一种家蚕茧衍生具有蜂窝状结构的钴-锰共掺杂碳材料双功能电解水催化剂
张 明 李 涛 王 娟 潘 逸 马仕杰 朱 罕 杜明亮
(1浙江理工大学材料与纺织学院,先进纺织材料与制备技术教育部重点实验室,杭州 310018)
(2江南大学化学与材料工程学院,合成与生物胶体教育部重点实验室,无锡 214122)
0 Introduction
In the next few decades,fossil-fuel crisis,demand for energyresourcesand environmentalpollution continue to worsen,the clean,environmental friendly and renewable energy sources would play a supreme important role.Hydrogen fuel,as one of the most ideal clean energy,has recently aroused considerable attention because its high heating value and pollutionfree[1-3].Currently,electrocatalytic water splitting has been recognized as one of the most promising method for producing hydrogen.Nevertheless,the overall water splitting efficiency isgreatly influenced by the hydrogen evolution reaction (HER)and oxygen evolution reaction (OER)as a result of multistep protoncoupled electron transfer process[4-6].Therefore,much effort has been made to accelerate the HER and OER reaction kinetics and dropping the requirement of overpotential.To the best of our knowledge,Pt-based electrocatalysts are known as the most effective HER catalysts[7]and Ru or Ir-based electrocatalysts exhibit the most effective OER catalytic activity[8-11].However,the widespread application has been significantly hindered by the limited reserves and high costs.Hence,one of the main challenges in renewable energy research has been developing sustainable,cost-effective and earth-abundant electrocatalysts.
In a variety of electrode materials,graphene,which has high specific surface area of all carbon materials is extensively used in the fields of electrocatalytic water splitting because of its high specific surfacearea,superb electricalconductivity,high mechanical strength and low cost[12-15].Furthermore,N-doped graphene materials demonstrate higher catalytic activity in electrocatalysts[16-18].Recent studies suggest that among these specimens,pyridinic and graphitic nitrogen both serve as active site which play an important role in the catalytic process[19-21].Nevertheless,N-doped graphene materials almost are used as monofunctional catalysts,and enormous potential of bifunctional or multifunctional catalysts have not been widely investigated so far.In addition,at present,preparations ofN-doped graphene materials by hydrothermal reaction and thermolysis with extra N resource into their precursor are inefficient.Thanks to the high N element content,many biomass[22-24],for example silk,are expected to be ideal precursors for porous N-doped graphene materials,which usually exhibit impressive catalytic performance for HER and OER[25].
As previous reported,some carbon materials incorporated with metals have been applied to electrocatalysis and demonstrated improved electrocatalytic performance[26-28].Recently,transition metal nanoparticles(Co,Mn,Ni,Cu,etc.)coated with carbon layers have been found to be one of most effective electrocatalysts[29-33]. Theoretically,the incorporated metal nanoparticles increase the active site greatly;moreover,certain multiple-metal co-incorporated systems can exhibit synergistic effects in transition metal clusters and further improve the catalytic activity,and the carbon layer on the surface of the metal can protect the metal from coming into being metallic oxides[34].However,the growth of transition metal in carbon materials derived from biomass and its electrochemical properties have not been thoroughly studied.
In this study,we develop a simple approach to synthesize porous and graphene-like carbon materials from Bombyx mori silk cocoons by an effective thermal carbonization strategy.Bombyx mori possesses an oriented β-sheet crystal structure(high-ordered silk II structure)with a lamellar-like layer and highly porous nonwoven structure.As reported,Bombyx mori silk cocoons are composed of sericin and fibroin.After the thermal carbonization process,the sericin are eliminated[24].Proteins that make up the silk fibroin consist of 18 types of amino acid.In addition,recent reports have demonstrated that activated by KCl solution,the silk nanostructure shows excellent HER performance.
In this work,we prepared honeycomb-like structured Co-Mn incorporated N-doped graphene materials by a facile thermal carbonization process of an abundant biomass,Bombyx mori silk cocoons,as catalyst for electrolysis of water,which demonstrate amazing HER and OER performance.These encouraging results may offer a facile and effective method for the preparation of bifunctional catalysts derived from natural sustainable biomass.
1 Experimental
1.1 Synthesis and method
Bombyx mori silk cocoons were first stripped with the outermost and the innermost and then washed by DI water three times to remove impurities.The cocoons were torned into small pieces.Secondly,cobalt nitrate and manganese acetate were dissolved into DMF with different proportion solution (the total mass fraction of Co and Mn is 0.5%,2%,3%and 5%,wCo∶wMn=1∶1).The cocoons were immersed into solution for 24 hours at room temperature and dried in a vacuum oven at 40℃.The cocoons were placed into a ceramic boat,carbonized at 800℃under Ar and NH3atmosphere(Ar:150 mL·min-1,NH3:10 mL·min-1)for 4 h with a heating rate of 5℃·min-1,and cooled down to room temperature naturally.The obtained products were washed with 0.5 mol·L-1H2SO4aqueous solution and dried at 60℃for 24 h in a vacuum oven,which were denoted as MCo-Mn/SCC (M is the total mass fraction of Co and Mn).
1.2 Characterizations
Field-emission scanning electron microscopic(FE-SEM,JEOL,Japan)measurements at an acceleration voltage of 3 kV were used to characterize the morphology of all the samples.Transmission electron microscopic(TEM)measurements at an acceleration voltage of 200 kV were carried out by a JEOL JSM-2100 microscope.Powder X-ray diffraction(XRD)patterns of the samples were recorded on a SIEMENS Diffraktometer equipped with Cu Kα radiation(λ=0.154 06 nm)at 35 kV in the 2θ range of 10°~80°.X-ray photoelectron spectroscopic(XPS)measurements were obtained using a Kratos Axis Ultra DLD.
1.3 Electrochemistry
The HER and OER electrochemical perform measurements were evaluated with a typical threeelectrode system using an electrochemical workstation(CHI 660D)in 0.5 mol·L-1H2SO4and 1 mol·L-1KOH electrolytes,respectively.The samples were cut into 1 cm×1 cm and directly used as the working electrode.A platinum gauze and a saturated calomel electrode were used as counter electrode and reference electrode.In all measurements,the potentials were reported versus a reversible hydrogen electrode (RHE).Linear sweep voltammetry(LSV)with a scan rate of 2 mV·s-1was recorded in 0.5 mol·L-1H2SO4and 1 mol·L-1KOH,respectively.Cyclic voltammetry (CV)was obtained to evaluate the long-term catalysis stability at a sweep rate of 50 mV·s-1.
2 Results and discussion
To further improve the electrochemical performance,we incorporated Co-Mn with different fraction.Compared with pure silk fibroin (Fig.S1),which demonstrates a small amount of pores on surface,the samples treated with the cobalt nitrate and manganese acetate and carbonization at 800℃under Ar/NH3atmosphere have a rough surface.The content of Co-Mn will obviously influence the morphology.At the same time,the metal particles are evenly dispersed on the surface of silk fibers.Fig.1a shows the lowmagnification SEM image of the as-obtained 0.5%Co-Mn/SCC,which possesses a morphology with metal particles covering the surface but without any apparent pores.With the metal concentration increased to 2%(Fig.1b),large amounts of pores are dispersed inhomogeneously on its surface.When the content of Co-Mn was further increased to 3% (Fig.1c),the pores with the size range of 50~150 nm are distributed uniformly on the surface of carbonized silk cocoons,forming honeycomb-like network structure. This continuous honeycomb-like morphology has been well recognized as the ideal structure for water electrolysis because of shortening the ion diffusion paths during the charge/discharge process,atthe same time honeycomb-like pores can act as ions storage buffer between the electrolyte solution and electrode material.In addition,metal nanoparticles can be observed distributed at the cross-link sections which play an important role of exposing more active sites to further improve the electrochemical performance.The sample of 5%Co-Mn/SCC is shown in Fig.1d,it can be observed that the original morphology collapses from the honeycomb-like network into a compact concave appearance,which is mainly ascribed to the excessive concentration of Co and Mn ions.The honeycomb-like network was further confirmed by TEM,as shown in in Fig.S2,nearly no porous structure was observed in 0.5%Co-Mn/SCC and 5%Co-Mn/SCC,however,2%Co-Mn/SCC and 3%Co-Mn/SCC form the network structure consisting of macropores with diameter of approximately 50 nm corresponding to the Fig.1c,and obviously,all samples exist graphene sheets and metalnanoparticles.Itis noteworthy that agglomeration occurs for the 5%Co-Mn/SCC metal and the porous structure begin collapse as shown in Fig.S2d.Through the Fig.1(e,f),we noticed that the nanoparticles are covered by graphitic carbon layers and the interlayer distance of the surrounded carbon is 0.37 nm,which are assigns to the(002)planeofcarbon.Tofurtherconfirm theporous characteristics of the resultant honeycomb-like carbon materials,nitrogen adsorption-desorption measurements were conducted.The N2adsorption-desorption isotherms obtained by the quenched solid density functional theory(QSDFT)method for the as-prepared samples are shown in Fig.S3.It can be found that all the samples exhibit combinedⅠandⅣtype adsorption-desorption isotherms with N2adsorption at relatively low pressure and slightly steep adsorption in the relative pressure range of 0.8~1.0 (Fig.S3),indicating the coexistence of the micropores,mesopores and/or macropores in the materials.The pure silk fibroin only exhibits a BET surface area of 10 m2·g-1.With the incorporation of Co-Mn,the surface area of the 0.5%Co-Mn/SCC,2%Co-Mn/SCC and 3%Co-Mn/SCC increases to 148,151 and 315 m2·g-1,respectively.However,5%Co-Mn/SCC exhibits the surface area of 105 m2·g-1,indicating the evolution of pore architectures of the carbon materials derived from Bombyx mori cocoons.
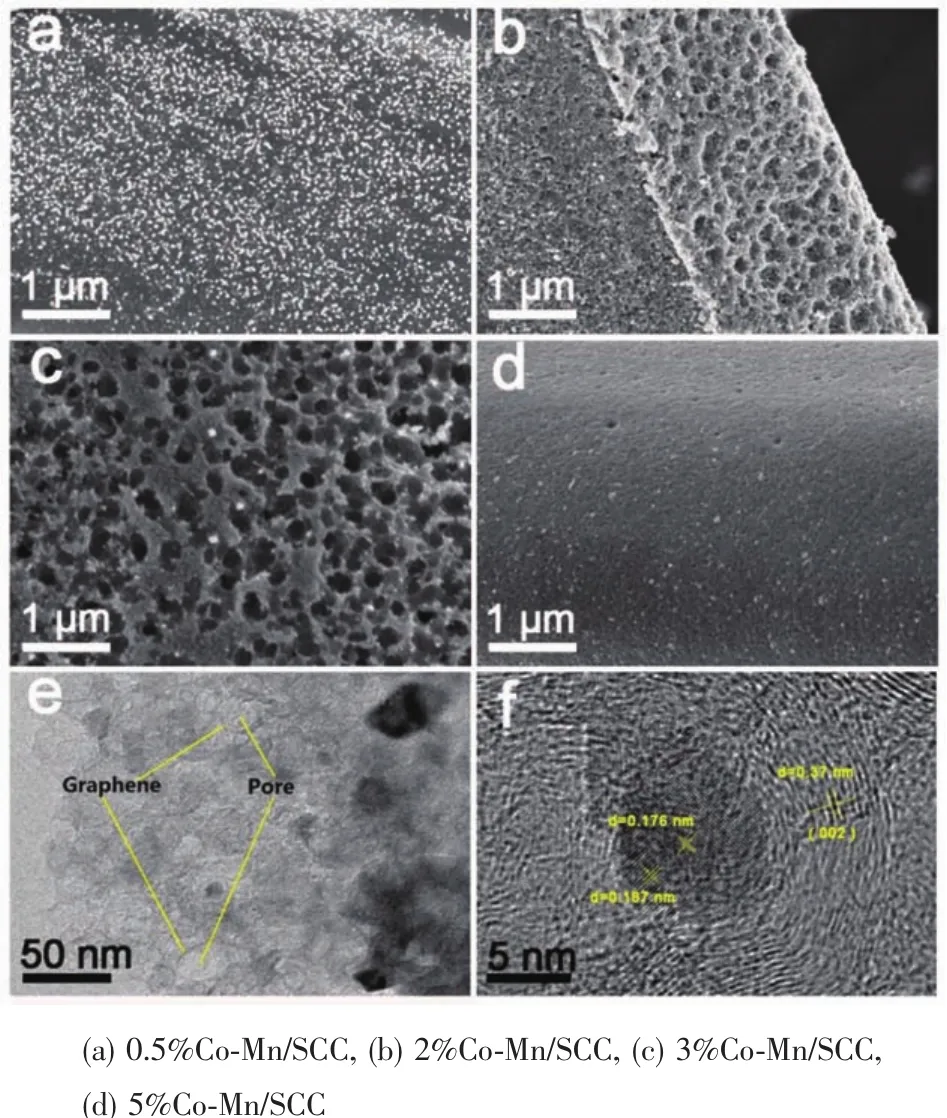
Fig.1 FE-SEM images of MCo-Mn/SCC(a~d)and TEM images of 3%Co-Mn/SCC(e,f)
The sample has been thoroughly investigated by STEM-EDS mapping(Fig.2(a,b)). Theresultsof elemental distribution indicate uniform distribution of C,N and O elements and with the line-scan EDX spectra (Fig.2c),we may conclude that part of the incorporated Co-Mn has already converted into metaloxide and after washed with 0.5 mol·L-1H2SO4aqueous solution,some have been removed,the mass fraction of Co-Mn is different from the original.To further investigate the microstructure of the carbonized silk cocoon and the crystalline structure of the Co-Mn oxide,the XRD patterns of the samples are presented in Fig.2d.All samples exhibit the same XRD pattern of Co3Mn7(PDF No.18-0407)(it is also confirmed by Fig.1f,d=0.176 and d=0.187 nm corresponding to the Co3Mn7)and(Co,Mn)(Mn,Co)2O4(PDF No.18-0409).Interestingly,all samples,excepting for 5%Co-Mn/SCC,exhibit a broad diffraction peak at around 24°corresponding to the (002)plane of graphite,demonstrating the amorphous nature and low graphitization degree.When the Co-Mn concentration increased from 0.5%to 3%,the peaks get stronger and sharper,indicating the improvement of crystallinity.However,the diffraction peak exhibit relatively lower intensity,corresponding to the lower crystallinity resulted from the metal agglomeration and over-doping of elements with excessive Co-Mn.
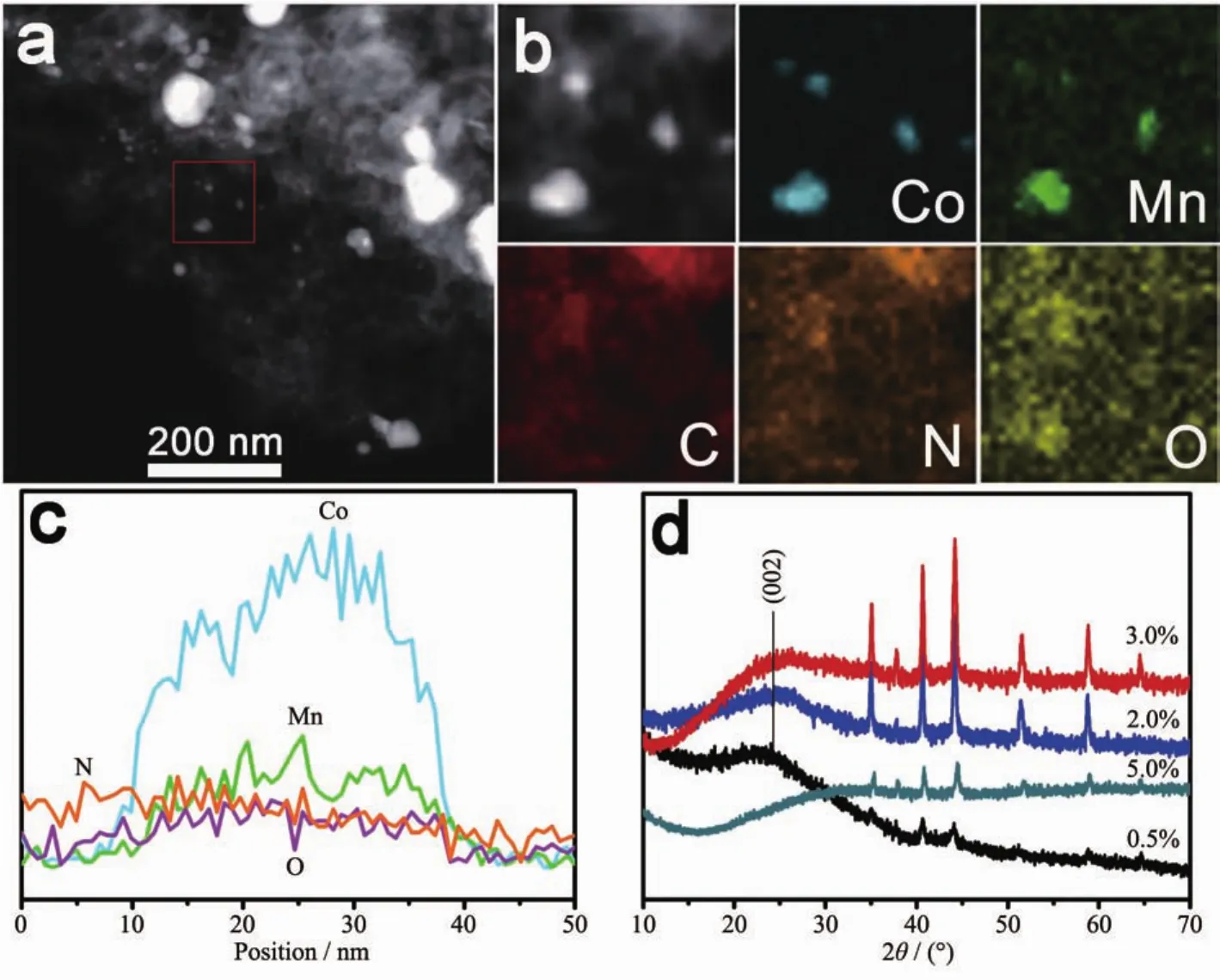
Fig.2 (a)HAADF-STEM image,(b)STEM-EDS mapping images and(c)line-scan EDX spectra of 3%Co-Mn/SCC;(d)XRD patterns of MCo-Mn/SCC
X-ray photoelectron spectroscopy(XPS)measurements were utilized to further investigate the chemical composition and surface chemistry of 3%Co-Mn/SCC.The XPS survey spectrum of the 3%Co-Mn/SCC presented in Fig.S4 displays the corresponding peaks of C,N,O,Co and Mn with no further evidence of any impurities.The high-resolution C1s spectrum could be fitted to three peaks (Fig.3a)located at approximately 284.7,285.1 and 285.9 eV,which corresponds to the graphite C bonds,the C-N and CH bonds resulting from displacement of the N atoms and the defects of the N-doped carbon fibers.Owing to nitrogen existence of N-doped silk carbon fibers(2.5%(w/w)as indicated in Fig.S5),the chemical states of nitrogen were further investigated by obtaining high-resolution N1s peaks(Fig.3b).Deconvolution of the high-resolution scan of the N1s electrons yielded three peaks at the binding energies of 401.6,400.3,and 398.6 eV,which are assigned to the graphitic nitrogen(N-Q),pyrrole-likenitrogen(N-5),andpyridinic-like nitrogen(N-6),respectively.Compared with N-5,N-Q and N-6 show more intense peaks.In other words,theactivenitrogen species(pyridinic-like nitrogen and graphitic nitrogen)[15]occupy the main part of nitrogen element.The different valence state of the Co species and Mn species of were also detected by Co2p and Mn2p spectrum(Fig.3(c,d)).It demonstrates that the Co species were mainly in the oxidation state of approximately+3.0 and Mn species correspond to metal-oxide.
The electrocatalytic activities for HER and OER were then using a typical three-electrode system with electrochemical measurements in 0.5 mol·L-1H2SO4and 1 mol·L-1KOH,respectively at room temperature.The overpotential at 10 mA·cm-2versus a reversible hydrogen electrode (RHE)is used to evaluate the HER performance.Fig.4a shows the linear sweep voltammetric(LSV)of 0.5%Co-Mn/SCC,2.0%Co-Mn/SCC,3.0%Co-Mn/SCC and 5.0%Co-Mn/SCC for HER.Obviously,the 3.0%Co-Mn/SCC reveal the best HER activity with a lower onset potential of 121 mV and an overpotential of 155 mV among four samples.According to Fig.S6,3.0%Co-Mn/SCC shows the best HER activity compared with 3.0%Co/SCC,3.0%Mn/SCC and SCC.It implies that,through the incorporation of Co and Mn,more active sites were exposed and with the formation of Co3Mn7,the HER performance is further improved.What′s more,the results imply that the HER performance was improved with the increase of Co-Mn concentration.However,the 5.0%Co-Mn/SCC catalyst exhibits poor activity,which is ascribed to the collapse of honeycomb-like network.The results suggest that the samples exhibit high electrocatalytic activity with only a small content of Co-Mn.
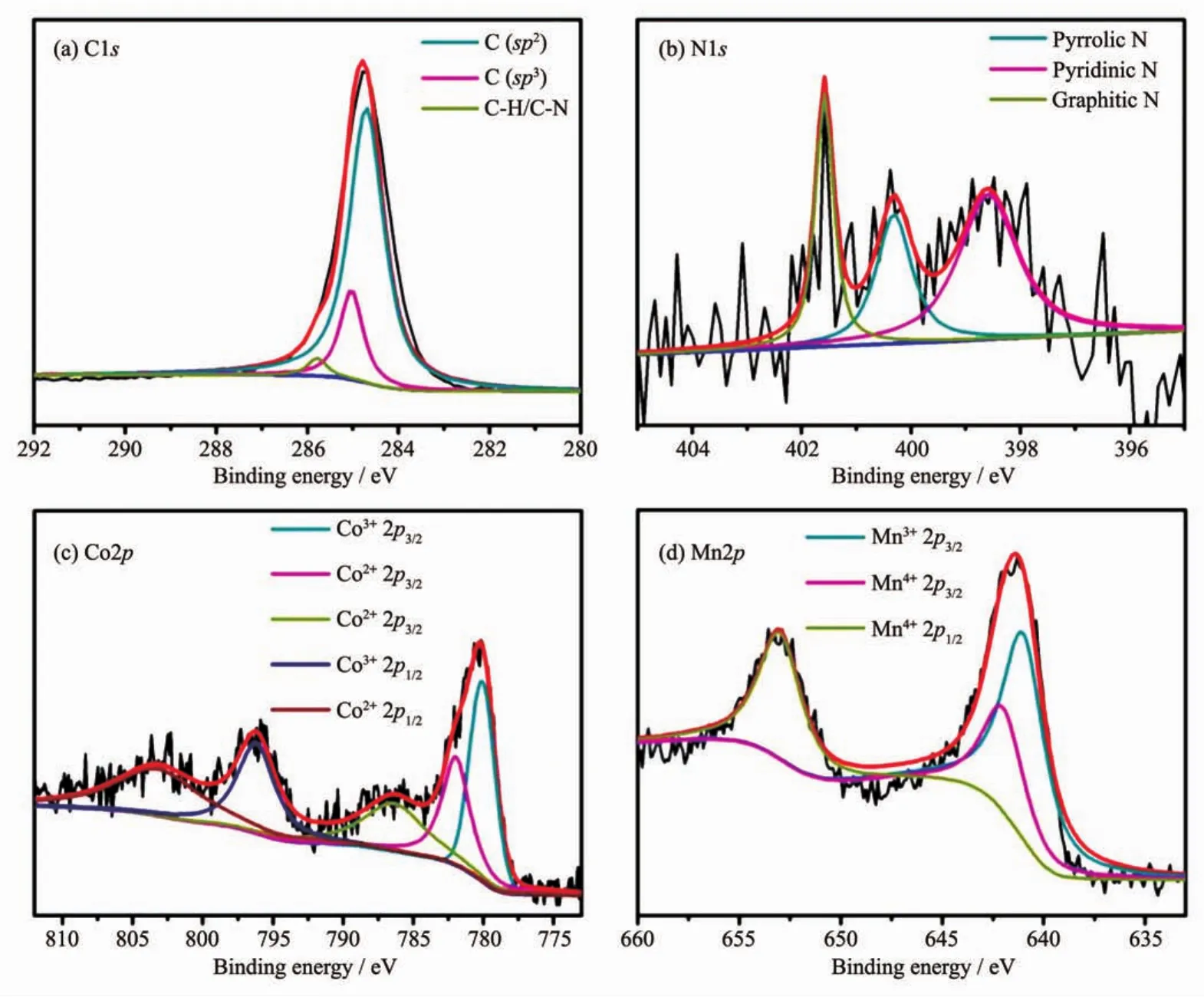
Fig.3 XPS spectra of the 3%Co-Mn/SCC
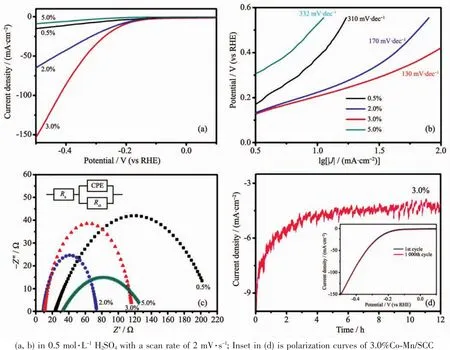
Fig.4 (a)Polarization curves,(b)corresponding Tafel slopes and(c)Nyquist plots performed at-0.25 V vs RHE of MCo-Mn/SCC;(d)Time-dependent current density of the 3.0%Co-Mn/SCC at a constant voltage of-0.2 V vs RHE
To further investigate the catalytic kinetics of HER process,the Tafel plots are calculated by the Tafel equation (η=a+blg|J|,where J is the current density,a and b are constant;and η is the onset potential)to evaluate the rate determining step(RDS)(Fig.4b).Tafel slopes of 0.5%Co-Mn/SCC,2.0%Co-Mn/SCC,3.0%Co-Mn/SCC and 5.0%Co-Mn/SCC are 310,170,130 and 332 mV·dec-1respectively.The 3.0%Co-Mn/SCC catalyst possesses the minimum Tafel slope,implying a drastic increase of HER currents with increasing electrode potential and hence better HER performance. Electrochemical impedance spectroscopy (EIS)is another index to evaluate the interface reactions and electrode kinetics of catalysts.As shown in Fig.4c,where Rsis the solution resistance and CPE is the parallel connection,the Nyquist plots of the 2.0%Co-Mn/SCC and 3.0%Co-Mn/SCC have smaller resistance and charge transfer resistance(Rct)value of 73 and 117 Ω,respectively,which denote high conductivity and fast electron transmission.In addition,after 12 hours continuous electrocatalytic operations,the current density of 3.0%Co-Mn/SCC show almostnegligible drop and afterlong-term cycling,the current density retention rate is 80%,the bubble accumulate on the catalyst surface at the first two hours,which is not conducive to the electron transport process,resulting in the current density attenuation(Fig.4d).After that,the number of bubbles is stable,and the current density is further stabilized.The polarization curves of 3.0%Co-Mn/SCC also shows a negligible decay in cathodic currents after 1 000 cycles,which demonstrates the superior cycle stability(inset in Fig.4d).
Additionally,we also explore the OER performance of M-Co-Mn/SCC (Fig.5).LSV measurements(Fig.5a)reveal that 2.0%Co-Mn/SCC exhibits the lowest onset potential of 1.5 V,which is very close to that of a high-surface-area commercial IrO2catalyst(≈1.47 V)with respect to a reversible hydrogen electrode(RHE)much lower than that of Pt/C catalysts(1.76 V)[29].Compared with 5.0%Co-Mn/SCC and 3.0%Co-Mn/SCC,2%Co-Mn/SCC exhibits the smaller Tafel slope of 143 mV·dec-1(Fig.5b),which is lower than that of Pt/C(170 mV·dec-1),indicating its rapid OER reaction kinetics.
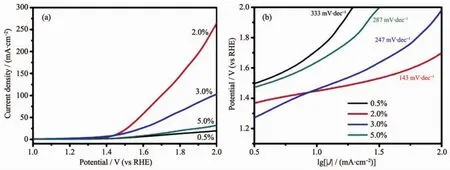
Fig.5 (a)Polarization curves and(b)corresponding Tafel slopes of MCo-Mn/SCC obtained in 1 mol·L-1KOH with a scan rate of 2 mV·s-1
In the present investigations,the change of ion concentration leads to the evolution of the morphology and structure of the Co-Mn/SCC,resulting in the oxygen-deficient structures and enhanced OER and HER activity.The material exhibit several advantages:(1)Catalysts are prepared by a carbon resource derived from silk cocoons,which are an environmental friendly and renewable biomass,without any extra N resource into precursor; (2)The honeycomb-like structured graphene materials show a hierarchical porous structure and a large specific surface area,and through the co-incorporation of Co and Mn,more active sites were exposed; (3)The honeycomb-like structured graphene materials were synthesized through a facile process and no templates or reagents were utilized; (4)The honeycomb-like structured graphene materials function as a bifunctional catalyst for electrocatalytic water splitting with high catalytic activity and outstanding stability as well as durability.
3 Conclusions
Porous and graphene-type honeycomb-like structured N-doped and Co-Mn incorporated carbon materials used as a bifunctional catalyst for electrocatalytic water splitting were designed and synthesized.Because of the high specific surface,the exposed Co-Mn active sites and the rich pyridine-N and graphitic-N,M-Co-Mn/SCC shows good HER and OER catalytic activity.In particular,3%Co-Mn/SCC exhibits high HER performance with a low onset potential of 121 mV,a low overpotential of 155 mV at 10 mA·cm-2and a Tafel slope of 130 mV·dec-1as well as long-term stability in acidic electrolyte,and the 2%Co-Mn/SCC shows great oxygen evolution reaction (OER)performance in 1 mol·L-1KOH with the lowest onset potential 1.5 mV and a Tafel slope of 143 mV·dec-1.These encouraging results may offer a facile and effective method for the preparation of bifunctional catalyst catalysts derived from naturally sustainable biomass.
Supporting information is available at http://www.wjhxxb.cn
[1]Li J S,Wang Y,Liu C H,et al.Nat.Commun.,2016,7:11204(8 Pages)
[2]Ray C,Dutta S,Negishi Y,et al.Chem.Commun.,2016,52(36):6095-6098
[3]Subbaraman R,Tripkovic D,Strmcnik D,et al.Science,2011,334(6060):1256-1260
[4]Youngblood W J,Lee S H A,Maeda K,et al.Acc.Chem.Res.,2009,42(12):1966-1973
[5]Kanan M W,Nocera D G.Science,2008,321(5892):1072-1075
[6]Zhu H,Yu D N,Zhang S G,et al.Small,2017,13:1700468
[7]Lv H F,Xi Z,Chen Z Z,et al.J.Am.Chem.Soc.,2015,137(18):5859-5862
[8]Jackson A,Viswanathan V,Forman A J,et al.ChemElectro Chem,2014,1(1):67-71
[9]Cid R E,de la Fuente J L G,Rojas S,et al.ChemCatChem,2013,5(12):3680-3689
[10]Pei J J,Mao J J,Liang X,et al.Chem.Commun.,2016,52(19):3793-3796
[11]Thomassen M S,Mokkelbost T,Sheridan E,et al.ECS Meeting:Vol.35.Zaghib K,Julienet C,Chiu W,et al.Ed.,New Jersey:Electrochemical Society,2011:271-279
[12]Bridewell V L,Kareacki C J,Kamat P V.ACS Sens.,2016,1(10):1203-1207
[13]Cui H J,Yu H M,Zheng J F,et al.Nanoscale,2016,8(5):2795-2803
[14]Chanda D,Hnat J,Dobrota A S,et al.Phys.Chem.Chem.Phys.,2015,17(40):26864-26874
[15]Yoo E,Okata T,Akita T,et al.Nano Lett.,2009,9(6):2255-2267
[16]Yang S B,Feng X L,Wang X C,et al.Angew.Chem.Int.Ed.,2011,50(23):5339-5343
[17]Subrahmanyam K S,Panchakarla L S,Govindaraj A,et al.J.Phys.Chem.C,2009,113(11):4257-4259
[18]Zhang J,Liu X,Blume R,et al.Science,2008,322(5898):73-77
[19]Liu G,Li X G,Ganesan P,et al.Electrochim.Acta,2010,55(8):2853-2858
[20]Zhao A Q,Masa J,Muhler M,et al.Electrochim.Acta,2013,98:139-145
[21]Ikeda T,Boero M,Huang S F,et al.J.Phys.Chem.C,2008,112(38):14706-14709
[22]Yan N,Chen X.Nature,2015,524(7564):155-157
[23]Wang R F,Wang K,Wang Z H,et al.J.Power Sources,2015,297:295-301
[24]Gao Y J,Chen X,Zhang J G,et al.ChemPlusChem,2015,80(10):1556-1564
[25]Liu X R,Zhang M,Yu D N,et al.Electrochim.Acta,2016,215:223-230
[26]Murthy A P,Theerthagiri J,Premnath K,et al.J.Phys.Chem.C,2017,121(21):11108-11116
[27]Kato M,Murotani T,Yagi I.Chem.Lett.,2016,45(10):1213-1215
[28]Wang J,Zhu H,Chen J D,et al.Int.J.Hydrogen Energy,2016,41(40):18044-18049
[29]Zhu H,Gu L,Yu D N,et al.Energy Environ.Sci.,2016,10(1):321-330
[30]Liu X,Meng C G,Han Y.J.Phys.Chem.C,2013,117(3):1350-1357
[31]Lu H S,Zhang H M,Liu R R,et al.Appl.Surf.Sci.,2017,392:402-409
[32]Li J X,Zou M Z,Wen W W,et al.J.Mater.Chem.A,2014,2(26):10257-10262
[33]Liu K,Song Y,Chen S W.Nanoscale,2015,7(3):1224-1232
[34]Yuasa M,Oyaizu K,Murata H,et al.Electrochemistry,2012,75(10):800-806

