Stability Analysis of a Lignocellulose Degrading Microbial Consortium
Zheng Guo-xiang, Li Jian, Zhou Chen-yang and Chong Yu-ting
1 College of Engineering, Northeast Agricultural University, Harbin 150030, China
2 Key Laboratory of Pig-breeding Facilities Engineering, Ministry of Agriculture, Harbin 150030, China
3 Heilongjiang Key Laboratory of Technology and Equipment for the Utilization of Agricultural Renewable Resources, Harbin 150030, China
Introduction
Rice straw is an important carbon resource for the biorefinery industry, its use for bio-energy-biogas is also a very promising way, and is thus considered a sustainable and environmentally friendly alternative to the fossil energy (Kamm and Kamm, 2004; Edwardet al., 2007).The use of microbial populations in decomposing lignocellulosic biomass to produce highcalorific value products is an important research area in alleviating energy crisis and effectively utilizing the cellulose resources (Barkhaet al., 2016;Zhouet al., 2009).
In natural, lignocellulosic biomass is decomposed under aerobic or anaerobic conditions due to synergy of various microorganisms, which will avoid feedback regulation and metabolic inhibition in contrast to single strain (Kumaret al., 2008; Mohamedet al.,2015; Miqueletoet al., 2010).In recent years, many lignocellulose degradation complex consortia were successfully obtained by limiting-culture and directional construction (Tanget al., 2015; Barkha 2016;Liet al., 2009; Gaoet al., 2009).Harutaet al.(2002)obtained a structural stable and complex lignocellulolytic microbial consortium with high degradable activity on various cellulosic materials from rice straw compost by successive enrichment culture.Study on the dynamic changes of genetic components and functional stability of microbial consortia could provide necessary basis for subsequent theoretical and applied researchs (Bandal, 2003).The application of molecular ecology technology can avoid the cumbersome process in traditional separation and cultivation, directly to the relationships between microbial consortia structure and environment, DGGE technology is one of the commonly used molecular ecology technology (Gonget al., 2004).Liu (2009)studied the microbial community dynamics and determined the dominant bacteria in different rice stalk fermentation stages with PCR-DGGE technology.
The functional and structural stabilities of microbial consortia are considered to be important factors in biomass degradation capability and potential for biotechnological application (Wongwilaiwalinet al.,2010).In this study, the functional and structural stability of five different generations of reserved microbial consortium LZF-12 capable of degrading rice straw were evaluated through batch experiments and a molecular culture-independent approach.This study provided a valuable platform for the subsequent targeted regulation and control of fermentation system.The microbial consortia have potential in biotechnological application on lignocellulosic biomass degradation.
Materials and Methods
Culture and medium
The mesophilic microbial consortium LZF-12 capable of effectively degrading lignocelluloses was established by the Biomass Laboratory of Northeast Agricultural University (Chonget al., 2011).Rice straw was collected from local farm, pretreated with 1% (w/v) NaOH and dried at 50℃, used as the sole carbon source (Zheng, 2013).The microbial consortium was grown in peptone cellulose selection(PCS) medium (peptone, 5 g; yeast powder, 0.8 g;calcium carbonate, 2.0 g; sodium chloride, 5.0 g; dry straw, 10.0 g; distilled water, 1 000 mL) autoclaved at 121℃ for 15 min using standard methods (Wanget al., 2005).
The preserved 15th, 20th, 25th, 30th and 35th generations microbial consortia were inoculated in 500 mL petone cellulose substrate (PCS) medium containing 1% rice straw at a 5% inoculation amount,incubated at 35℃ under static condition.pH, dissolved oxygen (DO) value and liquid end products of the fermentation broth were monitored daily, the residual solid cellulosic substrates were determined gravimetrically incubated for 7 days with uninoculated media as the contrast group.
Analytical methods
DO and pH were monitored regularly using the Portable Dissolved Oxygen Analyzer (WTW oxi315i)and the portable pH meter (Accuracy: 0.01).The methods referred to Liet al(2006).
The microbial consortium was incubated in PCS medium containing 1% rice straw.Residual solid cellulosic substrates were analyzed after incubation with the microbial consortia for 7 days at 35℃ under the defined facultative anaerobic static condition.The residual substrates were then determined gravimetrically after dried at 105℃ for 2 days with uninoculated medium as a contrast group.Percent of residual weight was reported based on the total holocellulose content.Degradation ratio was described by the following equation (Kluzek-Turpeinen B,2000).

Where,Mtwas the total weight of the cellulosic materials before degradation andMrwas the weight of the residual substrates after degradation.The reactions were performed in triplicate and the averages of the results were reported.Volatile fat acid (VFA) was determined by a gas chromatography (GC-6890N,Agilent Inc., USA) equipped with a flame ionization detector and a 30 m×0.25 mm×0.25 μm fused-silica capillary column.Nitrogen was used as the carrier gas with split injection method, split ratio of 20 : 1.The details as described by Zhenget al( 2013).Retention time of ethanol, acetic acid, propionate and butyrate(analytically pure) were determined to obtain the peak sequence.A standard solution containing ethanol,acetic acid, propionic acid, butyric acid was diluted with deionized water to five different concentrations and measured to plot the standard curve.The concentrations of unknown alcohols and acids in the samples were determined according to external standard method (Chong, 2011).
(三)母猪产前准备 待产母猪分娩前5~7 d进入分娩舍。之前要将分娩舍清扫干净,用消毒液对地面、墙壁和圈栏进行消毒。墙壁、门窗有破损时应进行修补,防止贼风进入,保持温湿度良好(产房内温度为16℃~21℃,湿度为55%~57%)。地面要光滑,同时检查保育箱、电热板、水嘴、饲槽等功能是否正常。同时给待产母猪用温热的肥皂水清洗全身,清除污物,再用刺激性小的消毒水将全身消毒,分娩前用0.1%高锰酸钾清洗乳房和外阴部。在产房准备好接产用的消毒药水、抹布、碘酒、剪齿钳等接产用品,等待接产。
The total genomic DNA was extracted from the microbial consortia using bacterial genomic DNA extraction kit.The purified DNA was used as a template for amplification of the partial 16S rDNA fragment usingTaqDNA polymerase (Fermentas,Vilnius, Lithunia) according to the manufacturer's protocol with 338GC-F forward primer, which was attached to a GC clamp at the 5-terminus (5'-cgcccgc cgcgcgcggcgggcggggcgggggcacggggggactcctacggga ggca-3', GC clamp sequence is underlined), and 518R reverse primer (5'-attaccgcggctgctgg-3').Reactions were performed in a My Cycler thermal cycler (Bio-Rad Laboratories, Hercules, CA).The temperature profile consisted of 94℃ for 3 min, followed by 30 cycles of denaturation at 94℃ for 1 min, annealing at 55℃ for 1 min and extension at 72℃ for 2 min,followed by a final extension step at 72℃ for 3 min.
The pro files of amplified 16S rDNA fragments were analyzed by DGGE technology on DCodeTM system(Bio-Rad).The target bands were sequenced after cloning.Sequences were initially compared to the available databases using basic local alignment search tool (BLAST) server to determine their approximate phylogeny and analyze the genetic relationship and similarity.
Results
Stability analysis of microbial consortium LZF-12
The degradation rate of rice straw, the types and concentration of fermentation metabolites and genetic stability of the reaction system were used as measurable indicators to screen high efficient microbial consortia.After subculture, transfer experiments were conducted respectively to investigate the fermentation stability of rice straw microbial consortium LZF-12 using the degradation efficiency, pH, DO and terminal liquid products as key indicators.
Fig.1 showed pH changes of fermentation broth were similar in the 15th, 20th, 25th, 30th and 35th generation cultures with time.At the initial phase of fermentation (1 day), pH of different generations decreased rapidly from the initial 6.8 to 6.1, 6.2, 6.3,and 6.4, respectively.This could be explained by the production of organic acids from the degradation of easily compounds, as acids accumulate, the medium becomes acidified.After 1 day, pH increased from 6.3,6.1, 6.2, 6.4 and 6.3 to 7.0, 7.1, 7.2, 7.0 and 7.1 for each generation respectively, could be explained by the degradation of organic acids along with the growth and fermentation of microbial consortium LZF-12.On the 7th day, the rice straw was mostly degraded and pH was kept 7.0-7.2, it could be inferred that pH changes of the fermentation were closely related to the decomposition process of cellulose.There was no obvious difference in five pH change trends,suggesting the stability of the fermentation system in different generations of LZF-12.
DO in the 15th, 20th, 25th, 30th and 35th generation cultures were determined.Table 1 showed the changing trends of DO were basically same in five experimental groups.After inoculated 1 day, the decrease in DO significantly from initial 2.3 mg · L-1to 0.07-0.08 mg · L-1, which could be explained by the consumption of dissolved oxygen, due to the growth of facultative anaerobes.In 2-7 days, pH fluctuated slightly (0.07≤DO≤0.09), on the 7th day of the fermentation, DO were 0.07, 0.08, 0.08, 0.07 and 0.09,respectively, indicated that the fermentation of the microbial consortium adapted to micro-aerobic environment.
The component and concentration of the liquid end products could provide very important reference for controlling the normal operation of the reaction system.In view of the previous studies,the fermentation products of LZF-12 were mainly ethanol, acetic acid, butyric acid and a small amount of propionic acid, and the acetic acid made up about 70% of the total liquid product volume (Chong,2011).Therefore, in this experiment, the changes of acetic acid contents in the fermentation broth of different generations were analyzed dynamically(Fig.2).The main types of liquid end products did not change (the data was not listed) in different generation fermentation systems which were still composed of alcohol, acetic acid, butyric acid and propionic acid.The acetic acid showed small difference and slight variations in different generations.The increase of acetic acid concentrations from 0 to 2.2-2.4 g · L-1appeared to correspond to cellulose degradation amount within 6 days of the incubation.Then gradually decreased to 2.01-2.15 g · L-1, and the concentration of acetic acid in five experimental groups were all above 70% of the total liquid phase end product concentration.

Fig.1 Variation of pH of different subcultures of microbial system

Table 1 DO variation of different subcultures of microbial consortium LZF-12

Fig.2 Variation of acetic acid content of different subcultures of microbial system

Fig.3 % Residual weight of 15th, 20th, 25th, 30th and 35th generations of microbial consortium LZF-12
Microbial community structure
In order to assess the structural stability of the microbial consortium during subcultivation, 16S rDNA PCR products amplified with primer pairs collected from different cultured generations were analyzed by denaturing gradient gel electrophoresis to contrast to the community DGGE profiles.Sixteen bands were observed in DGGE pro files (Fig.4).
Comparison of the sequences originating from the numbered bands with GenBank (Table 2) revealed that LZF-12 was composed of five major bacteria.Bands 5, 9 and 10 originated fromClostridiumsp.AP81,Clostridium cellobioparumstrain DSM 1351 andClostridiumsp.T241, respectively.
Clostridiumstrains had a strong adaptation to ambient temperature and pH, and could produce extracellular hydrolytic enzymes decomposing proteins,sugars and lignocellulose to small molecular substances.Bands 7 and 8 originated fromClostridiumcellulolyticumH10 andC.cellulolyticum, gram negative and spore-forming, bacterium with flagella,could produce lignin modifying enzymes including extracellular oxidase, laccase, tyrosinase and peroxidase with a strong ability to degrade cellulose into acetic acid, H2, CO2and lactic acid (Wiegelet al., 2006).The sequences of bands 2, 3, 4, 6, 11 and 15 were identical to some uncultured bacteria which stably co-existed in the consortia, respectively.
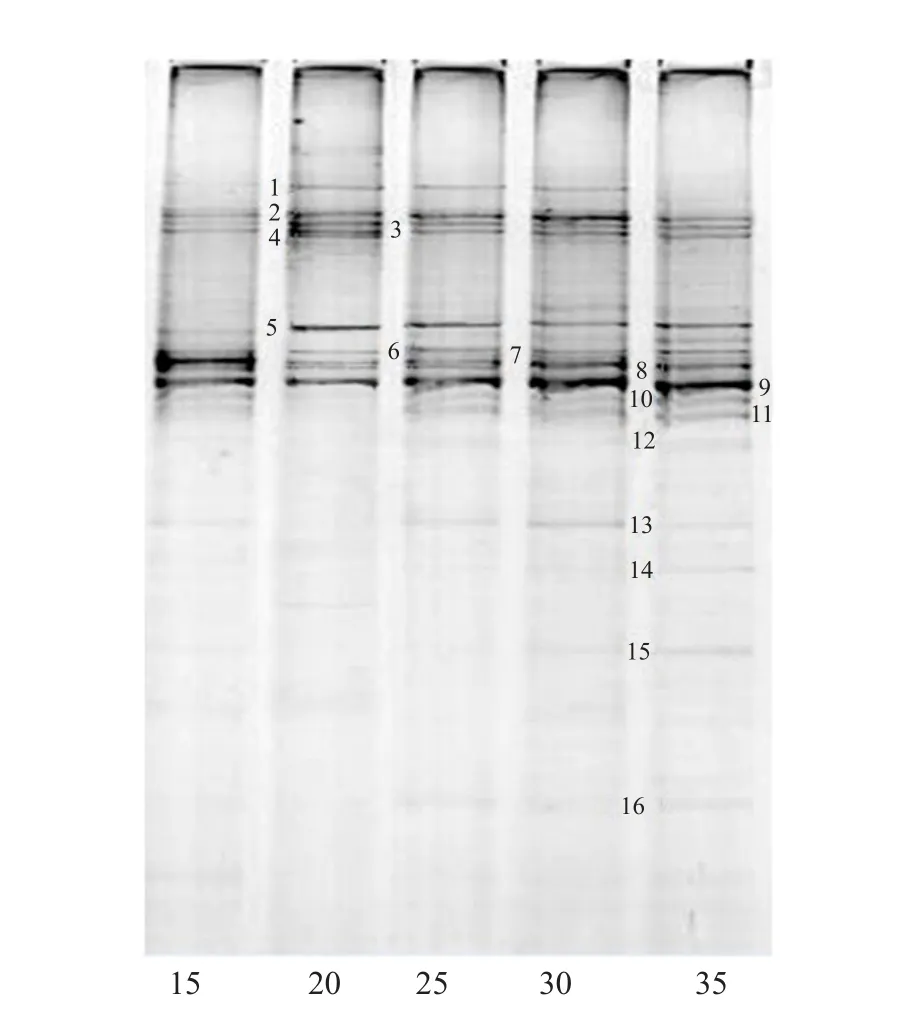
Fig.4 Structural stability of composite microbes in microbial consortium LZF-12 by denaturing gradient gel electrophoresis
Bands 13, 14 and 16 originated from unculturedVerrucomicrobiawere presented, in addition toPseudomonassp.F5OHPNU07IE0B8 (Band 1) andAcetivibriosp.WSC-27 (Band 12).Being one of the dominant bacterium,Verrucomicrobiaappeared in the later stage owing to oxygen depletion in reaction system and were good for lignocellulosic substrate degradation (Kuang, 2010).Pseudomonaswas rodshaped or slightly curved, gram negative, no spores and aerobic bacterium, used the dissolved oxygen at the initial stage of fermentation to create a microaerobic environment for the system, thus accelerated the lignocellulose degradation by anaerobic and facultative anaerobes.Furthermore,Acetivibriosp.WSC-27 was a mesophilic, chemoorganotrophic bacterium isolated from the waste sludge or pig manure, its main end products were acetates, in additional to a few of ethanol, CO2and H2.DGGE pattern pro file dynamics from different LZF-12 generations were reproducible,suggesting the relative stabilities of the microbial community structure and succession mechanism in the established consortia.

Table 2 Sequence similarity analysis of bands 1-16
Conclusions
These experimental results showed that efficient degradation of rice straw (>70%) could be achieved in batch cultures inoculated with microbial consortium LZF-12 from different generations.Acetic acid was the major aqueous products of five generations,ethanol, butyric acid and a small amount of propionic acid were also formed, but in low levels, the difference in liquid end products showed slightly variation and was the same with DO and pH in the whole fermentation process.Similar DGGE patterns among five generations (from generation 15 to 35) were observed, indicated that the structural and functional stability of the composite microbial co-existed in the consortium, which comprised mainly of approx.Five major composite members includingClostridium,Pseudomonas,Acetivibrio,Verrucomicrobiaand some uncultured unidentified bacteria.Their coexistence was assumed to be important for effective lignocellulose degradation by complex metabolic interaction.The balance of various types of metabolic relationships was considered to be essential for the stable co-existence of the composite members in the community, which resulted in efficient biomass degradation.The results also illustrated the high stability of the microbial community LZF-12 and allowed long-term storage of the seed culture for further experimental studies and application.
Barkha V, Vaibhav S, Pooja S,et al.2016.Exploring untapped energy potential of urban solid waste.Energy Ecology & Environment, 1(5):323-342.
Bandal C S.2003.Hemicellulose bioconversion.Journal of Industrial Microbiology and Biotechnology, 30: 279-291.
Chong Y T.2011.Screening and degradation characteristics of rice straw degrading composite strains.Northeast Agricultural University,Harbin.
Chong Y T, Li W Z, Zheng G X,et al.2011.Screening of rice straw degradation microbial system and its growth characteristics.Journal of Northeast Agricultural University, 42(8): 56-61.
Edward A B, Raphael L, Michael E H I.2007.The potential of cellulose and cellulosomes for cellulosic waste management.Current Opinion in Biotechnology, 18: 237-245.
Gao L W, Ma L, Zhang W F.2009.Estimation and utilization of crop straw nutrient resources in China.Journal of Agricultural Engineering, 25(7): 173-179.
Gong L M, Ren N Q, Xing D F.2004.DGGE/TGGE technology and its application in microbial molecular ecology.Journal of Microbiology,44(6): 845-848.
Harta S, Cui Z, Li M,et al.2002.Construction of a stable microbial community with high cellulose-degradation ability.Appl Microbiol Biotechnol, 59: 529-34.
Kamm B, Kamm M.2004.Principles of biorefineries.Appl Microbiol Biotechnol, 64: 137-145.
Kuang X Z, Qiu Y L, Shi X S,et al.2010.Isolation and characterization of a novel anaerobic fermentation bacterium.Journal of Anhui Agri Sci,38(17): 8840-8843.
Kumar R, Singh S, Singh O V.2008.Bioconversion of lignocellulosic biomass: biochemical and molecular perspectives.Ind Microbiol Biotechnol, 35: 377-91.
Li H, Liu L, Li M,et al.2009.Effects of pH, temperature, dissolved oxygen, and flow rate on phophorus release processes at the sediment and water interface in storm sewer.Journal of Analytical Methods in Chemistry, 10: 1-7.
Li W G, Li Q, He X X.2006.Research progress on returning straw to field.Hunan Agricultural Science, 1: 46-48.
Liu S.2009.Characteristics of degraded lignocellulose and its flora dynamics in complex strains.Northeast Agricultural University,Harbin.
Miqueleto A P, Dolosic C C,et al.2010.Influence of carbon sources and C/N ratio on EPS production in anaerobic sequencing batch biofilm reactors for wastewater treatment.Bioresource Technology, 101(4):1324-1330.
Mohamed T, Krishna K, Kadali A H,et al.2015.An effective microplate method (Biolog MT2) for screening native lignocellulosicstraw-degrading bacteria.Annals of Microbiology, 65(4): 2053-2064.
Tang H M, Xiao X P, Tang W G,et al.2015.Effect of winter covering crop residue incorporation on CH4and N2O emission from doublecropped paddy fields in southern China.Environmental Science &Pollution Research, 22(16): 12689.
Kluzek-Turpeinen B, Tuomala M, Hatakka A,et al.2003.Lingin degradation in a compost environment by the deuteromycetePaecilomyces in flatus.Applied Microbiology and Biotechnology, 61:374-379.
Wang W D, Cui Z J, Yang H Y,et al.2005.Stability of a composite microbial system WSC-6 with efficient cellulose degrading.China Environmental Science, 25(5): 567-571.
Wongwilaiwalin S, Rattanachomsri U, Laothanachareon T,et al.2010.Analysis of a thermophilic lignocellose degrading microbial consortium and multi-species lignocellulolytic enzyme system.Enzyme and Microbial Technology, 47: 283-290.
Wiegel J, Tanner R, Rainey F A.2006.An introduction to the family Clostridiaceae.The Prokaryote, 4: 654-678.
Zheng W L.2013.Optimization of fermentative factors and strengthening compost of rice straw degradation microbial system LZF-12.Northeast Agricultural University, Harbin.
Zhou J, Wang Y H, Chu J.2009.Optimization of cellulose mixture for efficient hydrolysis of steam-exploded corn storer by statistically designed experiments.Bioresource Technology, 100(2): 819-825.
 Journal of Northeast Agricultural University(English Edition)2018年1期
Journal of Northeast Agricultural University(English Edition)2018年1期
- Journal of Northeast Agricultural University(English Edition)的其它文章
- Effect of Inoculation Rhizobium and Response of Soybean-Rhizobium System to Insoluble Phosphate
- Characteristics and Degradation Mechanism of Fomesafen
- Comparative Study of Proximate, Chemical and Physicochemical Properties of Less Explored Tropical Leafy Vegetables
- A Comparative Study on Photosynthetic Characteristics of Dryopteris fragrans and Associated Plants in Wudalianchi City, Heilongjiang Province, China
- Effect of Mineral and Vitamin Supplementation on Performance and Haemotological Values in Broilers
- Comparative Research on Facultative Anaerobic Cellulose Decomposing Bacteria Screened from Soil and Rumen Content and Diet of Dairy Cow
