Characterization of a novel deep-sea microbial esterase EstC10 and its use in the generation of (R)-methyl 2-chloropropionate*
GONG Yanhui (公颜慧) MA Sanmei (马三梅) WANG Yongfei (王永飞) XU Yongkai (许永楷) SUN Aijun (孙爱君) ZHANG Yun (张云) HU Yunfeng (胡云峰) 5
1 Key Laboratory of Tropical Marine Bio-Resources and Ecology, South China Sea Institute of Oceanology, Chinese Academy of Sciences, Guangzhou 510301, China
2 Guangdong Key Laboratory of Marine Materia Medica, South China Sea Institute of Oceanology, Chinese Academy of Sciences,Guangzhou 510301, China
3 Department of Biotechnology, Jinan University, Guangzhou 510632, China
4 Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan 250014, China
5 South China Sea Bio-Resource Exploitation and Utilization Collaborative Innovation Center, Guangzhou 510275, China
1 INTRODUCTION
Chiral 2-chloropanoic acids and their ester derivatives are important building blocks for the synthesis of a great variety of chiral herbicides, chiral pharmaceuticals and chiral final chemicals (Schulze and Wubbolts, 1999; Kurata et al., 2004; Shen, 2005).For example, enantiomerically pure (R)-2-chloropanoic acid is utilized as a starting material in the synthesis of nutrition agent alanyl-glutamine (Breuer et al., 2004).Enantiomerically pure (S)-2-chloropanoic acid and its ester derivatives are crucial starting materials for the synthesis of (R)-2-phenoxypropionic acid, one crucial herbicide widely used in agriculture (Köhler et al.,1994; Kurata et al., 2004). It has been identified that(S)-2-phenoxypropionic acid exhibits potentially high toxicities and does not exert biological activities similar to itsR-enantiomer (Colton et al., 1995). So in order to avoid unexpected enantiomers and reduce the toxicities brought by unexpected enantiomers, the synthesis of chiral 2-chloropanoic acids and their ester derivatives has been a hot spot in asymmetric synthesis.
Chiral 2-chloropanoic acids and their ester derivatives contain one chiral carbon center connected with a chlorine atom and could be synthesized using traditional organic synthesis. However, traditional organic synthesis suff ers from some problems such as harsh working conditions and great pollution to the environment. Scientists are trying to generate chiral chemicals like chiral 2-chloropanoic acids and their ester derivatives using enzymes through green biocatalytic methods (Bui et al., 2000; Wang et al.,2014). Due to the lack of hydroxyl group, chiral 2-chloropanoic acids and their ester derivatives cannot be prepared from keto precursors through bioreduction using dehydrogenases (Moore et al., 2007).So the preparation of chiral 2-chloropanoic acids and their ester derivatives is mainly through kinetic resolutions catalyzed by esterases.
Esterases (EC 3.1.1.1) are one important class of hydrolases and have been widely utilized in industry(Koeller and Wong, 2001). Chiral alcohols and acids could be enzymatically prepared by using esterases through kinetic resolutions such as transesterifications, esterifications and hydrolysis reactions(Cambou and Klibanov, 1984; Ballesteros et al.,1989; Otero et al., 1990; Carta et al., 1991). Before our study, there were only few reports about the preparation of chiral 2-chloropanoic acids and their ester derivatives using esterases through kinetic resolutions, possibly because the structural difference on both sides of the ester bond was too small to be recognized by esterases. We previously functionally characterized one novel microbial esterase EST12-7 and utilized EST12-7 as a biocatalyst in the asymmetric synthesis of enantiomerically pure (R)-methyl 2-chloropropionate (Cao et al., 2016).
Because of the diverse environments of the oceans,many novel microorganisms and novel esterases should be able to be identified from the oceans. So we can isolate novel microorganisms and use novel microbial enzymes represented by esterases from the oceans as biocatalysts in diverse industries. Herein,we cloned and functionally characterized one novel esterase EstC10 fromBacillussp. CX01 isolated from the deep sea of the Western Pacific Ocean. Deep-sea microbial esterase EstC10 was further developed into another novel biocatalyst in the preparation of enantiomerically pure (R)-methyl 2-chloropropionate with high enantiomeric excess.
2 MATERIAL AND METHOD
2.1 Microorganisms and reagents
Bacillussp. CX01 was isolated from the deep sea of the Western Pacific Ocean. The plasmid pET28(a) was used as the protein expression vector. The strainsEscherichiacoliDH5α andE.coliBL21 (DE3) were used for cloning and protein expression, respectively.The reagents related to gene cloning were all obtained from the company of TransGen Biotech (Beijing,China).P-nitrophenyl (p-NP) esterases were purchased from Sigma (USA). (±)- methyl 2-chloropropionate and enantiomerically pure methyl 2-chloropropionate were purchased from Aladdin Industrial Corporation(Shanghai, China) and TCI Development Corporation(Shanghai, China), respectively. Other chemicals were of analytical grade.
2.2 Sequence analysis
The sequence homology of EstC10 was analyzed by the BLASTP program against the protein data banks at NCBI. DNAMAN 7.0 program was used to construct the multiple sequence alignments. Three dimensional model of enzyme was built and analyzed through the online Swiss-Model (https://www.swissmodel.expasy.org/) Phylogenetic tree analysis was conducted using the MEGA software version 5.Expasy (http://web.expasy.org/compute_pi/) were used to estimate the theoretical molecular weight andpIof EstC10.
2.3 Construction of cloning and expression vector
The gene encoding esterase EstC10 was cloned from the genome ofBacillussp. CX01 by using PCR with the following primers: 5'-CATGGATCCATGAAAATCGTCAAACCA-3'5'- CATCTCGAGTTATGTCTGCCAATCCAG-3'(the restriction sites ofBamHI andXhoI were underlined). PCR products were purified by using 0.8% agarose gel electrophoresis and cloned into pET28 (a) vectors by T4 ligase.Recombinant plasmids were transformed intoE.coliBL21 (DE3) competent cells for further protein expression.
2.4 Expression and purification of EstC10
The recombinantE.coliBL21 (DE3) cells were grown in LB medium supplemented with 50 μg/mL kanamycin at 37°C. To induce the target protein expression, when the OD600reached 0.6–0.8,isopropyl-β-D-thiogalactopyranoside (ITPG) at a final concentration of 0.3 mmol/L was added. After the protein was inducted at 22°C for 16 h, the cells were collected by centrifugation at 4 000 r/min for 20 min. Subsequently, the sediment were washed twice with 50 mmol/L phosphate buff er (pH 8.0),resuspended in 50 mmol/L Tris-HCl buff er (pH 8.0)and then disrupted by sonication on ice for 15 min.The supernatants were collected by centrifugation at 9 000 r/min for 30 min. The purification and desalination of target proteins were carried out by nickel-nitrilotriacetic acid agarose resin (QIAGEN,Hilden, CA, Germany), and PD-10 desalting columns(GE Healthcare Life Science, UK), respectively. The concentrations of purified proteins were measured by the Bradford method with bovine serum albumin as a standard. Purified esterase EstC10 was analyzed by SDS-PAGE. The enzyme powders were preparated by SCIENT-N10 freeze dryer and further stored at-20°C for following experiments.
2.5 Biochemical characterization of EstC10
The biochemical characterization was studied by enzymatic reaction systems containng 8 μL substrate(10 mmol/L, dissolved in acetonitrile) and 10-μL(0.16 μg/μL) purified EstC10 in 382 μL Tris-HCl buff er (50 mmol/L, pH 8.0). After the reactions were carried out at room temperature for 5 min, 100 μL ethanol was added to inbibit the reactions. Then the enzymatic activity was determined by measuring the absorbance ofp-NP at the wavelength of 405 nm immediately after the addition of ethanol. One unit of enzyme activity was defined as the amount of esterase required to release 1 μmol ofp-NP per minute.
Variousp-NP esters (p-NP C2–C12) were used to study the substrate specificity of EstC10 under standard reaction conditions. The optimal pH of of EstC10 was determined by incubating standard reactions in different pH buff ers: 50 mmol/L PBS (pH 6.0–8.0) and 50 mmol/L Tris-HCl (pH 8.0–10.0). The pH stability was researched by detecting the residual hydrolytic activity of EstC10 (same as the method mentioned above that measuring the absorbance ofp-NP at the wavelength of 405 nm) after incubating EstC10 in different pH buff ers for different times.Standard reaction systems were carried out at temperatures ranging from 10–55°C to investigate the optimal temperature of EstC10. The themo-stability of EstC10 was studied by incubating EstC10 for 15 min, 30 min, 45 min and 60 min at temperatures ranging from 30 to 50°C.The effect of organic solvents on the hydrolytic activity of EstC10 was studied by incubating the enzyme at 200 r/min for 3 h in the presence of different organic solvents at a final concentration of 10%, 20% and 50% (V/V),respectively. EstC10 was incubated at room temperature for 3 h in the presence of various metal ions at final concentrations of 2 mmol/L and 5 mmol/L or surfactants at final concentrations of 0.1% and 0.5% (W/V), respectively, to investigate the effect of metal ions and surfactants on the hydrolytic activity of EstC10.
2.6 Kinetic resolution of racemic methyl 2-chloropropionate by EstC10
Standard 500-μL reactions containing 10 mg enzyme powders, 80 mmol/L methyl 2-chloropropionate in 50 mmol/L buff er (pH 8.0) were carried out at 37°C for 15 min. Dodecane was added to the reaction samples at a final concentration of 5 mmol/L as an internal standard after the termination of the enzymatic resolution reactions. The products were extracted by adding 500-μL ethyl acetate and the organic phase was analyzed by chiral gas chromatograph (GC) equipped with112-6636 CYCLOSIL-B chiral capillary column(30 m×0.25 mm ID, 0.25 μm df) and H2flame ion detector. The split flow rate of carrier gas, nitrogen,was 1.20 mL/min. The temperature was 100°C, raised to 220°C at a rate of 15°C/min and maintained for 2 min. The temperature of injector and detector were 220 and 250°C, respectively. The enantiomeric excess(e.e.) and yield (Y) were calculated by the formulas described by Chen et al. (1982).
e.e.=([S-methyl 2-chloropropionate]-[R-methyl 2-chloropropionate]) / ([S-methyl 2-chloropropionate]+[R-methyl 2-chloropropionate]).e. andYrepresent the enantiomeric excess and yield ratio of methyl 2-chloropropionate, respectively.AandA0represent the content ofS-methyl 2-chloropropionate after and before reactions, respectively.
A series of reactions were performed at different pH buff ers ranging from 6.0 to 9.0 to determine the optimal pH for thepreparing of (R)-methyl 2-chloropropionate. The optimal temperature for preparing (R)-racemic methyl 2-chloropropionate was investigated by incubating the enzymatic reactions at temperatures ranging from 25°C to 50°C.The effect of organic solvents and surfactants on the preparation of (R)-methyl 2-chloropropionate were investigated by incubating the enzymatic reactions in the presence of different organic solvents at a final concentration of 10% (V/V) or various surfactants at a final concentration of 0.1% (W/V). different concentrations of racemic methyl 2-chloropropionate(ranging from 60 mmol/L to 120 mmol/L) were utilized to determine the optimal substrate concentration for the preparing (R)-methyl 2-chloropropionate. The best reaction time for preparing (R)-methyl 2-cholopropionate catalyzed by EstC10 was investigated by incubating the kinetic reactions at 37°C for different times.
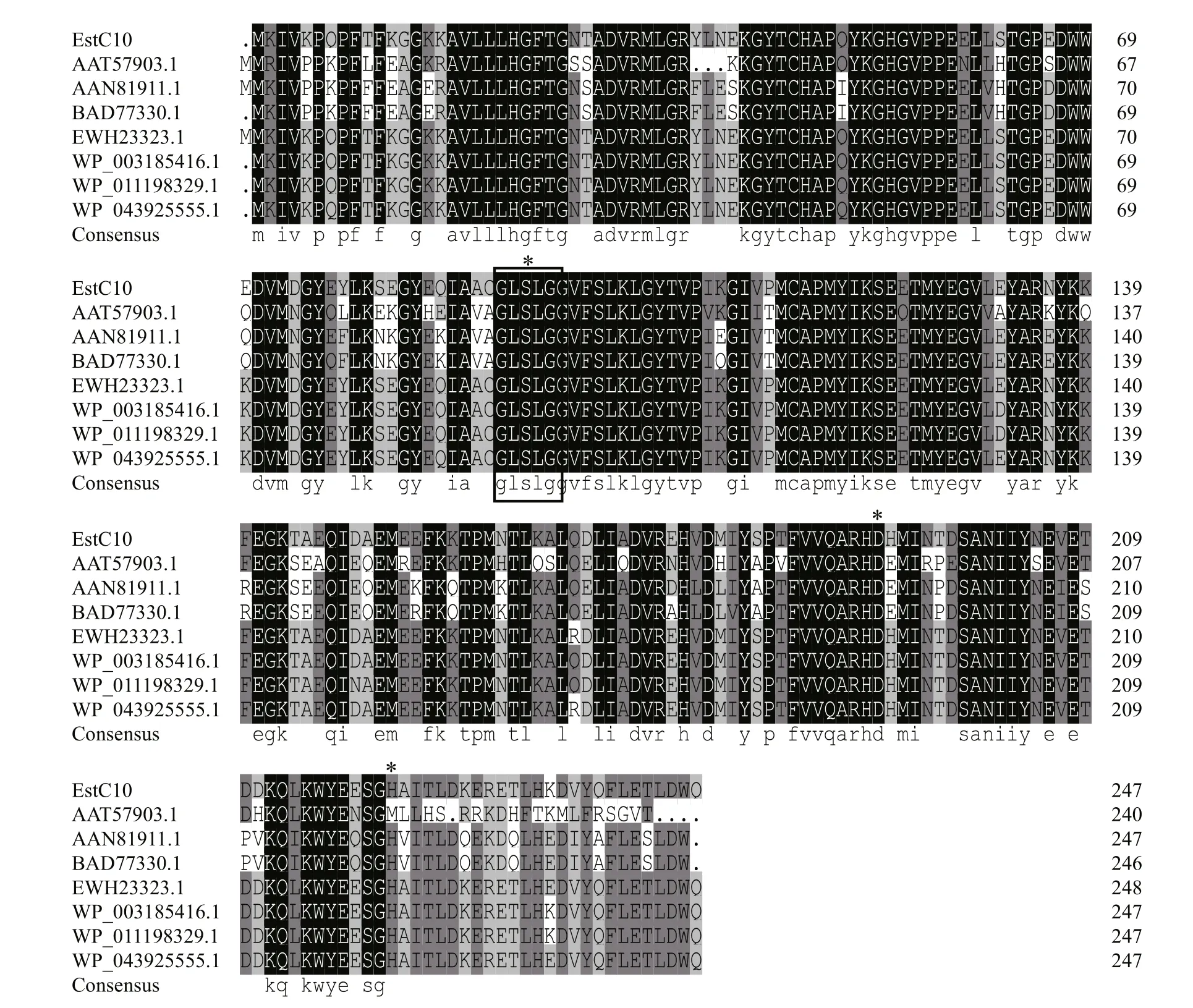
Fig.1 Multiple sequence alignment of EstC10 with some related esterases belonging to Family XIII
3 RESULT AND DISCUSSION
3.1 Sequence analysis of EstC10
A geneEstC10encoding an esterase was identified from the genome ofBacillussp. CX01 isolated from the deep sea of the Western Pacific Ocean. The GenBank accession number ofEstC10is KY478994.The protein sequence of EstC10 was blasted against the NCBI protein database. EstC10 exhibited 99%identity with one putative carboxylesterase(WP_003185416.1) fromBacillus, 98% identity with one putative carboxylesterase YVAK(WP_020452984.1) fromBacillusand 96% identity with one putative carboxylesterase (WP_026588800.1)fromBacillussp. NSP9.1. Although those proteins shared highly similarity with EstC10, the functionalities of those proteins have not been well characterized before. According to the phylogenetic tree analysis, EstC10 could be classified to family XIII of esterases. Multiple sequence alignment analysis indicated that EstC10 shared the pentapeptide GLSLG(Fig.1), which fits the serine α/β hydrolase consensus sequence Sm-X-Nu-X-Sm (Sm: small residue, X: any residue and Nu: nucleophile) (Nardini and Dijkstra,1999). Ser93, Asp192 and His222 were predicted to be the catalytic triad of EstC10 according to the treedimensional model (Rozeboom et al., 2014).

Fig.2 SDS-PAGE of purified EstC10
3.2 Expression and purification of EstC10
The theoretical molecular weight andpIwere calculated to be 31.4kD and 5.1, respectively. The recombinant EstC10 was successfully expressed inE.coliBL21 (DE3) and purified by Ni-NTA affinity chromatography (Fig.2).
3.3 Substrate specificity of EstC10
The hydrolytic activity of EstC10 was investigated with differentp-NP esterase as substrates (Fig.3). The experiments showed thatp-NP C2 was the preferred substrate of EstC10, with a specific activity of 310 U/mg. The hydrolytic activity of EstC10 was higher than those of EstA (176 U/mg) (Park et al., 2007),EM2L8 (156 U/mg) (Chu et al., 2008), and Est6(104 U/mg) (Jiang et al., 2012). The relative hydrolytic activity of EstC10 towardp-NP C4 andp-NP C6 were 84% and 56%, respectively. The relative activity towardp-NP (>C6) decreased rapidly. Those results indicated that EstC10 was a true esterase instead of a lipase (Arpigny and Jaeger, 1999).
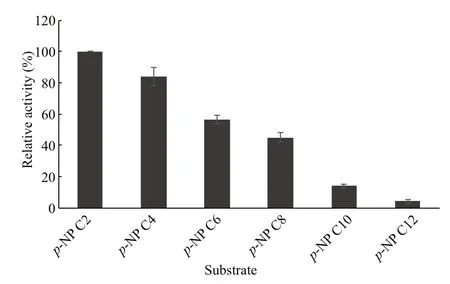
Fig.3 Hydrolytic activity of EstC10 toward p-NP esters with different acyl chain lengths (C2–C4)
3.4 effect of pH on the hydrolytic activity of EstC10
As shown in Fig.4a, the optimal pH of EstC10 was 8.0. Esterases BSE04211 from Bacillus (Liang et al.,2016a), MT6 (Deng et al., 2016), Est12-7 (Cao et al.,2016) and ScsEst01 from a South China Sea sediment metagenome (Zhang et al., 2015) had similar optimal pH. EstC10 exhibited high hydrolytic activities from pH 7.0 to 9.0. The hydrolytic activity of EstC10 dropped sharply under acidic or alkaline condition.Additionally, the hydrolytic activity of EstC10 in PBS buff er was better than that in Tris-HCl buff er under the same pH. EstC10 exhibited good stability from pH 6.0 to 9.0, with the residual hydrolytic activities being over 50% of the maximum hydrolytic activity(Fig.4b). However, EstC10 did not exhibit good stability at pH lower than 6.0 or higher than 9.0.
3.5 effect of temperature on the hydrolytic activity of EstC10
The optimal temperature was 35°C (Fig.4c), which was lower than that of esterases isolated from marine environment, BSE04211 (40°C) (Liang et al., 2016a),BSE01281(50°C) (Liang et al., 2016b) and E29(45°C) (Li, 2016). EstC10 exhibited high stability activities from 30°C to 45°C, at which the relative hydrolytic activities were above 80%. EstC10 performed excellent good stability at temperature between 30°C and 45°C (Fig.4d). After incubating EstC10 at 45°C for 90 min, the residual hydrolytic activity of EstC10 was above 65%. Its thermostability dropped rapidly when the temperature was over 50°C, with 10% of its original hydrolytic activity left after incubation at 50°C for 15 min.
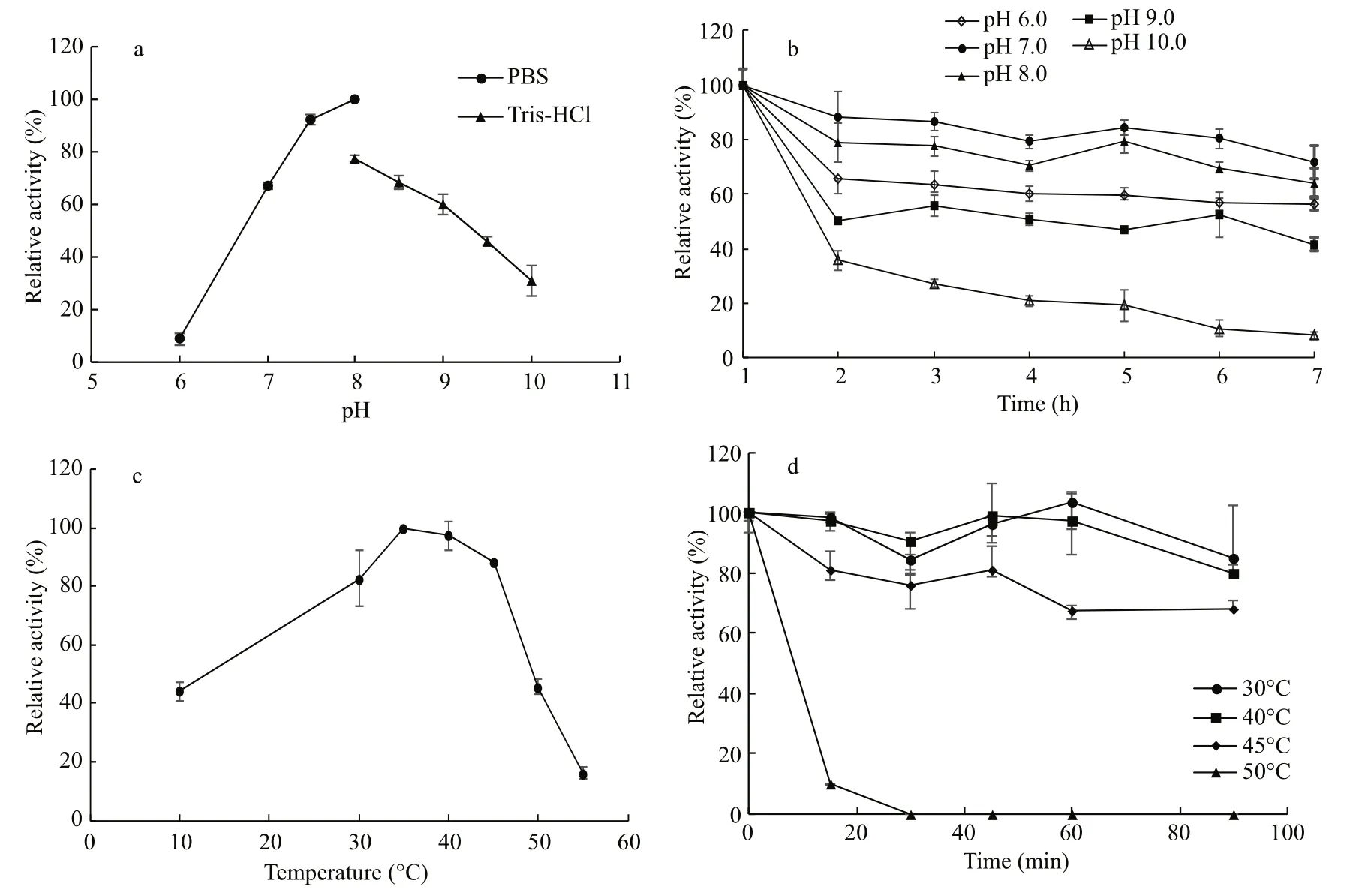
Fig.4 effect of pH and temperature on the activity of EstC10

Table 1 effect of organic solvents on the hydrolytic activity of EstC10
3.6 effect of organic solvents on the hydrolytic activity of EstC10
As shown in Table 1, 50% (V/V) isooctane and n-hexane were observed to have stimulating effects on its hydrolytic activity. The similar phenomenon was also observed from esterase RppE01 (Ma et al.,2013). However, other organic solvents in this study performed inhibiting effect on the hydrolytic activity of EstC10, especially dimethylbenzene, 1-pentanol,tetrahydrofuran and cyclohexanone.
3.7 effect of metal ions on the hydrolytic activity of EstC10
According to the data from Table 2, the hydrolytic activity of EstC10 was not affected by Na+, Ba2+, K+,Mg2+or Ca2+. However, the hydrolytic activity of EstC10 was inhibited by the presence of Cu2+and Zn2+. Esterase EstZF172 (Xu et al., 2015), one esterase fromGeobacillussp. JM6 (Zhu et al., 2015), EstOF4(Rao et al., 2013) and EstCE1 (Elend et al., 2006)exhibited similar results.
3.8 effect of surfactants on the hydrolytic activity of EstC10

Fig.5 effect of pH on the preparation of ( R)-methyl 2-chloropropionate (a); effect of temperature on the preparation of ( R)-methyl 2-chloropropionate (b); effect of substrate concentration on the preparation of ( R)-methyl 2-chloropropionate(c); effect of reaction time on the preparation of ( R)-methyl 2-chloropropionate (d)
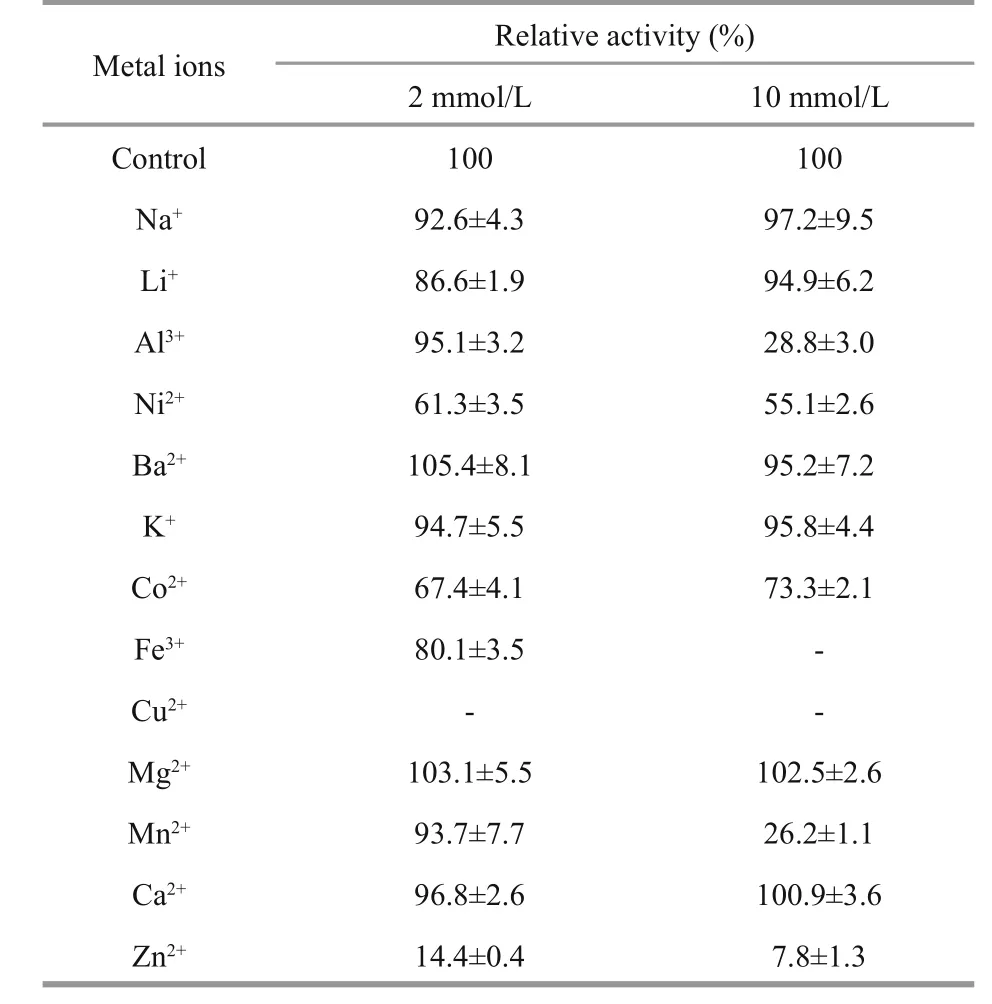
Table 2 effect of metal ions on the hydrolytic activity of EstC10
Results in Table 3 indicated that tween-20 and sodium tripolyphosphate at a concentration of 0.5%had stimulating effect on the hydrolytic activity of EstC10. However, Tween-80 and TritonX-100 exhibited negative effects on its hydrolytic activity. In addition, the hydrolytic activity of EstC10 was strongly inhibited by SDS, SDBS and CTAB.

Table 3 effect of surfactants on the hydrolytic activity of EstC10
3.9 effect of pH on the preparation of ( R)-methyl 2-chloropropionate
The effect of pH on the kinetic resolution of methyl 2-chloropropionate catalyzed by EstC10 was investigated by incubating standard reaction systems at pH ranging from 6.0–8.5 (Fig.5a). The optimal pH for the enzymatic resolution of racemic methyl 2-chloropropionate was found to be pH 8.0,with thee.e. being 83% and a yield being 33%.
3.10 effect of temperature on the preparation of( R)-methyl 2-chloropropionate by EstC10
Standard reaction systems were carried out atdifferent temperatures ranging from 25°C to 50°C to study the effect of temperature on the kinetic resolution of racemic methyl 2-chloropropionate catalyzed by EstC10 (Fig.5b). The higheste.e. (83%)was obtained at 37°C with a yield of 33%. So, 37°C was determine to be the optimal temperature for preparing (R)-methyl 2-chloropropionate catalyzed by EstC10.
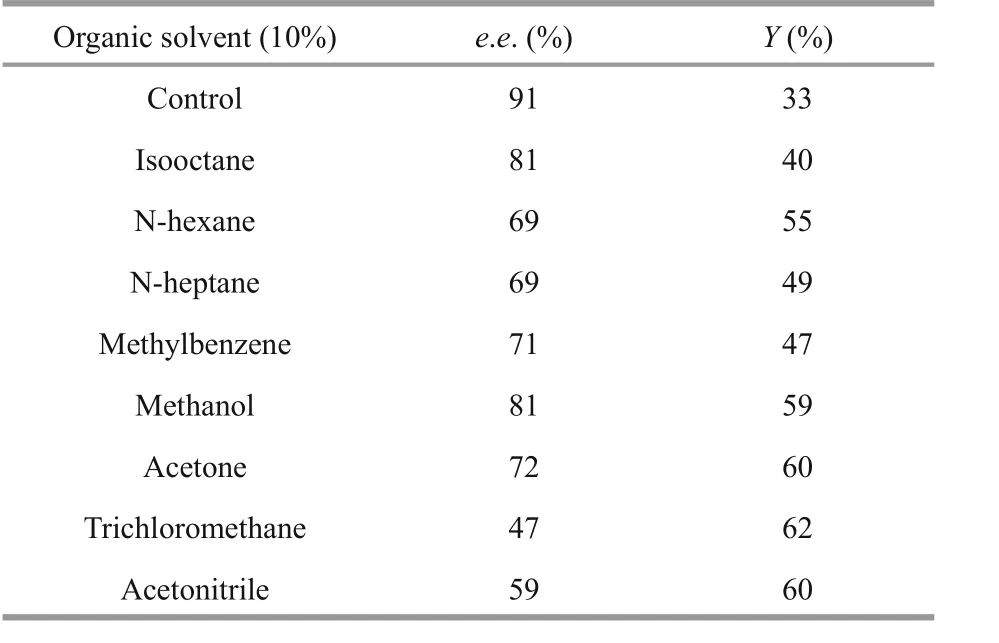
Table 4 effect of organic solvents on the preparation of ( R)-methyl 2-chloropropionate
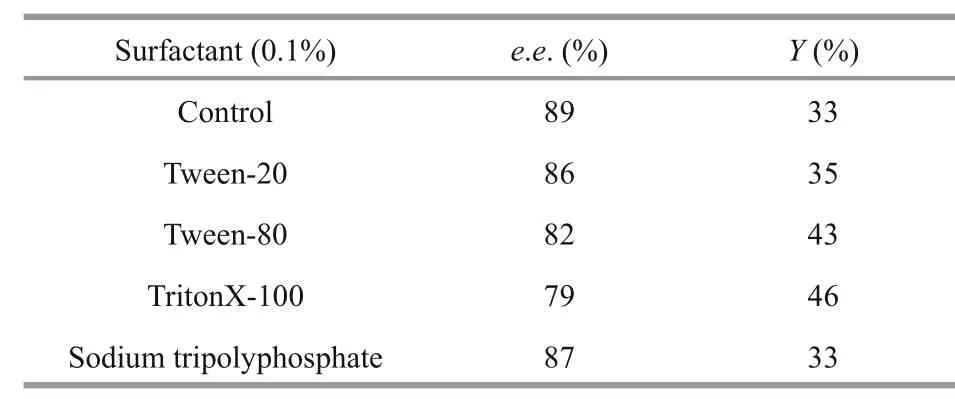
Table 5 effect of surfactants on the preparation of ( R)-methyl 2-chloropropionate
3.11 effect of organic solvents on the preparation of ( R)-methyl 2-chloropropionate by EstC10
different organic solvents were used to study the effect of organic solvents on the kinetic resolution of racemic methyl 2-chloropropionate catalyzed by EstC10 under the optimal temperature and pH (37°C,pH 8.0). As shown in Table 4, all organic solvents exhibited negative effects on the kinetic resolution of racemic methyl 2-chloropropionate by EstC10.Therefore, no organic solvent was added to further optimize the kinetic resolutions.
3.12 effect of surfactants on the preparation of ( R)-methyl 2-chloropropionate catalyzed by EstC10
Various surfactants at final concentrations of 0.1%,0.5% and 1% (W/V) were added into the standard resolution reaction systems to investigate the effect of surfactants on the kinetic resolution of racemic methyl 2-chloropropionate (Table 5). All tested surfactants had negative effects on the kinetic resolution of racemic methyl 2-chloropropionate. So no surfactant was utilized to further optimize the kinetic resolutions.
3.13 effect of substrate concentration on the preparation of ( R)-methyl 2-chloropropionate catalyzed by EstC10
The effect of substrate concentration on the preparation of ( R)-methyl 2-chloropropionate was investigated by adding substrate of concentrations ranging from 60 mmol/L to 120 mmol/L into the standard reaction systems (Fig.5c). The enantiomeric excess and yield of ( R)-methyl 2-chloropropionate generated could reach up to 93% and 33%,respectively, when the concentration of substrate was set at 80 mmol/L. With the increase of substrate concentration, the yield increased, but the enantiomeric excess dramatically decreased.Therefore, 80 mmol/L was determined to be the optimal substrate concentration for the kinetic resolution of racemic methyl 2-chloropropionate catalyzed by EstC10.
3.14 effect of reaction time on the preparation of( R)-methyl 2-chloropropionate by EstC10
The enzymatic kinetic resolution reactions were carried out at 37°C for different times to study the effect of reaction time on the kinetic resolution of racemic methyl 2-chloropropionate catalyzed by EstC10 (Fig.5d). The highest enantiomeric excess was obtained when the enzymatic kinetic resolution reaction was carried out for 30 min, with an enantiomeric excess of 99% and yield of 30%. When the reactions were carried out for longer times, both the enantiomeric excess and the yield basically remained unchanged.Therefore, 30 min was characterized to be the optimal reaction time for enzymatic kinetic resolution of methyl 2-chloropropionate.
3.15 Comparation of EstC10 and other esterases in the kinetic resolutions of racemic methyl 2-chloropropionate
Before this study, our research group functionally characterized one novel esterase Est12-7 fromPseudoncardiaantitumoradisand used Est12-7 as a novel biocatalyst in the enzymatic kinetic resolution of methyl 2-chloropropionate. Esterase Est12-7 could hydrolyze racemic methyl 2-chloropropionate and generate (R)-methyl 2-chloropropionate with high enantiomeric excess (e.e.>99%) and conversion (49%)under optimal conditions (50 mmol/L racemic substrate, pH 8.0, 30 min at 20°C) (Cao et al., 2016).In our work, we also functionally characterized one novel deep-sea microbial esterase EstC10. Esterase EstC10 was further used as a novel biocatalyst in the kinetic resolution of racemic methyl 2-chloropropionate and generated (R)-methyl 2-chloropropionate. Under optimal conditions (80 mmol/L racemic substrate, pH 8.0, 30 min at 37°C), the enantiomeric excess could reach 99%. However, the yield of (R)-methyl 2-chloropropionate generated by EstC10 was not quite high (30%) and remained constant even after the enzymatic reactions were incubated for longer time, possibly because the acid generated during kinetic resolutions denatured the biocatalyst.Remarkably, compared to Est12-7, the optimal substrate concentration of EstC10 was much higher,indicating esterase EstC10 is a novel biocatalyst which can bear higher substrate concentration and possesses very good potential in asymmetric synthesis. In addition, the sequence alignment of EstC10 with Est12-7 showed that these two enzymes were of very low sequence identities (10.39%), indicating that EstC10 was a novel promising biocatalyst identified from deep-sea microorganisms. Additionally, one lipase fromCandidacylindraceacould hydrolyze racemic methyl 2-chloropropionate and generate (S)-methyl 2-chloropropionate, with the enantiomeric excess and yield being 95% and 30%, respectively(Dahod and Siuta-Mangano, 1987).
4 CONCLUSION
In conclusion, we identified one novel esterase EstC10 fromBacillussp. CX01 isolated from the deep sea of the Western Pacific Ocean and characterized the functionalities of EstC10. At present, the reports about the kinetic resolution of racemic methyl 2-chloropropionate were quite rare.So further developed deep-sea microbial esterase EstC10 as a novel biocatalyst in the kinetic resolution of racemic methyl 2-chloropropionate and generate(R)-methyl 2-chloropropionate with high enantiomeric excess (>99%) after the optimization of process parameters such as pH, temperature, organic cosolvents, surfactants, substrate concentration and reaction time. Notably, the optimal substrate concentration (80 mmol/L) of esterase EstC10 was higher than that of another kinetic resolution catalyzed by esterase Est12-7 (50 mmol/L) (Cao et al., 2016).Thus, deep-sea microbial esterase EstC10 is a promising biocatalyst in the generation of (R)-methyl 2-chloropropionate as well of many other valuable chiral chemicals in industry. Some other technologies such as protein engineering may be necessary to further improve the enzymatic properties of biocatalyst EstC10.
5 ACKNOWLEDGEMENT
We thank the R/VKexueof the Chinese Academy of Sciences for collecting samples and WPOS sample center for providing samples.
Arpigny J L, Jaeger K E. 1999. Bacterial lipolytic enzymes:classification and properties.Biochem.J.,343: 177-183.
Ballesteros A, Bernabé M, Cruzado C, Martín-Lomas M,Otero C. 1989. Regioselective deacylation of 1,6-anhydroβ-D-galactopyranose derivatives catalyzed by soluble and immobilized lipases.Tetrahedron,45(22): 7 077-7 082,https://doi.org/10.1016/S0040-4020(01)89175-4.
Breuer M, Ditrich K, Habicher T, Hauer B, Keßeler M, Stürmer R, Zelinski T. 2004. Industrial methods for the production of optically active intermediates.Angew.Chem.Int.Ed.Engl.,43(7): 788-824.
Bui V P, Hansen T V, Stenstrøm Y, Hudlicky T. 2000. Direct biocatalytic synthesis of functionalized catechols: a green alternative to traditional methods with high effective mass yield.GreenChemistry,2(6): 263-265.
Cambou B, Klibanov A M. 1984. Comparison of different strategies for the lipase-catalyzed preparative resolution of racemic acids and alcohols: asymmetric hydrolysis,esterification, and transesterification.Biotechnologyand Bioengineering,26(12): 1 449-1 454.
Cao Y Y, Deng D, Sun A J, Zhang Y, Hu Y F. 2016. Functional characterization of a novel marine microbial esterase and its utilization in the enantioselective preparation of (R)-methyl 2-chloropropionate.Appl.Biochem.Biotechnol.,180(2): 210-227.
Carta G, Gainer J L, Benton A H. 1991. Enzymatic synthesis of esters using an immobilized lipase.Biotechnologyand Bioengineering,37(11): 1 004-1 009.
Chen C S, Fujimoto Y, Girdaukas G, Sih C J. 1982. Quantitative analyses of biochemical kinetic resolutions of enantiomers.J.Am.Chem.Soc.,104(25): 7 294-7 299.
Chu X M, He H Z, Guo C Q, Sun B L. 2008. Identification of two novel esterases from a marine metagenomic library derived from South China Sea.Appl.Microbiol.Biotechnol.,80(4): 615-625.
Colton I J, Ahmed S N, Kazlauskas R J. 1995. A 2-propanol treatment increases the enantioselectivity ofCandida rugosalipase toward esters of chiral carboxylic acids.J.Org.Chem.,60(1): 212-217.
Dahod S K, Siuta-Mangano P. 1987. Carbon tetrachloridepromoted stereo selective hydrolysis of methyl-2-chloropropionate by lipase.Biotechnologyand Bioengineering,30(8): 995-999.
Deng D, Zhang Y, Sun A J, Liang J Y, Hu Y F. 2016. Functional characterization of a novel marine microbial GDSL lipase and its utilization in the resolution of (±)-1-phenylethanol.Appl.Biochem.Biotechnol.,179(1): 75-93.
Elend C, Schmeisser C, Leggewie C, Babiak P, Carballeira J D, Steele H L, Reymond J L, Jaeger K E, Streit W R.2006. Isolation and biochemical characterization of two novel metagenome-derived esterases.Appl.Environ.Microbiol.,72(5): 3 637-3 645.
Jiang X W, Xu X W, Huo Y Y, Wu Y H, Zhu X F, Zhang X Q,Wu M. 2012. Identification and characterization of novel esterases from a deep-sea sediment metagenome.Arch.Microbiol.,194(3): 207-214.
Koeller M K, Wong C H. 2001. Enzymes for chemical synthesis.Nature,409(6817): 232-240.
Köhler J E H, Hohla M, Richters M, König W A. 1994. A molecular-dynamics simulation of the complex formation between methyl (R)/(S)-2-chloropropionate and heptakis(3-O-acetyl-2, 6-di-O-pentyl)-β-cyclodextrin.BerichtederdeutschenchemischenGesellschaft,127(1):119-126.
Kurata A, Kurihara T, Kamachi H, Esaki A N. 2004.Asymmetric reduction of 2-chloroacrylic acid to (S)-2-chloropropionic acid by a novel reductase fromBurkholderiasp. WS.Tetrahedron:Asymmetry,15(18):2 837-2 839, https://doi.org/10.1016/j.tetasy.2004.06.035.
Li Z Y, Rong Z, Wang Z, Huo Y Y, Meng F X, Wang C S, Cui H L, Xu X W. 2016. Cloning, expression and characterization of a novel esterase (E29) from a marine bacterium Altererythrobacter luteolus SW109T.Microbiology,43(5): 1 051-1 059, https://doi.org/10.13344/j.microbiol.china.150974. (in Chinese with English abstract)
Liang J Y, Sun A J, Zhang Y, Deng D, Wang Y F, Ma S M, Hu Y F. 2016a. Functional characterization of a novel microbial esterase identified from the Indian Ocean and its use in the stereoselective preparation of (R)-methyl mandelate.Chin.J.Oceanol.Limnol.,34(6): 1 269-1 277.
Liang J Y, Zhang Y, Sun A J, Deng D, Hu Y F. 2016b.Enantioselective resolution of (±)-1-phenylethanol and(±)-1-phenylethyl acetate by a novel esterase fromBacillussp. SCSIO 15121.Appl.Biochem.Biotechnol.,178(3): 558-575.
Ma B D, Yu H L, Pan J, Li J Y, Ju X, Xu J H. 2013. A thermostable and organic-solvent tolerant esterase fromPseudomonasputidaECU1011: catalytic properties and performance in kinetic resolution of α-hydroxy acids.BioresourceTechnology,133: 354-360, https://doi.org/10.1016/j.biortech.2013.01.089.
Moore J C, Pollard D J, Kosjek B, Devine P N. 2007. Advances in the enzymatic reduction of ketones.Acc.Chem.Res.,40(12): 1 412-1 419.
Nardini M, Dijkstra B W. 1999. α/β Hydrolase fold enzymes:the family keeps growing.CurrentOpinioninStructural Biology,9(6): 732-737, https://doi.org/10.1016/S0959-440X(99)00037-8.
Otero C, Pastor E, Ballesteros A. 1990. Synthesis of monobutyrylglycerol by transesterification with soluble and immobilized lipases.Appl.Biochem.Biotechnol.,26(1): 36-44.
Park H J, Jeon H J, Kang S G, Lee J H, Lee S A, Kim H K.2007. Functional expression and refolding of new alkaline esterase, EM2L8 from deep-sea sediment metagenome.Protein.Expr.Purif.,52(2): 340-347, https://doi.org/10.1016/j.pep.2006.10.010.
Rao L, Xue Y F, Zheng Y Y, Lu J R, Ma Y H. 2013. A novel alkaliphilicbacillusesterase belongs to the 13thbacterial lipolytic enzyme family.PLoSOne,8(4): e60645, https://doi.org/10.1371/journal.pone.0060645.
Rozeboom H J, Godinho L F, Nardini M, Quax W J, Dijkstra B W. 2014. Crystal structures of twoBacilluscarboxylesterases with different enantioselectivities.Biochim.Biophys.Acta,1844(3): 567-575, https://doi.org/10.1016/j.bbapap.2014.01.003.
Schulze B, Wubbolts M G. 1999. Biocatalysis for industrial production of fine chemicals.CurrentOpinionin Biotechnology,10(6): 609-615, https://doi.org/10.1016/S0958-1669(99)00042-7.
Shen G Y. 2005. 2-chloropropionic acid.FineandSpecialty Chemicals,13(13): 19-20, 30. (in Chinese with English abstract)
Wang M, Bao W J, Wang J, Wang K, Xu J J, Chen H Y, Xia X H. 2014. A green approach to the synthesis of novel“Desert rose stone”-like nanobiocatalytic system with excellent enzyme activity and stability.Sci.Rep.,4: 6 606.
Xu F X, Chen S Y, Xu G, Wu J P, Yang L R. 2015. Discovery and expression of aPseudomonassp. esterase as a novel biocatalyst for the efficient biosynthesis of a chiral intermediate of pregabalin.BiotechnologyandBioprocess Engineering,20(3): 473-487.
Zhang H, Li F C, Chen H X, Zhao J, Yan J F, Jiang P, Li R G,Zhu B L. 2015. Cloning, expression and characterization of a novel esterase from a South China Sea sediment metagenome.Chin.J.Oceanol.Limnol.,33(4): 819-827.
Zhu Y B, Zheng W G, Ni H, Liu H, Xiao A F, Cai H N. 2015.Molecular cloning and characterization of a new and highly thermostable esterase fromGeobacillussp. JM6.J.BasicMicrobiol.,55(10): 1 219-1 231.
 Journal of Oceanology and Limnology2018年2期
Journal of Oceanology and Limnology2018年2期
- Journal of Oceanology and Limnology的其它文章
- An aftereffect of global warming on tropical Pacific decadal variability*
- Analysis of monthly variability of thermocline in the South China Sea*
- A numerical study of the South China Sea Warm Current during winter monsoon relaxation*
- Predicting the sinkage of a moving tracked mining vehicle using a new rheological formulation for soft deep-sea sediment*
- Chemical characterization of fractions of dissolved humic substances from a marginal sea—a case from the Southern Yellow Sea*
- The morphological and molecular detection for the presence of toxic Cylindrospermopsis (Nostocales, Cyanobacteria) in Beijing city, China*
