Synthesis, Crystal Structure, Antitumor Activities and Docking Study of 1-(2-(1H- Indol-3-yl)ethyl)-3-(2-methoxyphenyl)urea①
HU Chun-Hong WEI Dui-Dui, b SUN Xio-Fei LIN Rui-Li HU Min-Min TANG Lei WANG Zhen HE Din
Synthesis, Crystal Structure, Antitumor Activities and Docking Study of 1-(2-(1- Indol-3-yl)ethyl)-3-(2-methoxyphenyl)urea①
HU Chun-HongaWEI Dui-Duia, bSUN Xiao-FeiaLIN Rui-LiaHU Min-MinaTANG LeiaWANG ZhenaHE Diana②
a(730000)b(730000)
The title compound, 1-(2-(1-indol-3-yl)ethyl)-3-(2-methoxyphenyl)urea (C18H19N3O2,M= 309.36) has been synthesized, and its structure was characterized by1H-NMR,13C-NMR, ESI-MS and single-crystal X-ray diffraction. It crystallizes in the monoclinic space group21/with= 16.2774(15),= 11.1082(10),= 9.0819(3) Å,= 103.09(9)°,= 1599.5(3) Å3,= 4,= 293(2) K,(Mo) = 0.086 mm-1,D= 1.285 g/cm3,(000) = 656 and= 0.981. 5973 reflections were measured (7.04≤2≤52.04°), and 3143 were unique (int= 0.0393,sigma= 0.0546) and used in all calculations. The final= 0.0756 (> 2()) and= 0.1976 (all data). The antitumor activity of the title compound was analyzed by MTT assay. Meanwhile, to rationalize its potencies in the CDK4 target, the title compound was docked into CDK4 protein and the interactions with the active site residues were analyzed.
crystal structure, synthesis, antitumor activity, molecular docking;
1 INTRODUCTION
Fascaplysin (1,Scheme 1) is a fused benzoyl- linked-carbolinium alkaloid isolated from marine spongecollected in the South Pacific near theIsland as a unique antimicrobial pigment[1]. It is reported to possess antimicrobial, antimalarial, and antiacetylcholinstere activities[2-5]. Recently,fascaplysin has exhibited potent cytotoxicity against small cell lung cancer cells and induces cell cycle arrest in G0/G1 at lower concentrations and in S-phase at higher concentra- tions as the specific inhibitor of CDK4-cyclin D1 (IC50for CDK4-cyclin D1 = 0.35 μM)[6, 7]. CyclinD1 and CDK4 genes are often amplified or overex- pressed in many types of cancers, intimating that CDK4-cyclin D1 kinase would be a good break- through point in the cancer treatment[8].
Nonetheless, it is reported that fascaplysin itself has limited potential as an anti-cancer drug due to its toxic side effects. A possible explanation is the potency for its planar structure to intercalate with DNA[9]. So many researchers devote their efforts to design a series of non-planar fascaplysin-based derivatives[10-12]. Hence, to devise a potent, non-toxic (non-planar) CDK4 inhibitor based on fascaplysin, our strategy is to open C and D rings of fascaplysin and the urea linkages bond can increase the rigidity of the title compound, as shown in Scheme 1.Then the title compound was designed and synthesized. The single-crystal X-ray diffraction analyses indicate that the title compound is not coplanar from a molecular level.Docking analysis has been per- formed to find out the binding affinity between the title compound and cyclin dependent kinases enzyme (PDB ID: 2w96).In this paper, we describe the synthesis of the title compound in a proper way (Scheme 2), and also focus on its crystal structure and antitumor activity.
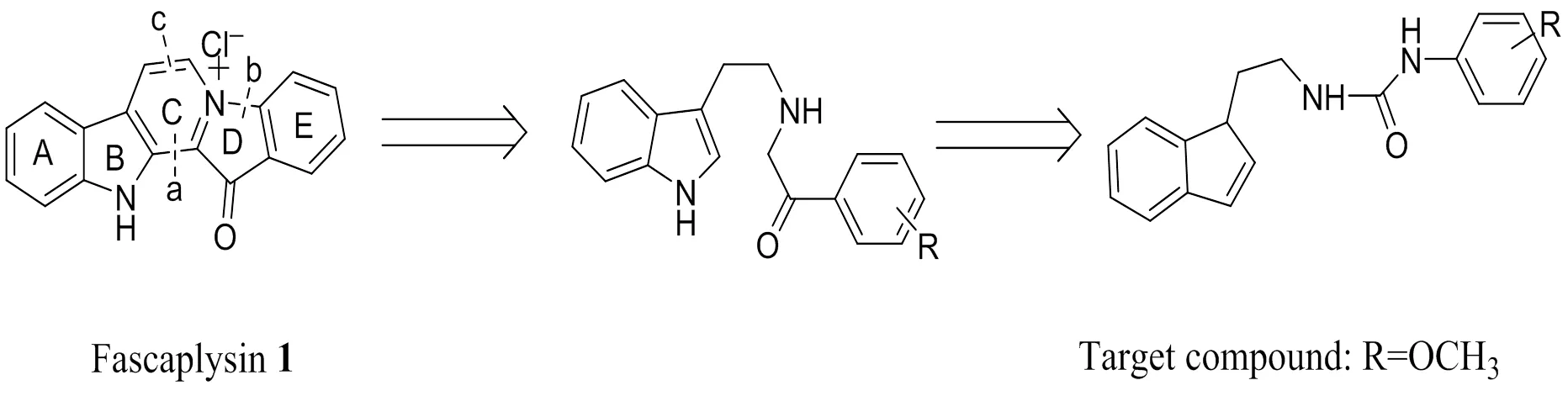
Scheme 1. Fascaplysin 1 and the target compound
2 EXPERIMENTAL
2. 1 Instruments and reagents
All organic solvents and materials were obtained from commercial suppliers and used with further purification. Melting points were determined by using the electrothermal PIF YRT-3 apparatus without correction.1H-NMR and13C-NMR (ppm) spectra were measured on a Varian Mercury (400 MHz) using TMS as the internal standard. Mass spectra were recorded on a VGZAB-HS (70ev) spectrometer with ESI source as ionization. Crys- tallographic data of this compound were collected by a Super Nova, Dual, Cu at zero, Eos diffractometer.Molecular modeling and protein structure optimiza- tion were performed for crystal structure of CDK4 (PDB ID: 2w96), and FlexX software was used for flexible docking.
2. 2 General procedure
Compound 2 (4 mmol, 0.492 g) and triethylamine (14 mmol, 1.41 g) were treated with bis(trichloro- methyl) carbonate (2 mmol, 0.59 g) in 1,2-dichlo- roethyl (20 mL) and stirred for 30 min in an ice-bath. Then the mixture was stirred for 4 h at 85°C. After full reaction, the mixture was obtained by filtration and the filtrate was evaporated, and then compound 3 as obtained (0.70 g, 80% yield). Compound 3 was dissolved in 1,2-dichloroethane (20 mL), to which tryptamine (4 mmol, 0.64 g) and triethylamine(5 mmol, 0.50 g) were added and stirred for 24 h, and the reaction was monitored by TLC. After filtration, the filtrate was concentrated under reduced pressure, and the residue was purified via silica gel column chromatography (petroleum ether:dichlorome- thane:ethyl acetate = 4:2:1).Then compound 4 (white solid, 60% yield) was obtained,m.p.: 154°C.1H NMR (400 MHz, DMSO-d6, TMS, ppm):10.83 (s, 1H, NH), 8.11 (dd,= 7.4, 2.3 Hz, 1H, Ar-H), 7.92 (s, 1H, NH), 7.56 (d, J = 7.8 Hz, 1H, Ar-H), 7.34 (d,= 8.1 Hz, 1H, Ar-H), 7.17 (s, 1H, Ar-H), 7.09~7.04 (m, 1H, Ar-H), 7.01~6.97 (m, 1H, Ar-H), 6.95 (dd, J = 3.3, 1.6 Hz, 1H, Ar-H), 6.94~6.93 (m, 1H, Ar-H), 6.91 (s, 1H, NH), 6.86~6.82 (m, 1H, Ar-H), 3.81 (s, 3H, CH3), 3.43~3.35 (m, 2H, CH2-NH), 2.84 (t, J = 7.1 Hz, 2H, CH2).13C NMR (100 MHz, DMSO-d6,TMS, ppm):155.75, 147.81, 136.82, 130.12, 127.79, 123.27, 121.44, 121.36, 120.99, 118.86, 118.75, 118.53, 112.36, 111.89, 111.05, 56.13, 41.28, 26.39. ESI-MS: Calcd. for C18H19N3O2[M+H]+:310.1477. Found: 310.1556.

Scheme 2. Reagents and conditions: (a) BTC, TEA, ClCH2CH2Cl, 85 ℃, 4 h; (b) TEA, ClCH2CH2Cl, rt 24 h
2. 3 Structure determination
The clear white crystal of the title compound was obtained by recrystallization from ethyl acetate-petroleum ether (1:6 = v/v). The crystal with dimensions of 0.21mm × 0.17mm × 0.15mm was chosen for X-ray diffraction analysis. The data were collected on a Super Nova, Dual, Cu at zero, Eos diffractometer equipped with graphite-monochroma- tic Moradiation (= 0.7107 Å) at 293(2) K. The structure was solved by direct methods with ShelXS[13]and refined with ShelXL[14]refinement package using Least-squares minimization. All hydrogen atoms were positioned geometrically and refined using a riding model, withU(H) = nU(carrier atom), where n = 1.5 for the methyl hydrogens groups and 1.2 for the C(H) groups, C(H, H) groups and all N(H) groups. The final(reflections) = 0.0756,= 0.1263 (= 1/[2(F2) + (0.0362)2+ 0.00], where= (F2+ 2F2)/3),= 0.982, (Δ/)max= 0.000, (Δ/)max= 0.18 and (Δ/)min= –0.22 e/Å3.
2. 5 Molecular docking
The X-ray crystallography structure of CDK4 inhibitor protein is downloaded from protein data bank (PDB ID: 2w96)[15],and further the water molecule was removed, hydrogen was added, and energy was minimized using FlexX2.1.8 software in LeadIT. The ligand was set for docking using a 3D structure in a quantitative way and then the molecule was allowed for semi-flexible docking. And the docking result was viewed with the PYMOL.
2. 4 Pharmacological tests
The bioactivities of the title compound were tested by MTT assay[16]. HeLa, A549, HepG2 and WI-38 were selected for the experiment with fascaplysin as the positive control. All cell lines were kept at 37°C in 5% CO2in RPMI-1640 medium, supplemented with 10% fetal calf serum. The solutions of the compound with different concentrations were added into 96-well plates after 24 h with fascaplysin as the positive group. After 48 h, 10 μL of MTT solution (5 mg/mL in PBS) was added into each well and incubated for 4 h. Then, the medium was removed and 150 μL DMSO was added to dissolve the blue-colored formazan in 10 min. The absorbance was detected at 570 nm to calculate the inhibition rate (%) and the IC50was defined.
3 RESULTS AND DISCUSSION
3. 1 Synthesis and characterization
The structure of the title compound 5 was confirmed by the1H NMR,13C NMR and EI-MS spectra. In the1H NMR spectrum, the ratio of each integral area is consistent with expected number of protons in each group.1H NMR exhibited a characteristic single at10.83 ppm assigned to the proton of NH group of indole ring. In addition, the signals at7.92 and 7.56 ppm were also assigned to the proton of NH group ofurea linkage. In the13C NMR spectrum, the carbon atoms of carboxyl can be identified. The distinctive peak at 155.75 was corresponding to the carbonyl carbon.And the ESI-MS spectrum showed the molecular ion peak as the base peak at/310.1556 [M+H]+.
推动土地确权的一个重要原因是出现了村社集体侵犯农民土地承包权的现象,而且这种侵犯往往是在绝大多数村民的强烈要求下由村社集体实施的,这种侵犯引发了个别农户上访。为防止农民土地承包权被侵犯及减少农民上访,国家试图通过限制村社集体调整土地的权力来减少土地调整中的矛盾与冲突。这样一来,后果就是村社集体失去回应农民生产生活诉求的能力,一个积极、主动的具有主体性的集体变成一个被动的客体,成为被各种规范所束缚的缺少活力的执行上级命令完成上级任务的下级。充当国家与农民对接平台的灵活的基层组织因此不再存在,国家直接面对分散的农户,并因此使得国家失去了对基层简约有效的治理能力。
3. 2 Crystal structure description of the compound

The selected bond lengths, bond angles and torsional angles are shown in Tables 1 and 2, respectively. And the hydrogen bonds are given in Table 3. The molecular structure of the title compound with atomic numbering scheme is shown in Fig. 1, and Fig. 2 depicts the molecular packing and hydrogen bonds in a unit cell. In the crystal, the bond lengths of C(11)–N(2) (1.353(2) Å), C(11)–N(3) (1.374(5) Å) and C(1)–N(1) (1.367(6) Å) are obviously shorter than the typical C–N (1.47 Å) due to the P-π conjugating effort, whereas it approxi- mates to the distance for C=N bond (1.350 Å). In addition, the sum of N(2)–C(11)–O(1), N(3)–C(11)– O(1) and N(2)–C(11)–N(3) angles is 360°, which means sp2 hybridization state of the C(11) atom. What’s more, the indole ring plane (C(1)–C(2)– C(3)–C(4)–C(5)–C(6)–C(7)–C(8)–N(1)) and the phenyl ring (C(12)–C(17) are twisted with the dihedral angle being 76.661(135)°,which means that benzene plane and the indole ring plane are not coplanar. Besides, the torsion angle of N(3)–C(11)– N(2)–C(10)–N(3) is 175.8(4)°. Furthermore, the intermolecular hydrogen-bonding interaction N(2)– H(2)···O(1) and N(3)–H(3)···O(1) in the crystal link two molecules together. Moreover, they were further linked together to form an extensive network by C(10)–H(10A)×××π (H(10)–Cg(2) = 2.66 Å), C(18)– H(18C)×××π (H(18C)–Cg(3) = 2.88 Å), C(17)– H(17)×××π (H(17)–Cg(3) = 2.95 Å)and weak π-π stacking. Among them, the hydrogen bonds between nitrogen atom as donors and oxygen atoms as acceptors, N(2)–H(2)×××O(1), with N(2) to O(1) distance of 2.889 Å help to stabilize the structure to the extreme.
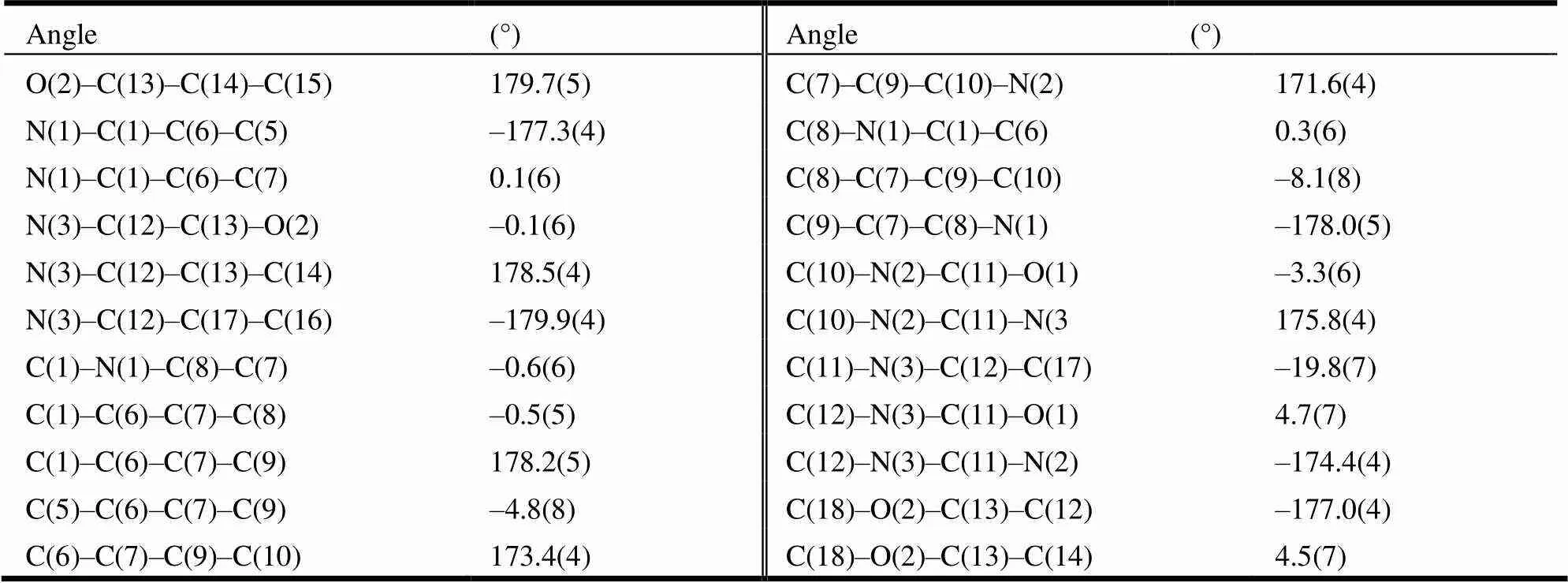
Table 2. Torsional Angles (°) for the Target Compound

Table 3. Hydrogen Bond Lengths (Å) and Bond Angles (°)
Symmetry codes: #1:, –+1/2,–1/2; #2:, –+1/2,–1/2; #3:1–,, 1#4: –, 1–, –. Cg (2):C(1)~C(6); Cg(3):C(12)~C(17)

Fig. 1. Structure of the title compound
Fig. 2. Fragment of the crystal packing of the title compound
3. 3 Binding site analysis
In order to understand the binding confirmation of the compound, the molecular docking study was performed to fit fascaplysin and the title compound into the active center of 2w96. The title compound and fascaplysin in complex with CDK4 were selected specially for interaction analysis. Docking results revealed that fascaplysin was observed to form hydrogen bond with H atom of Val 96 and the O atom of Asp97 and the title compound was observed to form hydrogen bond with H atom of Val 96 and Ile12. Both of the two compounds form hydrogen bond between the indole NH and the carbonyl group of valine 96. Glu94, His95, Val96, Asp97, and Asp99 were considered essential of inhibitory active in the previous study[17]. To obtain more detailed informa- tion on the interactions between the two compounds and CDK4,binding free energies were calculated using Autodock software. Fascaplysin and the title compound exhibited low binding energies of –3.79 and –3.56 kcal/mol. Such negative values for binding energies indicate high binding affinity and thermo- dynamic stability. We can therefore predict that the title compound would also have good CDK4- inhibitory activity. The interactions involved in the active site region between the protein and the title compound and fasacaplysin were displayed in Fig. 3, showing the binding mode of the title compound interacting with CDK4 protein. These preliminary results suggest that the title compound might exhibit inhibitory activity against CDK4.
3. 4 Biological activities
The antiproliferative activity of the title compound was evaluated against three different tumor cells and toxicity for normal cell with fascaplysin as the positive control, and the results are summarized in Table 4. The preliminary screening results indicate that the title compound presents good inhibition profile against A549, Hela and HepG2 with the values of IC50to be 77.02, 100.04, 51.07 μM. Moreover, the inhibitory to HepG2 was the most remarkable and the toxicity to WI-38 was much lower compared that to the fascaplysin.
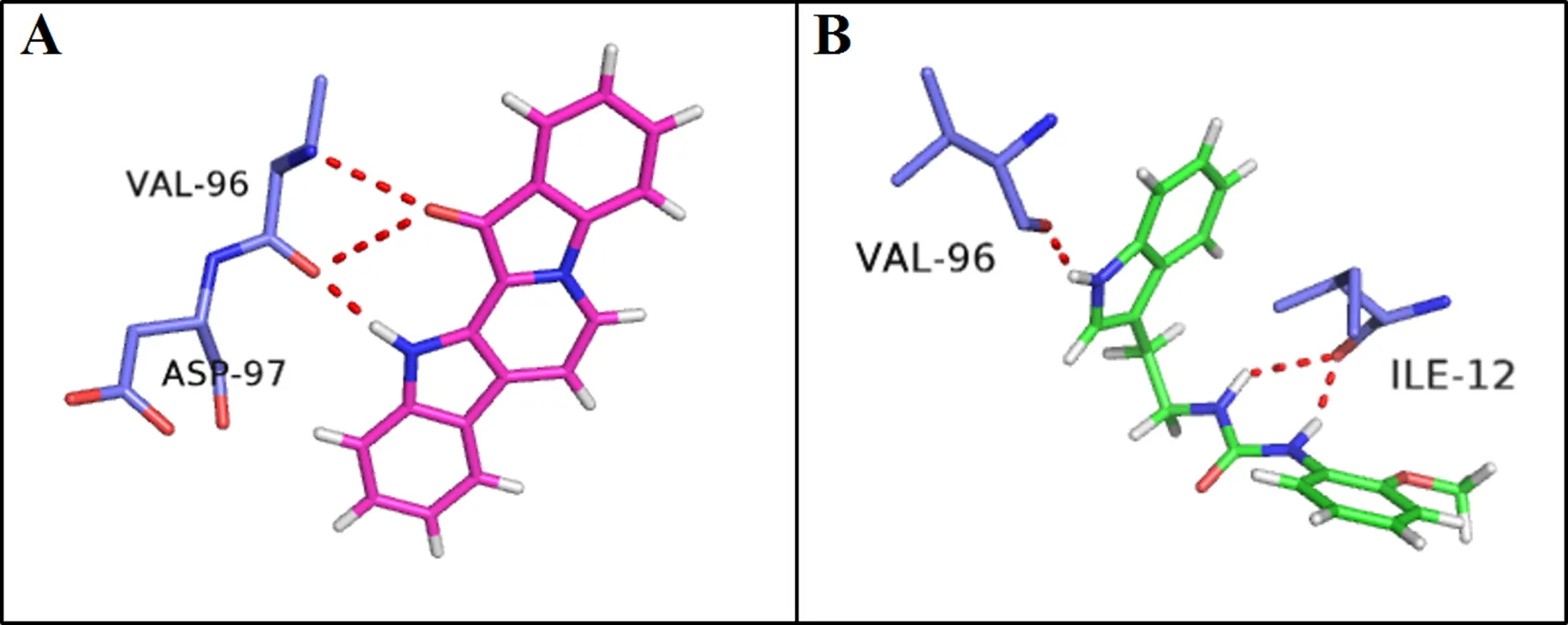
Fig. 3. Fascaplysin (A) and the title compound (B) docked with the CDK4 model

Table 4. Inhibition of the Cell Growth (μM)
4 CONCLUSION
In the above study, the title compound has been synthesized and characterized by the1H NMR,13C NMR and EI-MS spectra. The crystal structure of the title compound has been determined. The biological activity test shows that the title compound has better activity against HepG2 with IC50of51.07 μM and has lower toxicity to WI-38. Docking study shows thatthe title compound exhibits inhibitory activity against CDK4. Thus, it can be concluded that this kind of compound was worth exploring and further studies on the structure activity relationships and structural modifications of these compounds are under way.
(1) Roll, D. M.; Ireland, C. M.; Lu, H. S. M.; Clardy, J. Fascaplysin, an unusual antimicrobial pigment from the marine sponge fascaplysinopsis.. 1988, 53, 4201−4202.
(2) Segraves, N. L.; Robinson, S. J.; Garcia, D.; Said, S. A.; Fu, X.; Schmitz, F. J.; Pietraszkiewicz, H.; Valeriote, F. A.; Crews, P. Comparison of fascaplysin and related alkaloids: a study of structures, cytotoxicities, and sources.2004, 67, 783−792.
(3) Bharate, S. B.; Manda, S.; Mupparapu, N.; Battini, N.; Vishwakarma, R. A. Chemistry and biology of fascaplysin, a potent marine-derived CDK-4 inhibitor.. 2012, 12, 650−664.
(4) Bharate, S. B.; Manda, S.; Joshi, P.; Singh, B.; Vishwakarma, R. A. Total synthesis and anti-cholinesterase activity of marine-derived bis-indole alkaloid fascaplysin.. 2012, 3, 1098−1103.
(5) Dian, H. E.; Yang, Z. Q.; Hou, M.Synthesis, crystal structure and antitumor activities of N-(2-(1-indol-3-yl)ethyl)-2-nitroaniline.. 2014, 12, 1784−1788.
(6) Soni, R.; Muller, L.; Furet, P.; Schoepfer, J.; Stephan, C.; Zumstein-Mecker, S.; Fretz, H.; Chaudhuri, B. Inhibition of cyclin-dependent kinase 4 (CDK4) by fascaplysin, a marine natural product.2000, 275, 877−884.
(7) Fretz, H.; Ucci-Stoll, K.; Hug, P.; Schoepfer, J.; Lang, M. Investigations on the reactivity of fascaplysin, part I, aromatic electrophilic substitutions occur at position 9.2000, 83, 3064−3068.
(8) Aubry, C.; Patel, A.; Mahale, S.; Chaudhuri, B.; Marchal, J. D.; Sutcliffe, M. J.; Jenkins, P. R. The design and synthesis of novel 3-[2-indol-1-yl-ethyl]-1H-indole derivatives as selective inhibitors of CDK4.2005, 46, 1423−1425.
(9) Hörmann, A.; Chaudhuri, B.; Fretz, H. DNA binding properties of the marine sponge pigment fascaplysin. 2001, 9, 917−921.
(10) Aubry, C.; Jenkins, P. R.; Mahale, S.; Chaudhuri, B.; Marchal, J. D.; Sutcliffe, M. J. Design, synthesis and biological activity of new CDK4-specific inhibitors, based on fascaplysin.2006, 4, 787−801.
(11) Garcia, M. D.; Wilson, A. J.; Emmerson, D. P.; Jenkins, P. R.; Mahale, S.; Chaudhuri, B. Synthesis, crystal structure and biological activity of-carboline based selective CDK4-cyclin D1 inhibitors.2006, 4, 4478−4484.
(12) Jenkins, P. R.; Wilson, J.; Emmerson, D.; Garcia, M. D.; Smith, M. R.; Gray, S. J.; Chaudhuri, B. Design, synthesis and biological evaluation of new tryptamine and tetrahydro--carboline-based selective inhibitors of CDK4.2008, 16, 7728−7739.
(13) Sheldrick, G. M.. University of Göttingen, Germany 1997.
(14) Sheldrick, G. M.. University of Göttingen, Germany 1997.
(15) Day, P. J.; Cleasby, A.; Tickle, I. J. Crystal structure of human CDK4 in complex with a D-type cyclin.2009, 106 4166.
(16) Liu, X. F.; Zhang, Y.; Li, J.; Zhao, J. S.; Xi, N.; He, D. Synthesis and crystal structure of N-((3-(2-nitrophenyl)propanoyl)oxy)-N-phenylbenzamide.2015, 34, 688–694.
(17) Sivashanmugam M, Raghunath C, Vetrivel U. Virtual screening studies reveal linarin as a potential natural inhibitor targeting CDK4 in retinoblastoma.2013,4 256–264.
19 September 2017;
5 December 2017 (CCDC 1574685)
① This paper was supported by the Lanzhou Science and Technology Bureau Program Funds (2016-3-108)
. E-mail: hed@lzu.edu.cn, Tel.: +8613008738922
10.14102/j.cnki.0254-5861.2011-1832
- 结构化学的其它文章
- Crystal Structures, Luminescent Properties and Hirshfeld Surface Analyses of Zn(II) and Cd(II) Compounds Based on 1-(2-Carboxylphenyl)-3-(pyridin-2-yl)pyrazole①
- Investigation of the Substituent Effects on π-Type Pnicogen Bond Interaction①
- Structural and Mechanistic Studies of γ-Fe2O3 Nanoparticle as Capecitabine Drug Nanocarrier①
- Theoretical Study on the Mechanism of a New Synthesis Reaction of 1,3,5-Substituted-1,2,4-triazoles by Carboxylic Acids, Amidines, and Hydrazines①
- Local Structure Mediation and Photoluminescence of Ce3+- and Eu3+-Codoped YAG Nanophosphors①
- Syntheses, Crystal Structures and Characterization of Two Coordination Polymers Based on Mixed Ligands①

