The biological clock: future of neurological disorders therapy
Dear editors,
The seminal, discoveries by Jeffrey Connor Hall, Michael Rosbash and Michael Warren Young have earned the Nobel Prize in Physiology and Medicine 2017 for revealing a crucial physiological mechanism explaining biological clock, with important implications for human health and diseases. The work explains the interplay between the biological clock, the transcriptional feedback loop, and neuroscience, where they identified genes and proteins that work together both in humans and other animals.This article describes the link between biological clock disruption and consequent neurodegeneration and also highlights the signi ficance of biological clock modulators for possible clinical interventions in neurological disorders.
The biological clock of ~24 hours is an internal timekeeping mechanism which controls most of the body functions. The hypothalamic suprachiasmatic nucleus (SCN) is the master regulator of the biological clock, which coordinates functioning of various organs viz., brain, liver, kidney, and heart. Variations in sleep, metabolism, and hormone together determine daily circadian oscillation patterns. Disruption of the biological clock(e.g., people working in night shift) significantly increases the risk of developing various diseases such as neurodegenerative disorders, metabolic disorders, cardiovascular disease, and cancer (Cermakian and Boivin, 2003), which suggests that biological clock-controlled actions play indispensable roles in human physiology.
Clock disruption correlates with progressive neurodegeneration:Studies have shown that patients with neurodegenerative brain disorders such as Alzheimer’s disease (AD), Parkinson’s disease (PD), and Huntington’s disease (HD) are associated with disrupted biological clock (Musiek, 2015). The biological clock abnormalities comprised delayed sleep, impaired cortisol and thermoregulation rhythm, the reduction of melatonin levels during the night, and impaired expression of the CLOCK genes(e.g., Bmal1, Rev-Erb, Per1, Per2, Cry1 and Cry2). As shown inFigure 1, the biological clock is controlled at molecular level via feedback loop mechanism by a group of CLOCK genes in~24 hours. The CLOCK genes control a variety of biological functions viz., sleep-wake cycle, cognitive functions, immune responses, and response to oxidative stress. Importantly, disruption of the CLOCK leads to increased oxidative stress, in flammation and synaptic loss that contribute directly to neurodegeneration and loss of cognitive functions. In this context, Karatsoreos and coworkers have demonstrated a connection between clock dysfunction and neurodegeneration, wherein chronic disruption of CLOCK in mice via shifting light:dark (20:4) cycles leads to loss of cognitive functions, suggesting that impaired biological clock contribute in neurodegeneration (Karatsoreos et al., 2011).The findings are supported by the loss of expression and mutual correlations of the CLOCK genes in clinical and preclinical AD(Wu et al., 2006). Functional disruption of the SCN might be responsible for the loss of expression patterns of the CLOCK genes(Wu et al., 2006). Studies have shown that during AD the melatonin levels are profoundly depleted while its supplementation provides neural protection in experimental ischemia, AD, and PD (Reiter et al., 2004). A report by Song et al. (2015) showed that five familial AD mutations (5XFAD) mice (an experimental model of AD) have abundant amyloid deposits in their brain,and the amyloid β (Aβ) critically induce impairment of the biological clock. In addition, the biological clock also controls the functioning of lymphoid tissues viz., spleen, lymph nodes,and resident macrophages (e.g., microglia). In our study, we demonstrated that Aβ and lipopolysaccharide initiate neurotoxic inflammatory response through microglial activation, and we proposed that TLR4 antagonism, inhibition of JNK/p38-MAPK and CD40 stimulation could provide neuroprotection (Gaikwad et al., 2017). Further, evidences suggest a tight interplay between immune system and biological clock can control the disease outcome (Dumbell et al., 2016). In this complex scenario, it is possible that the microglia-mediated excessive production of inflammatory cytokines could have an influence on biological clock and sleep, which might play a role in regulation of neurodegenerative disorders. However, more detailed investigation of this system is warranted.
Clock dysfunction in fluences the pathogenesis of neurological disorders:Pathophysiological mechanisms of impairment of biological clock and neurodegeneration have been well documented in the AD (Musiek, 2015). Neuronal loss in the SCN and loss of melatonin production by pineal gland are the major contributors in impairment of biological clock in the AD patients (Wu et al., 2006). It is clear that the prolonged disrupted biological clock negatively influences health via impairment of immune responses, stress responses and metabolism in the brain (Musiek,2015), which may exacerbate the pathogenesis of neurodegenerative disorders. Notably, single nucleotide polymorphisms (SNPs)of bmal1 and per1 are associated with increased risk of PD.Furthermore, the clock genes (e.g., presenilin-2) regulate the expression of other genes that have direct implication in the pathogenesis of neurodegenerative disorders. Evidence suggests that the impaired biological clock may contribute to pathogenesis of neurodegenerative disorders through impaired metabolism and increased oxidative stress in the brain, a well-known contributor for neurodegeneration. The biological clock regulates oxidative stress via melatonin, an efficient scavenger of free radicals (Reiter et al., 2004). Restoration of the biological clock in a mouse model of neurodegenerative disease using pharmacological intervention, scheduled-feeding as well as management of sleep-wake cycles shown to rejuvenate oscillation of CLOCK genes in the SCN which leads to improvements in neurological function (Pallier et al., 2007; Maywood et al., 2010). These observation affirms that impairment of biological clock contributes to the pathogenesis of neurodegenerative disorders.
Clock targeted intervention for neurodegenerative disorders:As the impaired biological clock is involved in the pathogenesis of neurodegenerative disorders, restoring the biological clock could ameliorate the symptoms, or prevent the disease.In this context, a number of biological clock targeted therapies have been investigated for possible therapeutic interventions.In patients with PD, daily light exposure has shown to improve sleep/wake rhythms through reducing daytime sleepiness and increasing daytime activity (Videnovic et al., 2017). Further,light exposure regimens lead to improvements in daily living activities in patients with PD and severe dementia (Forbes et al.,2014). Timed light therapy achieves improvements in cognitive functions mechanistically via master clock restoration, which helps protect against oxidative stress and in flammation (Figure 1). Strategies directed at normalizing biological clock might provide novel therapeutic interventions. Therefore, the biological clock could be a novel therapeutic target and regulators of the master clock (e.g., light, melatonin, food intake pattern) could be employed in the future to treat neurological disorders. However,there is still no sufficient evidence to conclude bene fits of light therapy on long-lasting cognitive or motor functions.
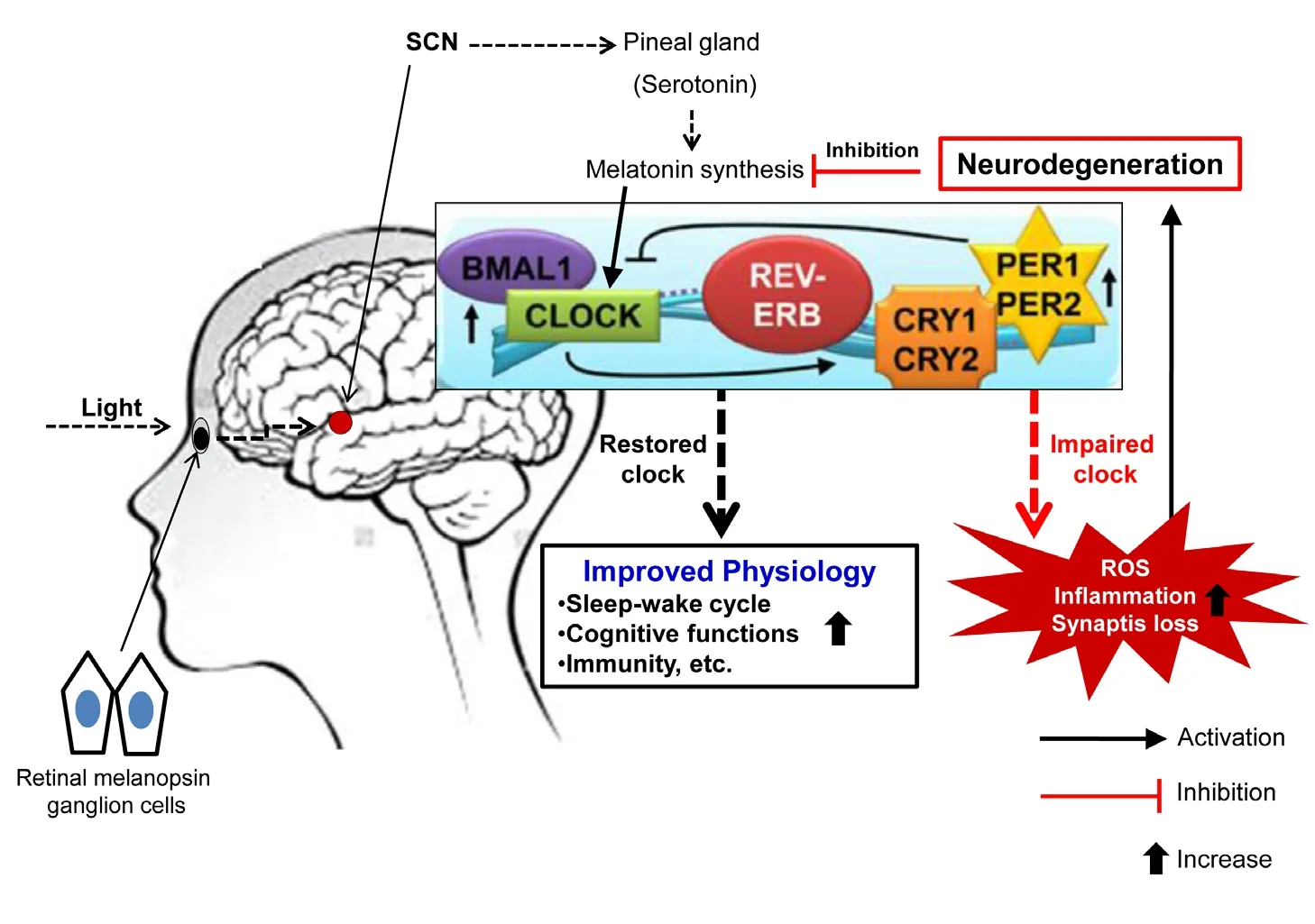
Figure 1 The biological clock impairment in fluences neurodegeneration and the potential effect of timed light therapy on restoration of biological clock in patients with neurodegenerative disorders.
In conclusion, the biological clock has an in fluence on human physiology, which is involved in the pathogenesis of neurological disorders. Thus environmental and pharmacological interventions to restore the master clock could be an effective strategy to ameliorate or prevent neurological disorders.
The study was financially supported by DST-SERB (PDF/2016/001369). The Author acknowledges Dr. Birendra Prusty, Dr. A. Raj Kumar Patro and Dr.Diwakar Singh for helpful discussion.
Sagar Gaikwad*
Institute of Life Sciences, NALCO Square, Bhubaneswar, India
orcid:0000-0003-0240-1357 (Sagar Gaikwad)
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-Shar-eAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under identical terms.
Open peer review reports:
Reviewer 1: Alessandra Bitto, University of Messina, Italy.
Comments to author: The article is generally sound and of interest.
Reviewer 2: Willian Orlando Castillo, Universidade de Sao Paulo, Brazil.
Comments to author: It has its merit and represents a valuable contribution to the literature.
Reviewer 3: Sage Arbor, Marian University College of Osteopathic Medicine, USA.
Comments to author: The topic of targeting the circadian clock to ameliorate neurodegeneration is a interesting topic.
Reviewer 4:Paulina Carriba, Cardiff University, UK.
Comments to author: This article is a perspective on the in fluence of the biological clock with neurological alterations. The article is principally very interesting, well-organized and correctly written.
Cermakian N, Boivin DB (2003) A molecular perspective of human circadian rhythm disorders. Brain Res Brain Res Rev 42:204-220.
Dumbell R, Matveeva O, Oster H (2016) Circadian clocks, stress, and immunity. Front Endocrinol (Lausanne) 7:37.
Forbes D, Blake CM, Thiessen EJ, Peacock S, Hawranik P (2014) Light therapy for improving cognition, activities of daily living, sleep, challenging behaviour, and psychiatric disturbances in dementia. Cochrane Database Syst Rev:CD003946.
Gaikwad S, Patel D, Agrawal-Rajput R (2017) CD40 negatively regulates ATP-TLR4-activated in flammasome in microglia. Cell Mol Neurobiol 37:351-359.
Karatsoreos IN, Bhagat S, Bloss EB, Morrison JH, McEwen BS (2011)Disruption of circadian clocks has rami fications for metabolism, brain,and behavior. Proc Natl Acad Sci U S A 108:1657-1662.
Maywood ES, Fraenkel E, McAllister CJ, Wood N, Reddy AB, Hastings MH, Morton AJ (2010) Disruption of peripheral circadian timekeeping in a mouse model of Huntington’s disease and its restoration by temporally scheduled feeding. J Neurosci 30:10199-10204.
Musiek ES (2015) Circadian clock disruption in neurodegenerative diseases: cause and effect? Front Pharmacol 6:29.
Pallier PN, Maywood ES, Zheng Z, Chesham JE, Inyushkin AN, Dyball R,Hastings MH, Morton AJ (2007) Pharmacological imposition of sleep slows cognitive decline and reverses dysregulation of circadian gene expression in a transgenic mouse model of Huntington’s disease. J Neurosci 27:7869-7878.
Reiter RJ, Tan DX, Pappolla MA (2004) Melatonin relieves the neural oxidative burden that contributes to dementias. Ann N Y Acad Sci 1035:179-196.
Song H, Moon M, Choe HK, Han DH, Jang C, Kim A, Cho S, Kim K,Mook-Jung I (2015) Abeta-induced degradation of BMAL1 and CBP leads to circadian rhythm disruption in Alzheimer’s disease. Mol Neurodegener 10:13.
Videnovic A, Klerman EB, Wang W, Marconi A, Kuhta T, Zee PC (2017)Timed light therapy for sleep and daytime sleepiness associated with Parkinson disease: a randomized clinical trial. JAMA Neurol 74:411-418.
Wu YH, Fischer DF, Kalsbeek A, Garidou-Boof ML, van der Vliet J, van Heijningen C, Liu RY, Zhou JN, Swaab DF (2006) Pineal clock gene oscillation is disturbed in Alzheimer’s disease, due to functional disconnection from the “master clock”. FASEB J 20:1874-1876.
- 中国神经再生研究(英文版)的其它文章
- Optic radiation injury in patients with aneurismal subarachnoid hemorrhage: a preliminary diffusion tensor imaging report
- Cerebral ischemia and neuroregeneration
- SNARE complex in axonal guidance and neuroregeneration
- Heterozygous carriers of galactocerebrosidase mutations that cause Krabbe disease have impaired microglial function and defective repair of myelin damage
- The relaxin peptide family – potential future hope for neuroprotective therapy? A short review
- Roles of neural regeneration in memory pharmacology