BDE-28及BDE-99对斑马鱼早期生命阶段HPT、HPG和HPA轴功能基因表达水平的影响
靳亚茹,刘红玲,* ,韩志华,花小雪
1. 南京大学环境学院 污染控制与资源化研究国家重点实验室,南京 210023 2. 环境保护部南京环境科学研究所,南京 210042
多溴联苯醚(poly brominated diphenyl ethers, PBDEs)作为一种曾被广泛应用的溴代阻燃剂[1],已被禁止生产和使用近10年[2],但仍能在许多环境介质包括空气[3]、水体[4]、土壤[5]、灰尘、底泥[6]和各种生物体[7-10]甚至人体母乳、血液、头发、胎盘以及婴儿血液[11-15]中检出它们的存在。其中2,4,4'-三溴联苯醚(2,4,4'-tribromodiphenyl ether, BDE-28)和2,2',4,4',5-五溴联苯醚(2,2',4,4',5-pentabromodiphenyl ether, BDE-99)是在长江下游底泥中检出浓度最高的PBDE类物质,甚至超过了2,2’,4,4’-四溴联苯醚(2,2',4,4'-tetrabromodiphenyl ether, BDE-47)[16]。另外,在长江的水生生物如虾类、蟹类、杂食性鱼类、植食性鱼类和肉食性鱼类中也都有检出BDE-28和BDE-99,其中BDE-28的检出比例尤其高(10.9%),超过了国外一般检出水平[17]。因此BDE-28和BDE-99是否会对水生生物中造成负效应引起了我们的关注。
动物实验研究发现,研究较多的BDE-47会导致鸟类、鱼类和哺乳动物的神经发育毒性、生殖毒性和内分泌紊乱[18-23],而且较低溴代的BDE-28和更高溴代的BDE-99也会对部分生物甚至人体健康产生不良影响。例如,已有毒性研究发现BDE-28影响斑马鱼幼鱼的生长发育且对小孩的神经发育有不良影响[24-25],而且BDE-99也会对红耳龟(red-eared sliders)、大鼠和鸟类的生长发育、神经行为以及生殖行为有负面效应[20,26-30]。由于PBDE类物质与甲状腺激素(thyroid hormone, TH)的结构相似,所以它们被认为是潜在的甲状腺内分泌干扰物。研究较多的BDE-47被报道作用于大鼠甲状腺受体,改变人体中T3和T4浓度并对斑马鱼下丘脑-垂体-甲状腺(hypothalamus-pituitary-thyroidal, HPT)轴基因表达有影响[31-33]。另外,也有文献表明人体内TH浓度与体内BDE-28和BDE-99浓度相关[34]。而且有研究表明BDE-99暴露能改变青年大鳞大麻哈鱼(Chinook salmon)和大鼠体内甲状腺激素水平,并影响大鼠小脑颗粒神经元中甲状腺激素受体基因的表达[35-37]。
综上所述,BDE-28和BDE-99与其具有相似结构的物质BDE-47一样对生物体和人类有潜在的神经发育、生殖和甲状腺相关的内分泌干扰作用。然而,关于BDE-28和BDE-99对水生生物的内分泌干扰效应和机制研究还较少。同时,有学者提出影响甲状腺系统的PBDE同系物也能影响硬骨鱼的生殖系统[38]。例如,BDE-47喂食暴露会损害黑头呆鱼(fathead minnow)的甲状腺功能并影响其生殖发育[39]。类似地,DE-71摄入会同时影响斑马鱼的甲状腺激素浓度和产卵率[40]。Yu等[41-42]报道,在胚胎到成鱼阶段暴露于低浓度的DE-71不仅改变了HPT轴和TH相关基因的表达,而且改变了下丘脑-垂体-性腺(hypothalamus-pituitary-gonadal, HPG)轴和性激素相关基因的表达,并减少了斑马鱼的产卵量。在中国稀有鮈鲫(Chinese rare minnow)中,十溴联苯醚(decabromodiphenyl ether, BDE-209)暴露可能导致成鱼TH相关基因表达的改变并对雄性睾丸中精子发生产生抑制[43]。另外,有研究表明在斑马鱼中已经观察到下丘脑-垂体-肾上腺(hypothalamus-pituitary-adrenal, HPA)和HPG轴之间的交互作用,表明化学物质可以通过降低E2的浓度从而使HPA轴基因的表达下调并降低皮质醇的浓度[44]。并且在非哺乳类脊椎动物实验中也发现HPA轴及HPG轴之间存在交互作用[45]。除此之外也有实验证明,化合物对斑马鱼体内某一内分泌轴的影响也将间接影响斑马鱼的其他内分泌轴[44, 46]。在脊椎动物中,生殖、甲状腺和肾上腺内分泌系统主要由HPT、HPA和HPG轴控制,这3个系统通路通过协调激素的合成、分泌、运输和代谢来调节其动力学过程[32,44,47]。所以本文选择与人体基因有87%相似的斑马鱼为模式生物,展开BDE-28和BDE-99对斑马鱼HPT、HPG和HPA内分泌轴上相关基因影响研究,从而探究它们的内分泌干扰机制。
1 材料与方法(Materials and methods)
1.1 实验材料
BDE-28、BDE-99(纯度>99.2%),购自美国AccuStandard公司;二甲基亚砜(dimethyl sulfoxide, DMSO),分析纯,购自南京化学试剂有限公司,作助溶剂;RNA抽提试剂盒(RNeasy®Mini Kit)、cDNA逆转录试剂盒(Omniscript RT Kit)均购自德国Qiagen公司;实时定量PCR试剂盒(SYBR®Real time PCR Master Mix Plus Kits)购自日本东洋纺公司。
斑马鱼养殖系统(ZKH-BM09)购自中国青岛中科海水处理有限公司;多功能连续变倍体视显微镜(AZ100)购自日本尼康公司;智能光照培养箱购自宁波海曙赛福实验仪器厂;分析天平(AUY120)购自日本岛津公司;鱼孵化器(WT-2005)购自旺通五金电器厂;全功能酶标仪(Synergy H4)购自美国Biotek公司;q-RT-PCR仪(Applied Biosystems Stepone Plus Real-time PCR System)购自美国Applied biosystems公司。
1.2 斑马鱼驯化及胚胎收集
5月龄野生AB型成年斑马鱼是由本实验室在斑马鱼养殖系统(ZKH-BM09)自行繁殖并养殖成熟。该半自动斑马鱼养殖系统配备曝气充氧泵、紫外杀菌灯、系统过滤器、温度控制系统、光周期控制系统并可维持系统的水持续循环。系统运行期间,维持温度在27 ℃~29 ℃的自来水供斑马鱼生存,并维持系统光暗比14 h:10 h,每天喂食3次新鲜孵化的丰年虫。
光照刺激对斑马鱼产卵非常重要,一般产卵发生在清晨开灯后30 min以内。由于斑马鱼会自食其卵,因此我们选择底部带有小孔塑料板的孵化器来进行斑马鱼产卵。前1天晚将3条雌鱼和3条雄鱼放进一个底部带有孔塑料板的孵化器,并用孵化器自带的塑料隔板将雌鱼与雄鱼分开。第2天清晨开灯时,将孵化器中间的塑料隔板撤去,让雄鱼与雌鱼在光刺激下交配产卵。30 min后将鱼卵取出并置于培养皿中,使用统一配制充氧饱和的60 mg·L-1海盐水清洗胚胎后,置于体视显微镜下观察。剔除未发生卵裂以及卵裂不正常的胚胎,这些不正常的胚胎主要表现为初期无卵裂或卵裂细胞大小不均等。然后将斑马鱼胚胎培养于(28 ± 1) ℃恒温光照培养箱中。在斑马鱼胚胎受精后4 h(4 hours post fertilization, 4 hpf)统一进行暴露实验。
1.3 斑马鱼胚胎暴露实验
使用前将BDE-28和BDE-99分别配成高浓度的DMSO母液,实验中所用相应浓度的暴露液由高浓度母液经胚胎培养水稀释而得,并且保证最高浓度暴露液中DMSO不超过暴露溶液体积的0.1%。暴露液现用现配,设置BDE-28和BDE-99水暴露溶液的浓度为2 μg·L-1、20 μg·L-1和200 μg·L-1,对照为0.1%的DMSO溶液。使用25 mL烧杯作为本次实验的暴露容器,实验设置3次生物学平行,每个烧杯中加入20 mL的暴露液,20枚发育正常的胚胎,放置于光照培养箱中。另外,为了防止溶液蒸发,覆盖保鲜膜于暴露容器上,有实验表明覆盖保鲜膜不会影响斑马鱼胚胎/幼鱼的正常生长和发育[32]。暴露期间及时将停止发育或死亡的胚胎和幼鱼用一次性吸管吸出,以免影响其他胚胎/幼鱼的生长。在胚胎受精后120 h(120 hpf),将各浓度对应的斑马鱼幼鱼收取到1.5 mL无RNA酶的离心管中,尽量将水分吸干,加入RNAlater试剂对样品进行固定(确保幼鱼样品浸没在RNAlater溶液中),置于-80 ℃超低温冰箱中待后续实验。
1.4 斑马鱼幼鱼总RNA抽提、逆转录和q-RT-PCR实验
本研究使用q-RT-PCR技术对HPT、HPG及HPA轴相关基因表达进行检验,因此首先需要对所取得的样品进行总RNA提取和逆转录。各浓度组孵化出的斑马鱼样品使用RNA抽提试剂盒进行总RNA提取。RNA抽提结束,使用酶标仪TAKE 3软件测定总RNA的量和OD260/OD280的比值,比值显示1.9~2.1之间说明纯度较好。根据测得的RNA浓度,使用无RNA酶的水将所有样品稀释到相同的浓度后,尽快使用逆转录试剂盒将RNA逆转录为cDNA。RNA抽提与逆转录过程严格按照试剂盒所述方法。最后使用SYBR实时定量PCR试剂盒对逆转录后的cDNA进行目的基因的扩增和测定。所选择的基因以及引物序列如之前文献中所描述[48]。
该实验RT-PCR条件设置如下:初始变性时间10 min,扩增40个循环,扩增条件为首先升温至95 ℃维持15 s,然后降至60 ℃保持1 min。目标片段扩增后,画出溶解曲线确保所有cDNA样品均仅能扩增出唯一目标产物。
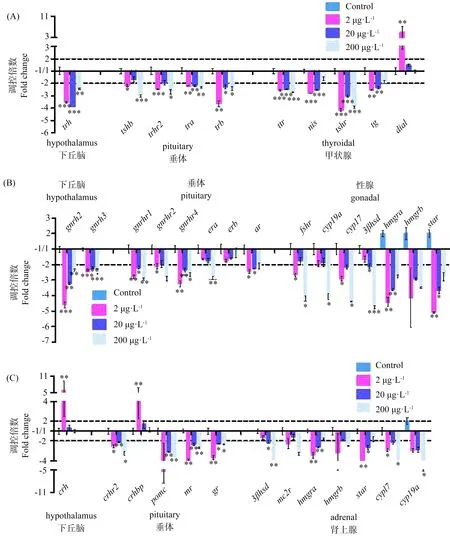
图1 斑马鱼胚胎暴露于2、20或200 μg·L-1的BDE-28至120 hpf时(A)下丘脑-垂体-甲状腺(HPT)、(B)下丘脑-垂体-性腺(HPG)和(C)下丘脑-垂体-肾上腺(HPA)轴的基因表达变化注:结果表示为3次重复的平均值±SD,*P <0.05和**P <0.01表明暴露组与对照组相比有显著性差异。Fig. 1 Expression of genes along the (A) HPT axis, (B) HPG axis, and (C) HPA axis of zebrafish embryo at 120 hpf following 2, 20 or 200 μg·L-1 BDE-28 exposureNote: Results were expressed as means±SD of three replicates. * P<0.05 and ** P<0.01 indicate significant differences in exposure groups compared with control group.
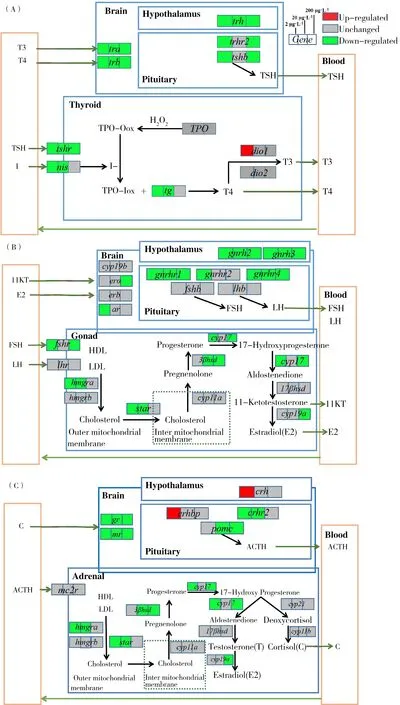
图2 暴露于2、20和200 μg·L-1的BDE-28后,斑马鱼幼鱼的3个轴((A)HPT轴、(B)HPG轴和(C)HPA轴)在各浓度下的效应结果和可能的毒性途径Fig. 2 Concentration dependent effects and proposed toxicity pathways along the three axes ((A) HPT axis, (B) HPG axis and (C) HPA axis) of zebrafish larvae exposed to 2, 20 and 200 μg·L-1 BDE-28
1.5 数据处理
使用LinRegPCR处理q-RT-PCR的原始数据,然后以持家基因18S rRNA作为mRNA表达内参,用空白对照组进行标准化,通过2-ΔΔCt方法计算基因的调控倍数[49]。基因调控数据使用Graph Pad Prism 5(GraphPad,San Diego,CA,USA)进行统计学分析。在GraphPad中使用单因素方差分析(ANOVA)并随后通过多重比较Tukey检验方法分析暴露组与对照组基因调控倍数之间的显著性差异,当P< 0.05时即表示存在显著性差异。
2 结果 (Results)
本研究使用q-RT-PCR技术研究了BDE-28和BDE-99对斑马鱼胚胎/幼鱼HPT、HPG和HPA轴上相关基因转录表达的影响。结果表明斑马鱼胚胎暴露于BDE-28和BDE-99在不同程度上引起3个内分泌轴上相关基因的表达变化,总的来说,BDE-28主要引起相关基因发生显著性下调而BDE-99引起相关基因表达发生显著性上调。
图1和图2展示了BDE-28暴露后HPT、HPG和HPA轴相关基因的表达情况。从图1(A)和图2(A)可以看出,与下丘脑相关的促甲状腺激素释放激素基因(trh)表达在2、20和200 μg·L-1分别显著下调3.53、3.89和2.43倍。与HPT轴相关的垂体基因的表达在BDE-28作用下都有下调趋势。促甲状腺激素β基因(tshb)和促甲状腺激素释放激素受体2基因(trhr2)在暴露于2 μg·L-1的BDE-28之后分别显著下调2.17和2.44倍,而在暴露于200 μg·L-1的BDE-28之后分别显著下调2.99和2.70倍。甲状腺激素受体α和β(trα and trβ)在3个暴露浓度下都发生了显著下调,在由低到高暴露浓度下trα和trβ分别下调2.20、2.16、2.31倍和3.64、2.30和2.42倍。同时,在3个暴露浓度下促甲状腺激素受体基因(tshr)分别显著下调了4.14、3.00和3.93倍。暴露于2和20 μg·L-1的BDE-28后,Na+/I同向载体(nis)的转录显著下调了2.79倍和2.54倍,而甲状腺球蛋白(tg)显著下调了2.53倍和2.32倍。脱碘酶1(dio1)在2 μg·L-1的BDE-28暴露下显著上调5.04倍。
BDE-28还对HPG轴相关基因的表达产生了影响,具体如图1(B)和图2(B)。下丘脑相关基因促性腺激素释放激素基因2和3(gnrh2和gnrh3)在2、20和200 μg·L-1的BDE-28暴露后分别显著下调4.59、3.22、2.34倍和2.43、2.18、2.60倍。垂体中相关基因基本都呈下调趋势,其中促性腺激素释放激素类似物1和4(gnrhr1和gnrhr4)在2、20和200 μg·L-1分别下调2.79、2.19、2.92倍和3.23、2.35、2.65倍,而gnrhr2只有在最低暴露浓度显著下调2.10倍。斑马鱼幼鱼体内雌激素受体基因a(era)在最高暴露浓度下显著下调2.84倍,而雄激素受体(ar)在低和中暴露浓度下分别下调2.44和2.26倍。BDE-28也使性腺中相关基因表现出不同程度地变化,如促滤泡激素受体(fshr)和雌激素合成的细胞色素P450(cyp17)在2、200 μg·L-1的暴露浓度下分别显著下调2.65、4.18倍和2.90、4.38倍,而芳香化酶a(cyp19a)在高浓度暴露下显著下调4.07倍。3β-羟类固醇脱氢酶(3βhsd)在中浓度下显著下调2.08倍,而在高浓度下显著下调4.75倍。羟甲基戊二酰辅酶a还原酶(hmgra)和类固醇合成急性调节蛋白基因(star)在低、中暴露浓度下分别显著下调3.41、2.59倍和4.08、2.65倍。
此外,BDE-28还影响HPA轴相关基因的表达(如图1(C)和图2(C))。在2 μg·L-1的BDE-28作用下下丘脑中促肾上腺皮质激素释放激素(crh)和垂体中促肾上腺皮质激素释放激素结合蛋白(crhbp)分别显著上调5.80倍和4.53倍。促肾上腺皮质激素释放激素受体2(crhr2)在低、中浓度下分别下调2.52和2.09倍。另外,垂体中阿片黑素促皮质素原(pomc)在中、高浓度下分别显著下调3.08和3.54倍。盐皮质激素受体(mr)和糖皮质激素受体(gr)在低、中、高暴露浓度下分别显著下调4.04、2.33、2.69倍和3.69、2.30、2.34倍。在肾上腺中,hmgra、hmgrb、3βhsd、star、cyp17和cyp19a表达情况跟上述HPG轴中一样,其余基因都没有显著性变化。
BDE-99与BDE-28的作用方式不同,BDE-99暴露后HPT、HPG和HPA轴相关的基因大部分呈下调状态(如图2),且大多基因效应强度与BDE-99浓度成反比,在较低浓度下具有较大的倍数变化。在HPT轴中(如图3(A)和图4(A)),下丘脑相关的基因trh在2 μg·L-1的BDE-99暴露下显著上调2.77倍,而在20或200 μg·L-1下没有显著变化。在垂体中,trhr2和tra在最低暴露浓度下分别显著上调4.20和3.91倍,其他基因与空白组相比没有显著性变化。在甲状腺中,nis、tshr、tg和dio1在最低暴露浓度下分别上调5.35、3.43、3.74和5.04倍。
BDE-99暴露后,HPG轴上基因也发生了变化(如图3(B)和图4(B))。下丘脑相关基因gnrh2和gnrh3在2 μg·L-1的BDE-99暴露下分别下调了3.00倍和4.83倍,而在较高浓度下没有显著变化。同样地,垂体相关基因gnrhr1、gnrhr2、erb、ar和fshb也在最低暴露浓度下分别显著上调2.50、3.46、2.18、2.78和3.93倍,而在其他浓度没有显著变化。性腺相关的基因cyp19a、lhr、hmgra、hmgrb、3βhsd3和cyp17在最低暴露浓度下分别显著上调3.81、4.06、2.69、4.02、4.04和3.01倍,其中3βhsd3 和cyp17在最高浓度下显著下调2.44和2.29倍。
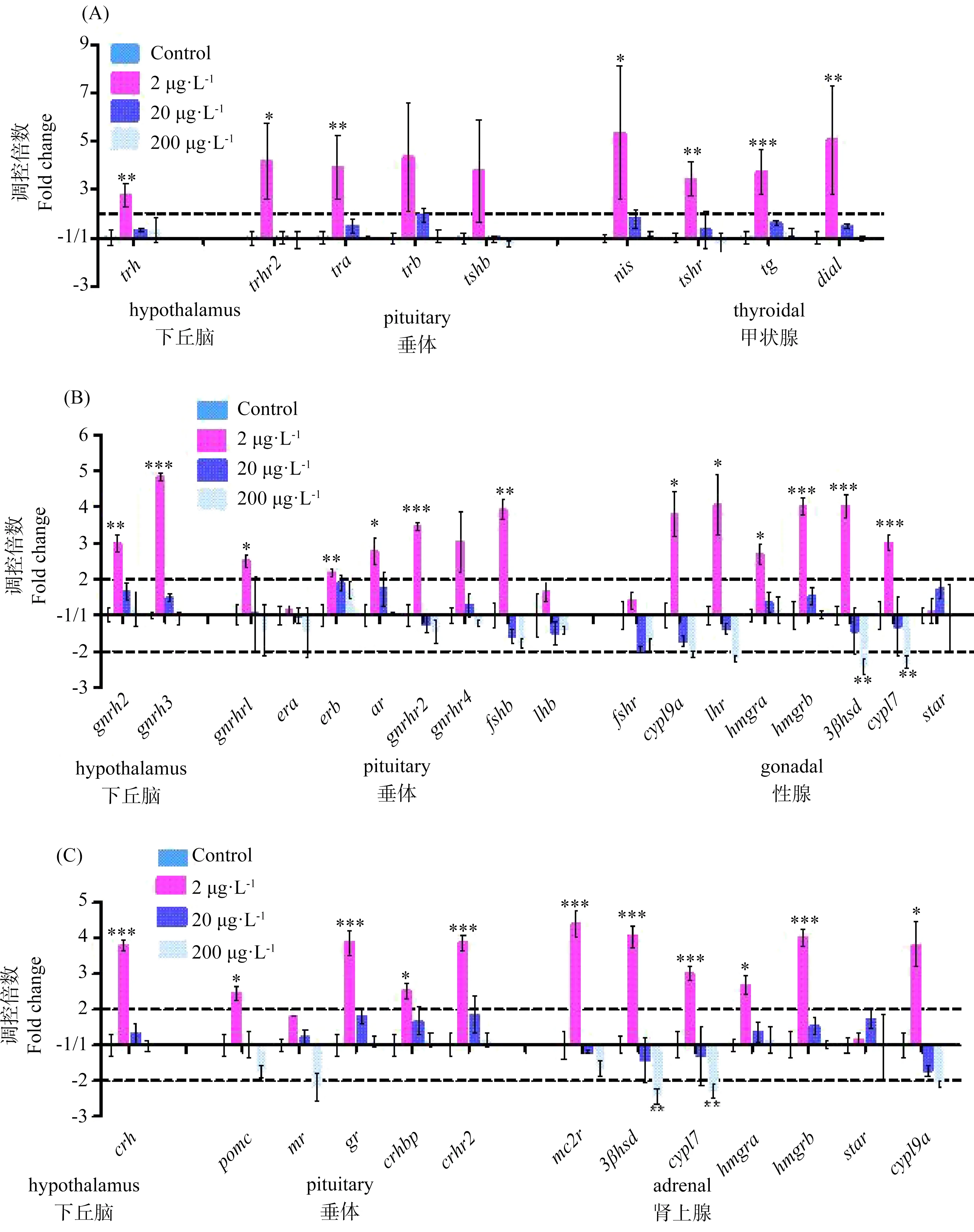
图3 斑马鱼胚胎暴露于2、20或200 μg·L-1的BDE-99至120 hpf时(A)下丘脑-垂体-甲状腺(HPT)、(B)下丘脑-垂体-性腺(HPG)和(C)下丘脑-垂体-肾上腺(HPA)轴的基因表达变化注:结果表示为3次重复的平均值±SD,* P <0.05和** P <0.01表明暴露组与对照组相比有显著性差异。Fig. 3 Expression of genes along the (A) HPT axis, (B) HPG axis, and (C) HPA axis of zebrafish embryo at 120 hpf following 2, 20 or 200 μg·L-1 BDE-99 exposureNote: Results were expressed as means±SD of three replicates. * P<0.05 and ** P<0.01 indicate significant differences in exposure groups compared with control group.
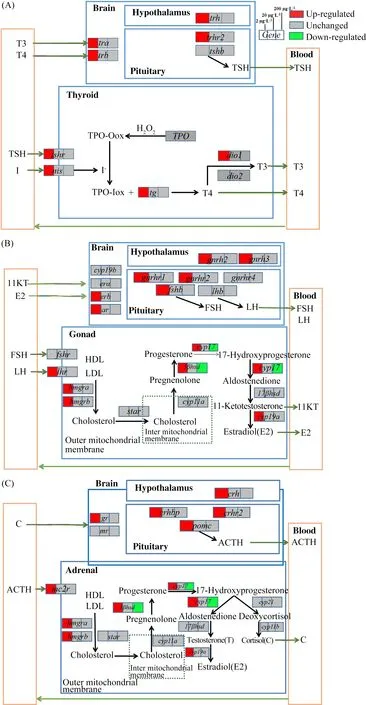
图4 暴露于2、20和200 μg·L-1的BDE-99后,斑马鱼幼鱼的3个轴((A)HPT轴、(B)HPG轴和(C)HPA轴)在各浓度下的效应结果和可能的毒性途径Fig. 4 Concentration dependent effects and proposed toxicity pathways along the three axes ((A) HPT axis, (B) HPG axis and (C) HPA axis) of zebrafish larvae exposed to 2, 20 and 200 μg·L-1 BDE-99
除此之外,BDE-99也对HPA轴相关的基因产生了影响(如图3(C)和图4(C))。与下丘脑相关基因crh只在最低暴露浓度下显著上调3.80倍,并且与垂体相关基因pomc、crhbp、crhr2和gr也只在最低暴露浓度下显著上调2.46、2.53、3.87和3.87倍,而下丘脑和垂体中其他基因在任何暴露浓度下都没有显著变化。与肾上腺相关基因mc2r、hmgra、hmgrb和cyp19a在2 μg·L-1的BDE-99暴露下分别显著上调4.40、2.69、4.02和3.81倍,而在其他暴露浓度无显著变化。3βhsd和cyp17在2 μg·L-1的BDE-99暴露下分别显著上调4.04和3.01倍,而在200 μg·L-1时分别显著下调2.44和2.29倍。
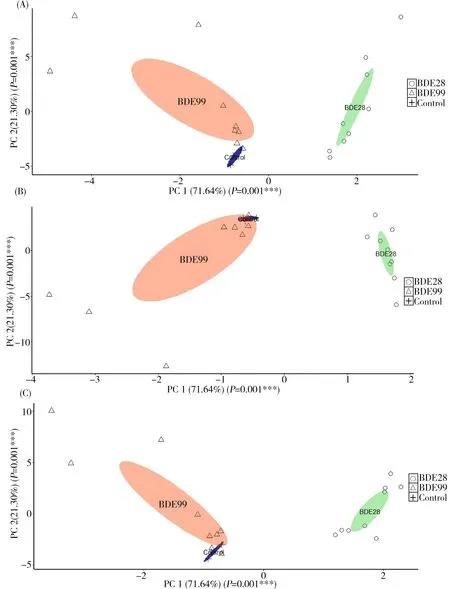
图5 BDE-28和BDE-99暴露后(A)HPT轴(B)HPG轴及(C)HPA轴相关基因表达情况的主成分分析注:每个点都代表相应物质暴露后基因的表达情况,而椭圆区域代表95%的置信区间。Fig. 5 Principal component analysis (PCA) of genes expression associated with (A) HPT (B) HPG (C) HPA axes after BDE-28/BDE-99 exposureNote: Each point represents the genes expression after exposure to the corresponding substance and the elliptical area represents the 95% confidence interval.
3 讨论 (Discussion)
本文研究了BDE-28和BDE-99对斑马鱼体内HPT轴的影响,结果发现BDE-28暴露后与甲状腺激素合成通路相关的基因nis、tg、tshr、tra和trb显著下调,这可能会使斑马鱼体内TH的合成量改变。脱碘酶1(dio1)显著上调导致T4更多地转化为有活性的T3,从而可能导致体内有活性的甲状腺激素水平失衡。这一结果与之前相关研究一致,之前研究表明人体妊娠期间体内BDE-28的存在与T4和T3浓度变化有关[34]。trh涉及下丘脑向垂体的信号传递过程,所以trh下调也导致垂体中相关基因trhr2和tshb显著下调,这些基因的调控变化也会最终影响TH的合成。BDE-99暴露使斑马鱼体内甲状腺合成通路相关的基因nis、tg、tshr、dio1、tra和trb显著上调,从而有可能使斑马鱼体内TH浓度升高。而下丘脑和垂体中相关基因trh与trhr2的上调传递给甲状腺相应的上调信号,从而也导致TH合成增加。之前有研究也表明人体内BDE-99含量与T3含量成正比关系[50]。但是,最近有研究报道BDE-99暴露降低青年大鳞大麻哈鱼和大鼠体内T3、T4浓度[35, 37]。本研究中BDE-99对斑马鱼HPT轴的作用与大鳞大麻哈鱼和大鼠相关研究结果的不一致可能是由生物种类差异导致的。
已有大量研究表明HPG轴与HPT轴有交互作用[38,44],所以BDE-28和BDE-99也可能直接或间接地作用于HPG。因此,本研究展开了2种物质对HPG轴基因表达影响的研究,结果显示暴露于BDE-28和BDE-99影响了HPG轴上的基因表达,从而可能影响了斑马鱼体内11KT和E2的合成。在暴露于BDE-28之后,下丘脑和垂体中基因gnrh2、gnrh3、gnrhr1、gnrhr2和gnrhr4显著下调,这可能导致性腺激素合成发生改变。且与性腺激素合成过程相关的基因fshr、hmgra、star、3βhsd、cyp17、cyp19a、era和ar都显著下调,这可能导致性腺激素合成减少。关于BDE-99的研究结果表明,在2 μg·L-1的BDE-99暴露后,lhr、hmgra、hmgrb、3βhsd、cyp17、cyp19a、erb和ar都显著上调,这可能导致E2和11KT合成和释放增加。而且另一项研究也表明BDE-99能刺激雄激素生成[51]。另外,之前关于斑马鱼的研究表明PBDE不仅改变了HPT轴和TH浓度中的基因的表达,而且改变了HPG轴基因的表达和性激素浓度并降低了成年鱼的产卵量[41-42]。本研究中BDE-28和BDE-99的研究结果也验证了这一结果,它们不仅影响斑马鱼HPT轴基因的表达,而且也可能直接或间接地影响HPG轴基因表达从而可能导致鱼类繁殖性能的破坏。
到目前为止,关于PBDE对HPA轴的影响基本上尚未被研究。而从本研究结果可以看出斑马鱼经过BDE-28和BDE-99暴露后,肾上腺中相关基因cyp17、3βhsd、cyp19a、hmgra、hmgrb以及mr和gr都有显著变化,这说明这2种物质可能导致肾上腺激素合成发生改变。有研究表明化学物质可以通过降低斑马鱼体内E2的浓度从而使HPA轴基因的表达下调并降低皮质激素的浓度[44]。所以本研究结果表明,BDE-28和BDE-99也可能通过HPG轴间接地作用于HPA轴。另外,已有学者证明在非哺乳类脊椎动物中的促肾上腺皮质激素释放激素(corticotropin releasing hormone, CRH)(激活HPA轴的主要神经激素)也是促甲状腺激素(thyroid stimulating hormone, TSH)的刺激因子[45]。在鱼类中,CRH比促甲状腺激素释放激素(thyrotropin releasing hormone, TRH)更能有效地刺激TSH的释放[45]。所以本研究中BDE-28和BDE-99对HPT轴的影响也可能是由于其对HPA轴的作用间接导致的。
通过BDE-28与BDE-99暴露后3个内分泌轴的基因表达情况的主成分分析(principal component analysis, PCA)发现,BDE-28和BDE-99暴露后,斑马鱼HPT(如图5(A))、HPG(如图5(B))和HPA(如图5(C))轴相关基因表达情况的椭圆区域都没有重叠部分。这一结果表明BDE-28和BDE-99可能通过不同的毒性通路影响斑马鱼的HPT、HPG和HPA轴。
参考文献(References):
[1]Talsness C E. Overview of toxicological aspects of polybrominated diphenyl ethers: A flame-retardant additive in several consumer products [J]. Environmental Research, 2008, 108(2): 158-167
[2]Ross P S, Couillard C M, Ikonomou M G, et al. Large and growing environmental reservoirs of deca-BDE present an emerging health risk for fish and marine mammals [J]. Marine Pollution Bulletin, 2009, 58(1): 7-10
[3]Bo S, Dodder N G, Ilora Basu A, et al. Concentrations and spatial variations of polybrominated diphenyl ethers and other organohalogen compounds in Great Lakes air [J]. Environmental Science & Technology, 2001, 35(6): 1078-1083
[4]Oros D R, Hoover D, Rodigari F, et al. Levels and distribution of polybrominated diphenyl ethers in water, surface sediments, and bivalves from the San Francisco Estuary [J]. Environmental Science & Technology, 2005, 39(1): 33-41
[5]Wu M H, Pei J C, Zheng M, et al. Polybrominated diphenyl ethers (PBDEs) in soil and outdoor dust from a multi-functional area of Shanghai: Levels, compositional profiles and interrelationships [J]. Chemosphere, 2015, 118(1): 87-95
[6]Wang J, Lin Z, Lin K, et al. Polybrominated diphenyl ethers in water, sediment, soil, and biological samples from different industrial areas in Zhejiang, China [J]. Journal of Hazardous Materials, 2011, 197(6): 211-219
[7]Luross J M, Alaee M, Sergeant D B, et al. Spatial distribution of polybrominated diphenyl ethers and polybrominated biphenyls in lake trout from the Laurentian Great Lakes [J]. Chemosphere, 2002, 46(5): 665-672
[8]Su G Y, Gao Z S, Yu Y, et al. Polybrominated diphenyl ethers and their methoxylated metabolites in anchovy (Coilia, sp.) from the Yangtze River Delta, China [J]. Environmental Science and Pollution Research, 2010, 17(3): 634-642
[9]Su G, Xia J, Liu H, et al. Dioxin-like potency of HO- and MeO- analogues of PBDEs’ the potential risk through consumption of fish from Eastern China [J]. Environmental Science & Technology, 2012, 46(19): 10781-10788
[10]Hu G C, Dai J Y, Xu Z C, et al. Bioaccumulation behavior of polybrominated diphenyl ethers (PBDEs) in the freshwater food chain of Baiyangdian Lake, North China [J]. Environment International, 2010, 36(4): 309-315
[11]de Wit C A. An overview of brominated flame retardants in the environment [J]. Chemosphere, 2002, 46(5): 583-624
[12]Hites R A. Polybrominated diphenyl ethers in the environment and in people: A meta-analysis of concentrations [J]. Environmental Science & Technology, 2004, 38(4): 945-956
[13]Norén K, Meironyté D. Certain organochlorine and organobromine contaminants in Swedish human milk in perspective of past 20-30 years [J]. Chemosphere, 2000, 40(9-11): 1111-1123
[14]Leonetti C, Butt C M, Hoffman K, et al. Concentrations of polybrominated diphenyl ethers (PBDEs) and 2,4,6-tribromophenol in human placental tissues [J]. Environment International, 2016, 88: 23-29
[15]Zheng J, Chen K H, Luo X J, et al. Polybrominated diphenyl ethers (PBDEs) in paired human hair and serum from e-waste recycling workers: Source apportionment of hair PBDEs and relationship between hair and serum [J]. Environmental Science & Technology, 2014, 48(1): 791-796
[16]Shen M, Yu Y, Zheng G J, et al. Polychlorinated biphenyls and polybrominated diphenyl ethers in surface sediments from the Yangtze River Delta [J]. Marine Pollution Bulletin, 2006, 52(10): 1299-1304
[17]Gao Z S, Jie X, Xian Q M, et al. Polybrominated diphenyl ethers (PBDEs) in aquatic biota from the lower reach of the Yangtze River, East China [J]. Chemosphere, 2009, 75(9): 1273-1279
[18]Thornton L M, Path E M, Nystrom G S, et al. Early life stage exposure to BDE-47 causes adverse effects on reproductive success and sexual differentiation in fathead minnows (Pimephales promelas) [J]. Environmental Science & Technology, 2016, 50(14): 7834-7841
[19]Currier H A, Letcher R J, Williams T D, et al. Effects of the bioaccumulative polybrominated diphenyl ether flame retardant congener BDE-47 on growth, development, and reproductive success in zebra finches [J]. Bulletin of Environmental Contamination and Toxicology, 2015, 94(2):140-145
[20]Eisenreich K M, Rowe C L. Dietary exposure of BDE-47 and BDE-99 and effects on behavior, bioenergetics, and thyroid function in juvenile red-eared sliders (Trachemys scripta elegans) and common snapping turtles (Chelydra serpentina) [J]. Environmental Toxicology & Chemistry, 2014, 33(12): 2810-2817
[21]Liu H, Tang S, Zheng X, et al. Bioaccumulation, biotransformation, and toxicity of BDE-47, 6-OH-BDE-47, and 6-MeO-BDE-47 in early life-stages of zebrafish (Danio rerio) [J]. Environmental Science & Technology, 2016, 49(3): 1823-1833
[22]Muirhead E K, Skillman A D, Hook S E, et al. Oral exposure of PBDE-47 in fish: Toxicokinetics and reproductive effects in Japanese medaka (Oryzias latipes) and fathead minnows (Pimephales promelas) [J]. Environmental Science & Technology, 2006, 40(2): 523-528
[23]Zhao J, Xu T, Yin D Q. Locomotor activity changes on zebrafish larvae with different 2,2',4,4'-tetrabromodiphenyl ether (PBDE-47) embryonic exposure modes [J]. Chemosphere, 2014, 94: 53-61
[24]Adgent M A, Hoffman K, Goldman B D, et al. Brominated flame retardants in breast milk and behavioral and cognitive development at 36 months [J]. Paediatric & Perinatal Epidemiology, 2014, 28(1): 48-57
[25]Shy C G, Huang H L, Changchien G P, et al. Neurodevelopment of infants with prenatal exposure to polybrominated diphenyl ethers [J]. Bulletin of Environmental Contamination and Toxicology, 2011, 87(6): 643-648
[26]Zhao W, Cheng J, Gu J, et al. Assessment of neurotoxic effects and brain region distribution in rat offspring prenatally co-exposed to low doses of BDE-99 and methylmercury [J]. Chemosphere, 2014, 112: 170-176
[27]Cheng J, Gu J, Ma J, et al. Neurobehavioural effects, redox responses and tissue distribution in rat offspring developmental exposure to BDE-99 [J]. Chemosphere, 2009, 75(7): 963-968
[28]Eng M L, Elliott J E, Macdougallshackleton S A, et al. Early exposure to 2,2′,4,4′,5-pentabromodiphenyl ether (BDE-99) affects mating behavior of zebra finches [J]. Toxicological Sciences, 2012, 127(1): 269-276
[29]Eng M L, Elliott J E, Williams T D. An assessment of the developmental toxicity of BDE-99 in the European starling using an integrated laboratory and field approach [J]. Ecotoxicology, 2014, 23(8): 1505-1516
[30]Viberg H, Fredriksson A, Eriksson P. Deranged spontaneous behaviour and decrease in cholinergic muscarinic receptors in hippocampus in the adult rat, after neonatal exposure to the brominated flame-retardant, 2,2′,4,4′,5-pentabromodiphenyl ether (PBDE 99) [J]. Environmental Toxicology and Pharmacology, 2005, 20(2): 283-288
[31]Chan W K, Chan K M. Disruption of the hypothalamic-pituitary-thyroid axis in zebrafish embryo-larvae following waterborne exposure to BDE-47, TBBPA and BPA [J]. Aquatic Toxicology, 2012, 108(1): 106-111
[32]Zheng X, Zhu Y, Liu C, et al. Accumulation and biotransformation of BDE-47 by zebrafish larvae and teratogenicity and expression of genes along the hypothalamus-pituitary-thyroid axis [J]. Environmental Science & Technology, 2012, 46(23): 12943-12951
[33]Suvorov A, Bissonnette C, Takser L, et al. Does 2,2',4,4'-tetrabromodiphenyl ether interact directly with thyroid receptor? [J]. Journal of Applied Toxicology, 2011, 31(2): 179-184
[34]Vuong A M, Webster G M, Romano M E, et al. Maternal polybrominated diphenyl ether (PBDE) exposure and thyroid hormones in maternal and cord sera: The HOME Study, Cincinnati, USA [J]. Environmental Health Perspectives, 2015, 123(10): 1079-1085
[35]Arkoosh M R, Gaest A L V, Strickland S A, et al. Alteration of thyroid hormone concentrations in juvenile Chinook salmon (Oncorhynchus tshawytscha) exposed to polybrominated diphenyl ethers, BDE-47 and BDE-99 [J]. Chemosphere, 2016, 171: 1-8
[36]Blanco J, Mulero M, López M, et al. BDE-99 deregulates BDNF, Bcl-2 and the mRNA expression of thyroid receptor isoforms in rat cerebellar granular neurons [J]. Toxicology, 2011, 290(2): 306-312
[37]Blanco J, Mulero M, Heredia L, et al. Perinatal exposure to BDE-99 causes learning disorders and decreases serum thyroid hormone levels and BDNF gene expression in hippocampus in rat offspring [J]. Toxicology, 2013, 308(2): 122-128
[38]Yu L, Han Z, Liu C. A review on the effects of PBDEs on thyroid and reproduction systems in fish [J]. General & Comparative Endocrinology, 2015, 219: 64-73
[39]Lema S C, Dickey J T, Schultz I R, et al. Dietary exposure to 2,2',4,4'-tetrabromodiphenyl ether (PBDE-47) alters thyroid status and thyroid hormone: Regulated gene transcription in the pituitary and brain [J]. Environmental Health Perspectives, 2008, 116(12): 1694-1699
[40]Kuiper R V, Vethaak A D, Cantón R F, et al. Toxicity of analytically cleaned pentabromodiphenylether after prolonged exposure in estuarine European flounder (Platichthys flesus), and partial life-cycle exposure in fresh water zebrafish (Danio rerio) [J]. Chemosphere, 2008, 73(2): 195-202
[41]Yu L, Lam J C, Guo Y, et al. Parental transfer of polybrominated diphenyl ethers (PBDEs) and thyroid endocrine disruption in zebrafish [J]. Environmental Science & Technology, 2011, 45(24): 10652-10659
[42]Yu L, Liu C, Chen Q, et al. Endocrine disruption and reproduction impairment in zebrafish after long-term exposure to DE-71 [J]. Environmental Toxicology & Chemistry, 2014, 33(6): 1354-1362
[43]Li W, Zhu L, Zha J, et al. Effects of decabromodiphenyl ether (BDE-209) on mRNA transcription of thyroid hormone pathway and spermatogenesis associated genes in Chinese rare minnow (Gobiocypris rarus) [J]. Environmental Toxicology, 2014, 29(1): 1-9
[44]Liu C, Zhang X, Deng J, et al. Effects of prochloraz or propylthiouracil on the cross-talk between the HPG, HPA, and HPT axes in zebrafish [J]. Environmental Science & Technology, 2011, 45(2): 769-775
[45]De G B, Van d G S, Darras V M, et al. Role of corticotropin-releasing hormone as a thyrotropin-releasing factor in non-mammalian vertebrates [J]. General and Comparative Endocrinology, 2006, 146(1): 62-68
[46]Liu C, Yu H, Zhang X. Zebrafish embryos/larvae for rapid determination of effects on hypothalamic-pituitary-thyroid (HPT) and hypothalamic-pituitary-interrenal (HPI) axis: mRNA expression [J]. Chemosphere, 2013, 93(10): 2327-2332
[47]Elsalini O A, von Gartzen J, Cramer M, et al. Zebrafish hhex, nk2.1a, and pax2.1 regulate thyroid growth and differentiation downstream of Nodal-dependent transcription factors [J]. Developmental Biology, 2003, 263(1): 67-80
[48]Ma Z, Song T, Su G, et al. Effects of tris (2-butoxyethyl) phosphate (TBOEP) on endocrine axes during development of early life stages of zebrafish (Danio rerio) [J]. Chemosphere, 2016, 144: 1920-1927
[49]Livak K J, Schmittgen T D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method [J]. Methods, 2001, 25(4): 402-408
[50]Huang F, Wen S, Li J, et al. The human body burden of polybrominated diphenyl ethers and their relationships with thyroid hormones in the general population in Northern China [J]. Science of the Total Environment, 2014, 466-467(1): 609-615
[51]Wang K L, Hsia S M, Mao I F, et al. Effects of polybrominated diphenyl ethers on steroidogenesis in rat Leydig cells [J]. Human Reproduction, 2011, 26(8): 2209-2217

