A New Zn(II) Coordination Polymer Constructed from 1,3,5-Benzenetricarboxylate Ligand Exhibiting Photoluminescence①
LI Jia-Xing YANG Hong-Li NI Meng ZHANG Shu-Hua ZHANG Xiu-Qing
A New Zn(II) Coordination Polymer Constructed from 1,3,5-Benzenetricarboxylate Ligand Exhibiting Photoluminescence①
LI Jia-Xing YANG Hong-Li NI Meng ZHANG Shu-Hua②ZHANG Xiu-Qing②
(()541004)

1,3,5-benzenetricarboxylic acid, Zn(II) complex, crystal structure, fluorescence;
1 INTRODUCTION
Coordination polymers attract interest because of their fascinating coordination architectures and po- tential applications, for example, in gas storage, cata- lysis, luminescence, biological activity and magnetic properties[1-6]. Generally, the self-assembly of coordi- nation polymers is mainly affected by the combi- nation of many factors, including temperature[7], the neutral ligands, the organic anions and the metal atoms[8-10]. Among these factors, great efforts have been devoted to the design of suitable organic ligands to construct new coordination polymers.
Organic ligands that contain carboxylic acid groups are frequently used in coordination polymers since the carboxylate group has excellent coordina- tion capability and flexible coordination patterns, which result in a wide diversity of structures. 1,3,5- Benzenetricarboxylic acid (H3BTC), a symmetric aromatic polycarboxylic acid derivative, has been used as a bridging ligand in the synthesis of novel coordination polymers[11-14]. Many workers have studied 1,3,5-benzenetricarboxylic acid complexes, so we want to further study the causes of their com- plexes[15, 16]. We explored the self-assembly of Zn(II) cation and H3BTC under hydrothermal conditions, and obtained a new coordination polymer {[Zn2(BTC)(HBTC)(dpa)(Hdpa)]·3H2O}n(1). We here report its synthesis, crystal structure and fluorescence property.
2 EXPERIMENTAL
All reagents and solvents were used as obtained commercially without further purification. The crystal structure was determined on an Agilent G8910A CCD diffractometer.The IR spectrum was recorded from a KBr pellet in the range of 4000~400 cm-1with a Bio-Rad FTS-7 spectrophotometer. The luminescence spectra for the solid samples were recorded at room temperature on a RF-4600 fluore-scence spectrophotometer under the same conditions. Powder X-ray diffraction (XRD) data were collected on a Bruker D8 Advance X-ray diffractometer with Curadiation (= 1.5418 Å).
2. 1 Synthesis and crystallization
A mixture of 1,3,5-benzenetricarboxylic acid (0.042 g, 0.2 mmol), Zn(NO3)2·6H2O (0.059 g, 0.2 mmol), dpa (0.017 g, 0.1 mmol), H2O (12 mL) and KOH (0.0285 g, 0.5 mmol) was placed in a 20 mL Teflon-lined stainless-steel vessel, heated to 413 K for 72 h and then cooled to room temperature. The final reaction mixture had a pH of 6.2. Colourless block shaped crystals of 1 were obtained (yield 37%, based on dpa). Analysis calculated for C38H26N6O12Zn2(%): C, 48.52; H, 2.82; N, 8.95. Found (%): C, 48.33; H, 2.76; N, 8.9. IR (KBr pellet, cm-1): 3412 (w), 1612 (s), 1517 (s), 1349 (s), 1199 (m), 1022 (m), 723 (m).
2. 2 Single-crystal X-ray structure determination
Single-crystal X-ray diffraction data for 1 were collected on an Agilent G8910A CCD diffractometer with Mo-radiation (= 0.71073 Å) at 293(2) K. Using Olex2[17], the structure was solved with the SHELXS[18]structure solution program using Patter- son Method and refined with the SHELXL[18]refine- ment package using Least-squares minimization. All non-hydrogen atoms were refined anisotropically. H atoms were treated by a mixture of independent and constrained refinement. For 1, a total of 6664 reflections were collected in the range of 3.31≤≤25.1°, of which 5880 were independent (int= 0.0208). The final= 0.0314 and= 0.0710 for observed reflections with> 2(), and= 0.0375,= 0.0749 for all data with (Δ)max= 0.574 and (Δ)min= –0.538 e·Å-3. Selected bond lengths and bond angles of 1 are shown in Table 1.
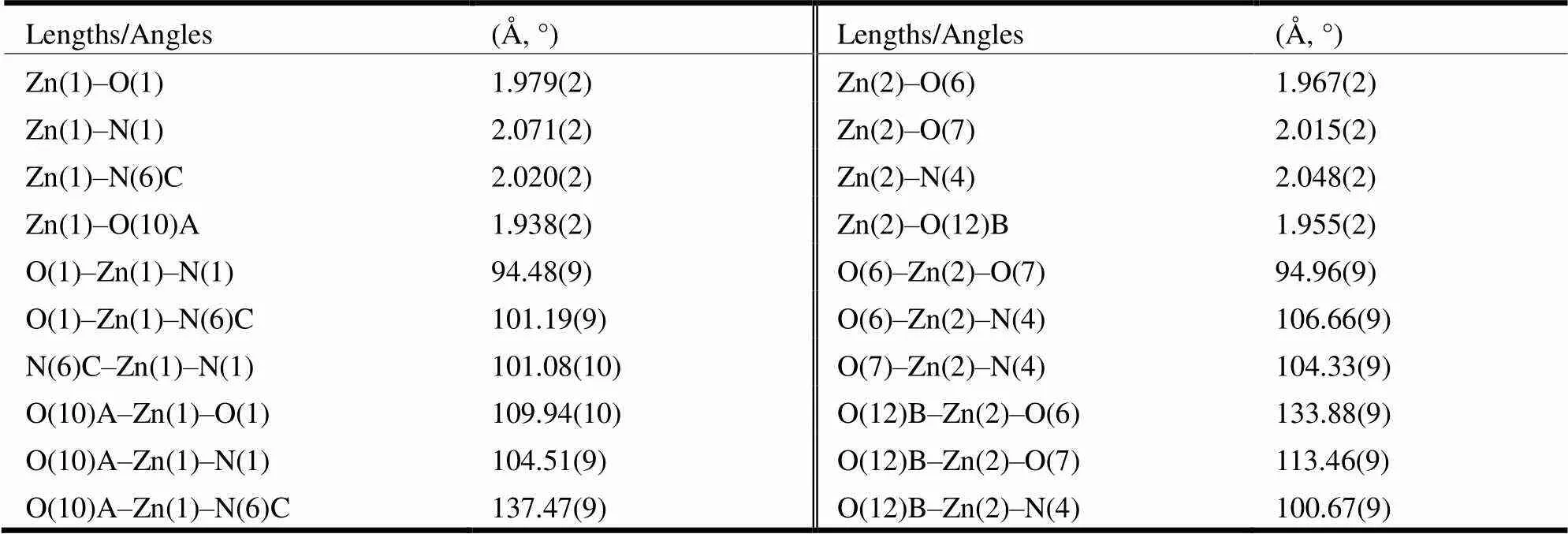
Table 1. Selected Bond Lengths and Bond Angles
Symmetry codes: (A) =+1,+1,; (B) = −, −−1, −; (C) = −, −, −+1
3 RESULTS AND DISCUSSION
3. 1 Structure analysis


Fig. 1(a). Molecular structure of complex 1
Fig. 1(b). Coordination environment of complex 1
The Zn centers are connected by BTC3−and dpa ligands to form a chain, and the two chains are connected by HBTC2-ligand to form a layer structure. The dpa ligand connects two Zn2+cations, and the Zn···Zn separation across the dpa ligand is 11.2191(3) Å. The one singly protonated HTBC2-ligand con- nects two Zn2+cations, and the Zn···Zn separation across the HBTC2-ligand is 7.9348 (5) Å. The completely deprotonated BTC3-ligand connects three Zn2+cations, and the Zn···Zn separations across the BTC3-ligand are 6.9332(4), 8.3741(3) and 9.4393(3) Å, respectively. The layers are connected by BTC3-ligand to form a bilayer structure (Fig. 1c).

Fig. 1(c). Bilayer structure of complex 1 (Hdpanot painted completely)
Complex 1 is stabilized by classical intrachain (N(2)–H(2)···O(8), N(3)–H(1)···O(14) and N(5)– H(5)···O(2)) hydrogen bonds between the 4,4΄-dipy- ridylamine molecules and carboxylate groups, and stabilized by classical intrachain (O(3)– H(3A)···O(15), O(13)–H(13A)···O(9), O(13)– H(13B)···O(5), O(14)–H(14A)···O(2), O(14)– H(14B)···O(4), O(15)–H(15A)···O(13), O(15)– H(15B)···O(8)) hydrogen bonds between carboxylate groups and free water molecules. The hydrogen bond lengths and bond angles for 1 are given in Table 2.

Table 2. Hydrogen Bond Lengths and Bond Angles
Symmetry codes: (i) = –, 1–, –; (ii) = 2–, 1–, 1–; (iii) = 1+, 1+,; (iv) = 1+,,
Moreover, there are-interactions in complex 1 between the pyridine ring of dpa ligand and the benzene ring of H3BTC ligand. The centroid- to-centroid distances between adjacent rings are 3.603(2), 3.6737(18), and 3.6737(18) Å for pyridine rings (N(3)C(24)C(25)C(26)C(27)C(28) and N(3΄)C(24΄)C(25΄)C(26΄)C(27΄)C(28΄) (2–, 1–, 1–)), benzene rings (C(2)C(3)C(4)C(6)C(7)C(9) and C(2΄)C(3΄)C(4΄)C(6΄)C(7΄)C(9΄) (–, 1–, –)), and (C(11)C(12)C(13)C(15)C(16)C(18) and C(11΄)C(12΄)C(13΄)C(15΄)C(16΄)C(18΄) (2–, 2–, 1–)), respectively. Thus, through hydrogen bonds and-interactions, the bilayer structure is further expanded into a three-dimensional structure (Fig. 1d) and plays an important role in stabilizing complex 1.

Fig. 1(d). Three-dimensional structure of complex 1 formed by hydrogen-bonding andinteractions
In the previously reported complexes [Zn- (HBTC)2(Hdpa)2] (2) and [Zn(BTC)(Hdpa)]n(3)[19], the HBTC2-has only one carboxyl group coordi- nation, and the Hdpa does not play the role in bridging Zn2+ions. But in complex 1, the HBTC2-and BTC3-have two and three carboxyl groups coordination, respectively. The dpa plays the role in bridging Zn2+ions. Compared with the structures, the structure of the complex is significantly affected by the pH of the reaction system[19].
3. 2 IR spectroscopic analysis
The IR spectrum of complex 1 shows a band of medium intensity near 3412 cm-1, which could be assigned to the(O–H) stretching of water molecules from residual humidity in the KBr pellets. Strong bands observed at 1612, 1517 and 1349 cm-1are associated with asymmetric(OCO)asand symmetric(OCO)sstretching vibrations of the carboxylic acid groups. The(OCO)as–(OCO)svalues are 95 and 263 cm-1, showing the presence of bridging and monodentate modes of carboxylate groups in the H3BTC ligand (Fig. 2).

Fig. 2. IR spectrum of complex 1
3. 3 Powder X-ray diffraction and thermal stability
A powder X-ray diffraction (PXRD) experiment was carried out for 1. Fig. 3 shows that the experi- mental pattern for the bulk of 1 matches basically the pattern simulated from its single-crystal data.

Fig. 3. Powder X-ray diffraction patterns
3. 4 Photoluminescence property
Luminescent property of 1 is investigated in the solid state at room temperature. To further analyze the nature of the emission band, the photolumine- scent properties of H3BTC and dpa have been investigated under the same experimental conditions. The luminescent emission spectra of 1, dpa and H3BTC are shown in Fig. 4. Intense photolumine- scence emissions for H3BTC and dpa are observed at 323 nm (ex= 290 nm) and 342 nm (ex= 290 nm), respectively. Complex 1 displays a photofluores- cence with two emissions around 364 and 393 nm upon excitation at 300 nm. In Zn(II)/Cd(II) coor- dination complexes, ligand-to-ligand charge transfer (LLCT) and ligand-to-metal charge transfer (LMCT) are commonly observed[20, 21]. The H3BTC plays a main role in luminescence of 1. Complex 1 displays red-shifts 41 nm (relative to H3BTC) and 51 nm (relative to dpa), respectively. The emissions for 1 were tentatively assigned to ligand-localized lumine- scence considering their emission features are similar to that of the free ligand. Comparing with the lumine- scence of complex 1 and the free ligand, the emission mechanism may be attributed to the intrali- gand*transition[22-24].

Fig. 4. Solid-state photoluminescence spectra at room temperature for 1 and the ligand
4 CONCLUSION
In summary, a coordination polymer {[Zn2(BTC)- (HBTC)(dpa)(Hdpa)]·3H2O}n(1) based on H3BTC and dpa ligands has been synthesized under hydro- thermal condition. Complex 1 displays a two-dimen- sional bilayer coordination polymer. Moreover, complex 1 exhibits photoluminescence property and may be a good candidate for potential luminescent materials.
(1) Erdem, O.; Yildiz, E. Synthesis and hydrogen gas storage properties of metal complexes including dicarboxylic acid derivatives.2015, 438, 1–4.
(2) Abu-Surrah, A. S.; Abu Safieh, K. A.; Ahmad, I. M.; Abdalla, M. Y.; Ayoub, M. T.; Qaroush, A. K.; Abu-Mahtheieh, A. M. New palladium(II) complexes bearing pyrazole-based Schiff base ligands: synthesis, characterization and cytotoxicity.2010, 45, 471–475.
(3) Su, F.; Lu, L. P.; Zhou, C. Y.; Wang, X. X.; Sun, L.; Han, C. A three-dimensional ZnIIcoordination polymer constructed from 1,10-biphenyl-2,20,4,40-tetracarboxylate and 1,4-bis(1H-imidazol-1-yl)benzene ligands exhibiting photoluminescence.2017,73, 72–77.
(4) Lu, L.; Wang, J.; Xie, B.; Wu, Y.; Wu, X. R.; Kumar, A. Syntheses, Hirshfeld surface analyses and magnetism of two complexes with flexible carboxylates.2017, 47, 1–8.
(5) Zhang, S. H.; Zhang, Y. D.; Zou, H. H.; Guo, J. J.; Li, H. P.; Song, Y.; Liang, H. A family of cubane cobalt and nickel clusters: Syntheses, structures and magnetic properties.2013, 396, 119–125.
(6) Zhang, H. Y.; Wang, W.; Chen, H.; Zhang, S. H.; Li, Y. Five novel dinuclear copper(II) complexes: crystal structures, properties, Hirshfeld surface analysis and vitro antitumor activity.2016, 453, 507–515.
(7) Zhang, S. H.; Zhao, R. X.; Li, G.; Zhang, H. Y.; Zhang, C. L.; Mullerb, G. Structural variation from heterometallic heptanuclear or heptanuclear to cubane clusters based on 2-hydroxy-3-ethoxy-benzaldehyde: effects of pH and temperature.2014, 4, 54837–54846.
(8) Cook, T. R.; Zheng, Y. R.; Stang, P. J. Metal-organic frameworks and self-assembled supramolecular coordination complexes: comparing and contrasting the design, synthesis, and functionality of metal-organic materials.2013, 113, 734–777.
(9) Almeida Paz, F. A.; Klinowski, J.; Vilela, S. M. F.; Tome´, J. P. C.; Cavaleiro, J. A. S.; Rocha, J. Liganddesign for functional metal-organic frameworks.2012, 41, 1088–1100.
(10) Tan, X. W.; Li, H. F.; Li, C. H. Two new isomeric zinc(II) metal-organic frameworks based on 1,5-bis(2-methyl-1H-imidazol-1-yl) pentane and 5-methylisophthalate ligands.2017, 73, 78–83.
(11) Wang, X.; Le, M.; Lin, H. Y.; Luan, J.; Liu, G. C.; Sui, F. F.; Chang, Z. H. Assembly, structures, photophysical properties and photocatalytic activities of a series of coordination polymers constructed from semi-rigid bis-pyridyl-bis-amide and benzenetricarboxylic acid.2015, 2, 373–387.
(12) Roy, M.; Sengupta, S.; Bala, S.; Bhattacharya, S.; Mondal, R. Systematic study of mutually inclusive influences of temperature and substitution on the coordination geometry of Co(II) in a series of coordination polymers and their properties.2016, 16, 3170–3179.
(13) Lin, J. M.; He, C. T.; Liu, Y.; Liao, P. Q.; Zhou, D. D.; Zhang, J. P.; Chen, X. M. A metal-organic framework with a pore size/shape suitable for strong binding and close packing of methane.2016, 55, 4674–4678.
(14) Liu, L.; Peng, Y. F.; Lv, X. X.; Li, K.; Li, B. L.; Wu, B. Construction of three coordination polymers based on tetranuclear copper(II) clusters: syntheses, structures and photocatalytic properties.2016, 18, 2490–2499.
(15) Lun, H. J.; Chen, Q.; Li, H. J.; Bai, Y. L.; Cui, S. S.; Li, H. Y.; Wang, S. B.; Li, Y. M. Synthesis, structure, and property of a two-dimensional layer coordination polymer on Ag(I).2017, 47, 801–805.
(16) Ma, H. F.; Lei, Q.; Wang, Y. L.; Yin, S. G.; Liu, Q. Y. Crystal structures and luminescence of two cadmium-carboxylate cluster-based compounds with mixed ligands.2017, 643, 477–482.
(17) Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program.2009, 42, 339–341.
(18) Sheldrick. G. M.. University of Göttingen, Germany 1997.
(19) Bravermana, M. A.; Supkowskib, R. M.; LaDucaa, R. L. Luminescent zinc and cadmium complexes incorporating 1,3,5-benzenetricarboxylate and a protonated kinked organodiimine: from a hydrogen-bonded layer motif to thermally robust two-dimensional coordination polymers.2007,180, 1852–1862.
(20) Zheng, S. L.; Chen, X. M. Recent advances in luminescent monomeric, multinuclear, and polymeric Zn(II) and Cd(II) coordination complexes.2004, 57, 703–712.
(21) Su, Z.; Fan, J.; Sun, W. Y. Novel two-fold interpenetrated Zn-based metal-organic framework with triple-stranded right-and left-handed helical chains.2013, 27, 18–21.
(22) Su, C. Y.; Goforth, A. M.; Smith, M. D.; Pellechia, P. J.; Loye, H. C. Exceptionally stable, hollow tubular metal-organic architectures: synthesis, characterization, and solid-state transformation study.2004, 126, 3576–3586.
(23) Wu, M. F.; Liu, Z. F.; Wang, S. H.; Chen, J.; Xu, G.; Zheng, F. K.; Guo, G. C.; Huang, J. S. Structures and photoluminescence of zinc(II) coordination polymersbased ongenerated 1-tetrazolate-5-propionic acid ligands.2011, 13, 6386–6392.
(24) Lu, Y. B.; Jin, S.; Jian, F. M.; Xie, Y. R.; Luo, G. T. Two novel 1-D helical chains Zn(II)/Cd(II) polymers based on tetrazolate-1-acetic acid: crystal structures, solid state fluorescence and thermal behaviors.2014,1061, 14–18.
26 June 2017;
1 November 2017 (CCDC 1491845)
①This work was supported by the Foundation of Guangxi Key Laboratory of electrochemical and Magneto-chemical Functional Materials (EMFM20161102)
Zhang Xiu-Qing, born in 1979, associate professor. E-mail: glutchem@163.com. Zhang Shu-Hua. E-mail: 909449982@qq.com
10.14102/j.cnki.0254-5861.2011-1764
- 结构化学的其它文章
- Crystal Structures, Luminescent Properties and Hirshfeld Surface Analyses of Zn(II) and Cd(II) Compounds Based on 1-(2-Carboxylphenyl)-3-(pyridin-2-yl)pyrazole①
- Investigation of the Substituent Effects on π-Type Pnicogen Bond Interaction①
- Structural and Mechanistic Studies of γ-Fe2O3 Nanoparticle as Capecitabine Drug Nanocarrier①
- Theoretical Study on the Mechanism of a New Synthesis Reaction of 1,3,5-Substituted-1,2,4-triazoles by Carboxylic Acids, Amidines, and Hydrazines①
- Local Structure Mediation and Photoluminescence of Ce3+- and Eu3+-Codoped YAG Nanophosphors①
- Syntheses, Crystal Structures and Characterization of Two Coordination Polymers Based on Mixed Ligands①

