaves and twigs between LD and HD were assessed by Student’s t tests.All data were normally distributed and met the assumption of homogeneity of variances.
Results
C and N concentrations,and C:N ratio by leaf age and stand density
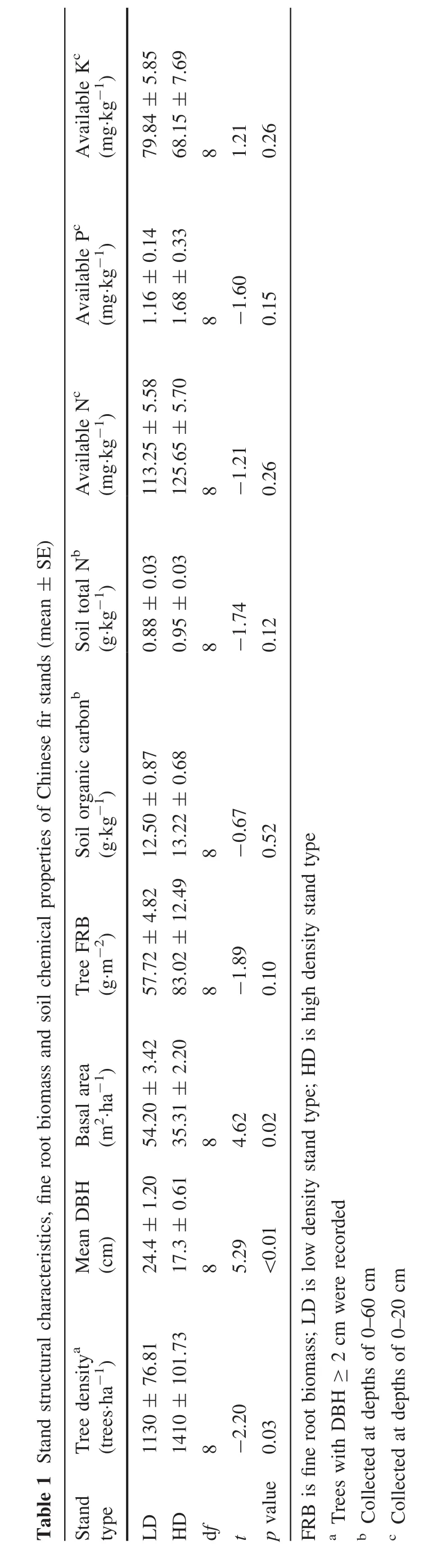
Leaf C concentrations were similar in HD and LD(p=0.185)(Fig.1a).Twig C concentrations were higher in LD(Fig.1d).HD showed lower N concentrations and higher C:N ratios in both leaves and twigs,but signi ficant differences were only found in old leaves and twigs(Fig.1b–f).
Leaf and twig age both showed signi ficant effects on the C,N concentrations and C:N ratio(Fig.1).Increase in C concentration and decrease in N concentration together led to an increase in C:N ratio with ageing.Signi ficant interactions between stand density and age were only found in twigs.With increasing age of both leaf and twig,HD showed more rapid decline in N concentration and faster increase in C:N ratio.
N resorption process in leaf and twig
N resorption by Chinese fir was affected by stand density.ANRRincreased rapidly after age 3 in both leaves and twigs in the LD stand.Leaf ANRRshowed a modest increase in the HD stand(Fig.2a).Twig ANRRshowed the highest value at Age-2(Fig.2b).The differences between trends in ANRRwith age between LD and HD could be attributed to the fact that Chinese fir increased ANRRin old leaves and relatively young twigs under high stand density.ANRRof Age-2 and Age-3 leaves in HD(11 and 14%)were marginally higher than those in LD(4 and 6%;p=0.077,0.082,respectively).The ANRRof Age-2 twigs in HD(25%)was signi ficantly higher than that in LD(6%),while there was no difference in ANRRat Age-3 between LD and HD twigs.However,LD showed signi ficantly higher ANRRthan HD in both leaf and twig litter.
NREwas similar for leaf and twig between LD and HD but the N resorption processes differed between them(Fig.3).Compared with HD,the N resorption ef ficiency in LD was mainly contributed by leaf and twig litter.Speci fically,the ANRRof leaf and twig litter in LD accounted for 76 and 64%of NRE,respectively.Whereas the ANRRof leaf litter in HD accounted for 44%of NRE.ANRRof twig litter in HD only accounted for 33%of NRE,while the ANRRof Age-2 twigs in HD contributed 50%of NRE.
Discussion
Lower N concentration of leaves at high stand density
Stand density showed a signi ficant effect on foliar N concentration.Foliar N concentration tended to be lower at high stand density.This result is consistent with previous studies and might be attributed to more intense competition for N at high stand density(Litton et al.2004;Turner et al.2009).Although reduced stand density after thinning might improve foliar N concentrations due to increased light availability(Medhurst and Beadle 2005),its effect would be weakened and might virtually disappear after the canopy re-closes,due to the dilution of nutrients caused by the development of the crown(Messina 1992;Turner et al.2009).Thus,more than ten years after thinning,the major determinant of foliar N in both LD and HD stands might be below-rather than above-ground.Our investigation indicated that soil N availability was similar in LD and HD stands(Table 1).But tree fine root biomass in HD was marginally higher than in LD(p=0.095).Considering that fine roots are the principal structures of plants involved in water and nutrient acquisition,more fine roots would be expected to strengthen the competitive ability of plants in nutrient absorption(Kochsiek et al.2013).Thus,compared with LD,the increased belowground investment in HD(Table 1)may indicate an intense competition for nutrients at high stand density(Casper and Jackson 1997;Litton et al.2004).
Higher sensitivity of old leaves and twigs to stand density
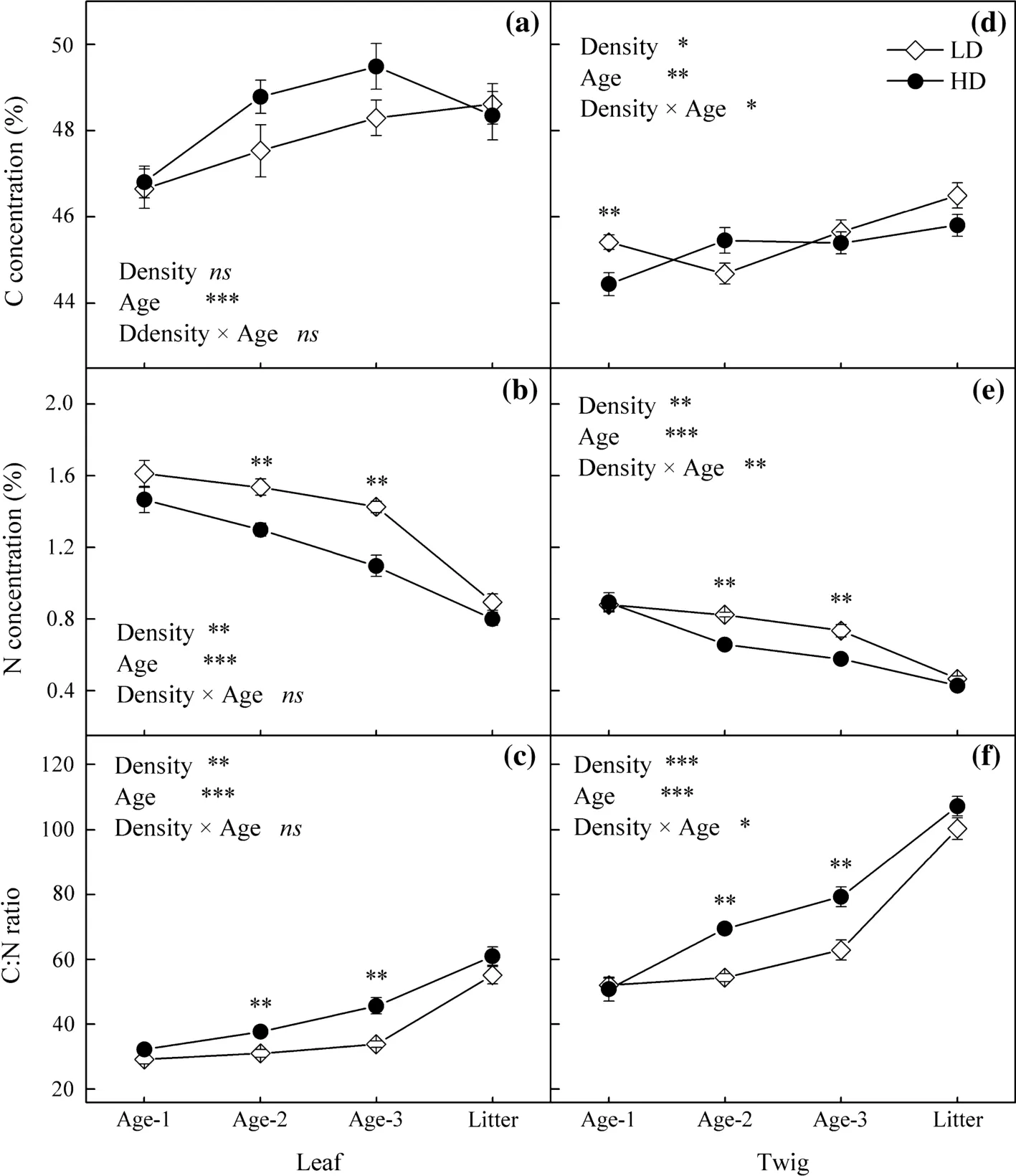
Fig.1 Changes of C and N concentrations,and C:N ratio in leaves and twigs with age in low density(LD)and high density stands(HD)of Chinese fir plantation in subtropical China.Error bars indicate standard errors.Asterisks denote signi ficant effects of the explanatory variables or signi ficant differences of C,N concentrations and C:N ratio between LD and HD:*p≤0.05;**p≤0.01;***p≤0.001,and ns,not signi ficant
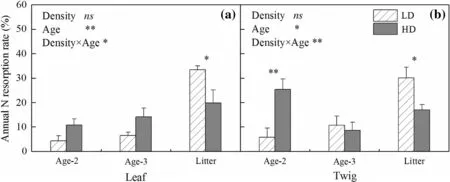
Fig.2 Annual N resorption rate(ANRR)of leaf a and twig b in low density(LD)and high density stands(HD)of Chinese fir plantation in subtropical China.Error bars indicate standard errors.Asterisks denote signi ficant effects of the explanatory variables or signi ficant differences of ANRR between LD and HD:*p≤0.05;**p≤0.01,and ns,not signi ficant
Leaf N concentrations differed between LD and HD but only in old leaves.Evergreen trees tend to maintain relatively favorable N status in active young leaves to achieve carbon gain,given that N can be used more ef ficiently in young leaves(Escudero and Mediavilla 2003;Schreeg et al.2014).Evergreens can transport N from old and senescing leaves to storage sites and new organs to support new growth,which has been veri fied as a key mechanism of N conservation and reuse in plants(Millard and Grelet 2010;Wang et al.2014).When N supply becomes limited,young leaves tend to maintain N concentration through N translocation from old leaves,leading to N decline in old leaves(Marschner 2011).Thus,old leaves might be more sensitive to environmental variation than young leaves.
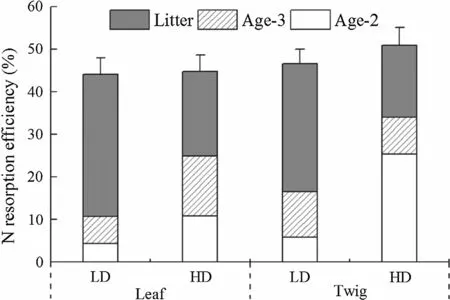
Fig.3 N resorption ef ficiency(NRE)and its process in leaf and twig in low density(LD)and high density stands(HD)of Chinese fir plantation in subtropical China.Error bars indicate standard errors of NRE
Our data show that the changes in N concentration in twigs with age and stand density were consistent with those of leaves in Chinese fir plantations.Differences in N concentration between LD and HD were found only in old twigs.These results suggest that twigs,especially the old ones,may also be an appropriate sampling tissue to indicate changes of N status and stand density of Chinese fir plantations.Similarly,Grove(1990)noted that twigs may be a better tissue than leaves for diagnosing nutrient de ficiencies for karri(Eucalyptus diversicolor F.Muell.).The twigs of Chinese fir appeared to be as active and sensitive as leaves,probably due to the fact that the twigs of Chinese fir generally grow and senesce together with leaves,and are also able to photosynthesize(Chen et al.2000,2015).
N resorption process of leaves and twigs affected by stand density
Our results showed that NREdid not vary with stand density.Thisimpliesthe relative stability ofNREat intraspeci fic level,as indicated by studies showing that resorption ef ficiency was unaffected by increasing nutrient availability(Aerts 1996;Chen et al.2015).Although we documented similar increasing trends with age in leaf ANRRbetween LD and HD stands,marginally higher ANRRin two-and three-year-old leaves and signi ficantly lower ANRRin leaf litter were recorded at high stand density.These results indicate that more N was translocated from old leaves to young ones at high stand density.Similarly,Douglas fir(Pseudotsuga menziesii)increased the redistribution of N from old to young leaves and shed the oldest leaves in response to sudden reduction in N availability(Turner 1977).Annual N resorption rates of twigs did not increase with age as did leaves at high stand density,but showed a maximum value at two years of age.This implies that twigs,especially one-year-old twigs,might be an N storage site for Chinese fir.This is supported by data showing that the bark tissues of twigs contain photosynthetic proteins,where much of the N is stored for conifer evergreen trees(Aschan and Pfanz 2003;Millard and Grelet 2010;Rennenberg et al.2010).
Conclusions
By analyzing the dynamics of C and N concentrations,and C:N ratios of leaves and twigs in two stand densities in 31-year-old Chinese fir plantations with similar soil conditions but different stand densities,we found that N concentrations of old leaves and twigs were more sensitive to stand density than were young ones.Although N resorption ef ficiency did not vary by stand density,the annual N resorption rates increased in old leaves and relatively young twigs at high stand density.These results showed that old leaves and twigs could be helpful in indicating the nutrient status of Chines fir plantations,and that using leaves and twigs of different ages may improve the effectiveness of nutrient analysis in Chinese fir plantation.
Foliar nutrient analysis is broadly applied in nutrient diagnosis and studies on plant stoichiometry.Considering that the sensitivity of nutrient concentrations in leaves and twigs may vary with age,the selection of sampling tissues should be adjusting depending on the research objectives rather than routinely selecting young leaves or twigs.When studying Chinese fir,young leaves and twigs might be a better choice to study the ecological stoichiometric characteristics across biomes due to their relative stability against environmental changes.But old leaves and twigs should be considered for studies of nutrient diagnosis due to their sensitivity to environmental change.
Testing the generality of these results could improve the use of foliar analysis as an indicator of nutrient status and environmental changes in evergreen tree species.
Acknowledgements The authors gratefully acknowledge the field support of Jianlei Wang,Hua Zhou and Junlong Yang.We greatly thank Stephanie Loh for providing language help.
Aerts R (1996)Nutrient resorption from senescing leaves of perennials:Are there general patterns?J Ecol 84(4):597–608
Aschan G,Pfanz H(2003)Non-foliar photosynthesis-a strategy of additional carbon acquisition.Flora 198(2):81–97
Brix H(1991)Mechanisms of response to fertilization.II.Utilization by trees and stands.In:Lousier JD,Brix H,Brockley R,Carter R,Marshall VG(eds)Improving forest fertilization decisionmaking in British Columbia.BCMoF,Victoria,pp 76–93
Bussotti F,Pollastrini M(2015)Evaluation of leaf features in forest trees:methods,techniques,obtainable information and limits.Ecol Indic 52:219–230
Carlyle JC(1995)Nutrient management in a Pinus radiata plantation after thinning:the effect of thinning and residues on nutrient distribution,mineral nitrogen fluxes,and extractable phosphorus.Can J For Res 25(8):1278–1291
Casper BB,Jackson RB(1997)Plant competition underground.Annu Rev Ecol Syst 28:545–570
Chen CY,Liao LP,Wang SL(2000)Ecology of Chinese fir plantation forest.Science Press,Beijing,pp 85–99(in Chinese)
Chen FS,Niklas KJ,Liu Y,Fang XM,Wan SZ,Wang H(2015)Nitrogen and phosphorus additions alter nutrient dynamics but not resorption ef ficiencies of Chinese fir leaves and twigs differing in age.Tree Physiol 35(10):1106–1117
Escudero A,Mediavilla S(2003)Decline in photosynthetic nitrogen use ef ficiency with leaf age and nitrogen resorption as determinants of leaf life span.J Ecol 91(5):880–889
Ewers FW,Schmid R(1981)Longevity of needle fascicles of Pinus longaeva(Bristlecone pine)and other North American pines.Oecologia 51(1):107–115
Fu X,Wang J,Di Y,Wang H(2015)Differences in fine-root biomass of trees and understory vegetation among stand types in subtropical forests.PLoS One 10(6):e0128894
Grove T(1990)Twig and foliar nutrient concentrations in relation to nitrogen and phosphorus supply in a eucalypt(Eucalyptus diversicolor F.Muell.)and an understorey legume(Bossiaea laidlawiana Tovey and Morris).Plant Soil 126(2):265–275
Hobbie SE,Gough L(2002)Foliar and soil nutrients in tundra on glacial landscapes of contrasting ages in northern Alaska.Oecologia 131(3):453–462
Inagaki Y,Kuramoto S,Torii A,Shinomiya Y,Fukata H(2008)Effects of thinning on leaf-fall and leaf-litter nitrogen concentration in hinoki cypress(Chamaecyparis obtusa Endlicher)plantation stands in Japan.For Ecol Manag 255(5):1859–1867
Jones JB,Case VW(1990)Sampling,handling,and analyzing plant tissue samples.In:Westerman RL(ed)Soil testing and plant analysis,3rd edn.SSSA,Madison,pp 389–427
Kochsiek A,Tan S,Russo SE(2013)Fine root dynamics in relation to nutrients in oligotrophic Bornean rain forest soils.Plant Ecol 214(6):869–882
Lemaire G,Jeuffroy M-H,Gastal F(2008)Diagnosis tool for plant and crop N status in vegetative stage:theory and practices for crop N management.Eur J Agron 28(4):614–624
Litton CM,Ryan MG,Knight DH(2004)Effects of tree density and stand age on carbon allocation patterns in post fire lodgepole pine.Ecol Appl 14(2):460–475
Luo T,Luo J,Pan Y(2005)Leaf traits and associated ecosystem characteristics across subtropical and timberline forests in the Gongga Mountains Eastern Tibetan Plateau.Oecologia 142(2):261–273
Luo J,Li H,Liu T,Polle A,Peng C,Luo ZB(2013)Nitrogen metabolism of two contrasting poplar species during acclimation to limiting nitrogen availability.J Exp Biol 64(14):4207–4224
Luyssaert S,Raitio H,Vervaeke P,Mertens J,Lust N(2002)Sampling procedure for the foliar analysis of deciduous trees.J Environ Monit 4(6):858–864
Marschner H(2011)Marschner’s mineral nutrition of higher plants.Academic press,London,pp 301–312
Medhurst JL,Beadle CL(2005)Photosynthetic capacity and foliar nitrogen distribution in Eucalyptus nitens is altered by highintensity thinning.Tree Physiol 25(8):981–991
Messina MG(1992)Response of Eucalyptus regnans F.Muell.to thinning and urea fertilization in New Zealand.For Ecol Manag 51(4):269–283
Millard P,Grelet G(2010)Nitrogen storage and remobilization by trees:ecophysiological relevance in a changing world.Tree Physiol 30(9):1083–1095
Mitchell A,Barclay H,Brix H,Pollard D,Benton R,DeJong R(1996)Biomass and nutrient element dynamics in Douglas- fir:effects of thinning and nitrogen fertilization over 18 years.Can J For Res 26(3):376–388
Moore JA,Mika PG,Shaw TM,Garrison-Johnston MI(2004)Foliar nutrient characteristics of four conifer species in the interior northwest United States.West J Appl For 19(1):13–24
Ollinger S,Smith M,Martin M,Hallett R,Goodale C,Aber J(2002)Regional variation in foliar chemistry and N cycling among forests of diverse history and composition. Ecology 83(2):339–355
Rennenberg H,Wildhagen H,Ehlting B(2010)Nitrogen nutrition of poplar trees.Plant Biol 12(2):275–291
Reuter DJ(1997)Plant analysis:an interpretation manual.CSIRO publishing,Australia,pp 3–27
Schreeg LA,Santiago LS,Wright SJ,Turner BL(2014)Stem,root,and older leaf N:P ratios are more responsive indicators of soil nutrient availability than new foliage.Ecology 95(8):2062–2068
Sterner RW,Elser JJ(2002)Ecological stoichiometry:the biology of elements from molecules to the biosphere.Princeton University Press,Princeton,pp 87–133
Thibodeau L,Raymond P,CamiréC,Munson AD(2000)Impact of precommercial thinning in balsam fir stands on soil nitrogen dynamics,microbial biomass,decomposition,and foliar nutrition.Can J For Res 30(2):229–238
Townsend AR,Cleveland CC,Asner GP,Bustamante M(2007)Controls over foliar N:P ratios in tropical rain forests.Ecology 88(1):107–118
Turner J(1977)Effect of nitrogen availability on nitrogen cycling in a Douglas- fir stand.For Sci 23(3):307–316
Turner MG,Smithwick EA,Tinker DB,Romme WH(2009)Variation in foliar nitrogen and aboveground net primary production in young post fire lodgepole pine.Can J For Res 39(5):1024–1035
Van den Driessche R(1974)Prediction of mineral nutrient status of trees by foliar analysis.Bot Rev 40(3):347–394
Wang Q,He T,Wang S,Liu L(2013)Carbon input manipulation affects soil respiration and microbial community composition in a subtropical coniferous forest.Agric For Meteorol 178:152–160
Wang M,Murphy MT,Moore TR(2014)Nutrient resorption of two evergreen shrubs in response to long-term fertilization in a bog.Oecologia 174(2):365–377
Will RE,Narahari NV,Shiver BD,Teskey RO(2005)Effects of planting density on canopy dynamics and stem growth for intensively managed loblolly pine stands.For Ecol Manag 205(1):29–41
Zhang J,Sheng W(2001)The study on decay of dead branches and leaves on living trees taken from crown into litter environment in a Chinese fir plantation,compared with decay in canopy.Sci Silv Sin 37(6):2–10(in Chinese)
 Journal of Forestry Research2018年1期
Journal of Forestry Research2018年1期
- Journal of Forestry Research的其它文章
- Vascular bundle connection between seed stalk and seed coat of Caragana arborescens
- Mini-cutting technique for Khaya anthotheca:selection of suitable IBA concentration and nutrient solution for its vegetative propagation
- Reconstructing the size of individual trees using log data from cut-to-length harvesters in Pinus radiata plantations:a case study in NSW,Australia
- Phenotypic variation in Phoebe bournei populations preserved in the primary distribution area
- Effects of soil drought stress on photosynthetic gas exchange traits and chlorophyll fluorescence in Forsythia suspensa
- Flavonoid content and radical scavenging activity in fruits of Chinese dwarf cherry(Cerasus humilis)genotypes
