Catgut implantation at acupoints increases the expression of glutamate aspartate transporter and glial glutamate transporter-1 in the brain of rats with spasticity after stroke
Rui-Qing Li, Ming-Yue wan, Jing Shi, Hui-Ling wang, Fei-Lai Liu, Cheng-Mei Liu, Jin Huang, Ren-Chao Liu, Le Ma, Xiao-Dong Feng,
1 Rehabilitation Center, First Affiliated Hospital of Henan University of Traditional Chinese Medicine, Zhengzhou, Henan Province, China
2 Major in Rehabilitation Medicine and Physiotherapy, Henan University of Traditional Chinese Medicine, Zhengzhou, Henan Province, China
3 Department of Oncology, Third People’s Hospital of Luoyang, Luoyang, Henan Province, China
Introduction
Stroke is a major cause of morbidity and mortality worldwide. The incidence of stroke is high in China, imparting a heavy burden on family and society (Shi, 2005). Numerous recent studies have focused on the diagnosis, treatment and pathogenesis of stroke, leading to a better understanding of the disease (Zhu et al., 2013; Qin et al., 2016).
Hemiplegic spasticity frequently occurs after stroke, and directly affects limb movement and reduces the quality of life of patients (Seo et al., 2013; Gunduz et al., 2014). Spasticity is a serious problem during recovery from hemiplegia (Zhang et al., 2009; Gao and Yang, 2014), and treating spasticity is a major challenge for rehabilitation medicine(Gong and Zhang, 2010). Gao and Yang (2014) showed that excessive excitatory transmission is the main cause of limb spasm after stroke. An important excitatory neurotransmitter, excessive glutamate increases the excitation of neurons,causing excitotoxicity (Hu and Chen, 2016), and thereby inducing or aggravating limb spasm after stroke. Therefore,reducing glutamate content in the brain is an effective way to relieve limb spasm after stroke.
A previous study showed that catgut implantation atDazhui(GV14),Guanyuan(CV4) andZhongwan(CV12)alleviates upper limb spasticity after stroke in rats (Feng et al., 2013a). Additionally, catgut implantation at the acupoints can ameliorate upper limb spasm after stroke and enhance body functions and activities of daily living in paralytics (Feng et al., 2013a). Both the Modified Ashworth Scale and isolated muscle tone in the treatment group were dramatically decreased after treatment. Therefore, in the present study, we investigate the mechanisms underlying the therapeutic effectiveness of catgut implantation at the acupoints by examining the expression of glutamate transporters.
Materials and Methods
Animals
Healthy male specific-pathogen-free Sprague-Dawley rats(n= 125), aged 42 days and weighing 260 ± 20 g, were supplied by Beijing Vital River Laboratory Animal Technology,Beijing, China (license number: SCXK (Jing) 2016-0011). All rats were housed at 22 ± 1°C, with a humidity of 50 ± 5% and noise < 60 dB. The rats were kept in individually ventilated cages in the Central Laboratory of the First Affiliated Hospital of Henan University of Traditional Chinese Medicine, and were given free access to food and water. The treatments conformed with the relevant ethical requirements, and the study was approved by the Animal Ethics Committee of the First Affiliated Hospital of Henan University of Traditional Chinese Medicine, China (approval number 8150150901). The rats were randomly divided into ischemia/reperfusion (IR) (n= 50), treatment (n= 50) and sham (n= 25) groups.
Production of the stroke model
We generated the middle cerebral artery occlusion (MCAO)model of stroke (Longa et al., 1989; Jiang et al., 2011; Uluç et al., 2011). Rats were fasted from food and water 24 hours before surgery. Nylon sutures were prepared before surgery.The rats were intraperitoneally anesthetized with 2% pentobarbital sodium (45 mg/kg), secured onto the operation table, and the necks were shaved and disinfected with iodophor. In the midline of the neck, an incision was made to expose and separate the left common carotid artery (CCA),internal carotid artery (ICA), and external carotid artery(ECA). After ligation of the CCA and ECA, the ICA was clipped with an arterial clamp. A small incision was made on the CCA near the bifurcation. The prepared nylon suture was inserted through the incision and into the ICA through the carotid bifurcation, and then stopped at the origin of the MCA. Subsequently, the nylon suture was bound to the CCA, and the incision was stitched. Reperfusion was performed 2 hours after surgery by removing the nylon suture.Functional assessments with the Zea Longa neurological deficit score and Modified Ashworth Scale were performed by the same investigator. Rats with Zea Longa scores of 1–3 and Modified Ashworth Scale scores of 1–4 were included.
Zea Longa neurological deficit score
Neurological deficit was evaluated 3 days after MCAO surgery and 7 days after treatment. The Zea Longa neurological deficit score scale (Longa et al., 1989; Cao and Cheng, 2001)is as follows: 0, no neurologic deficit symptoms, activity is completely normal; 1, mild neurologic deficit, unable to fully extend the opposite front paw; 2, moderate neurologic deficit, turning to the opposite side when crawling; 3, severe neurologic deficit, tilt towards the opposite side when crawling; 4, loss of consciousness, inability to crawl; 5, death.
The Modified Ashworth Scale
The Modified Ashworth Scale was used to evaluate muscle tone (Chen, 2009; Yang et al., 2013) 3 days after MCAO and 7 days after therapy. The scale is as follows: 0, no increase in muscle tension, activity is completely normal; 1, mild increase in muscle tension, with catch and release during flexion or extension; 2, moderate increase in muscle tension,with limbs moving easily and mild ataxia; 3, severe increase in muscle tension, with passive/active difficulty and moderate ataxia; 4, limited flexion or extension, severe ataxia.
Catgut implantation at acupoints
The treatment group received catgut implantation at the acupoints 3 days after MCAO surgery. After disinfection,the catguts (PGA absorbable surgical suture; Shanghai Pudong Jinhuan Medical Products Co., Ltd., China; license No.20000127) were cut into 0.5-cm segments and implanted atDazhui(GV14),Jizhong(GV6),Houhui,Guanyuan(CV4)andZhongwan(CV12) (Cui et al., 2010; Feng et al., 2013b)to a depth of 1 cm.Dazhui(GV14) is under the seventh cervical spine.Jizhong(GV6) is under the eleventh thoracic spine.Houhuiis in the anterior medial side of the sixth lumbar transverse process.Guanyuan(CV4) is 25 mm below the umbilicus.Zhongwan(CV12) is 20 mm above the umbilicus. The catguts are absorbed over a 2-week period. Rats in the IR and sham groups received the same handling, but did not undergo implantation. Acupoint positioning was in accordance with a previous study (Li, 2007).
2,3,5-Triphenyl tetrazolium chloride (TTC) staining
After anesthesia, the rats were perfused with normal saline through the heart. The brain was quickly removed, placed on ice, and cut into 2-mm slices. The slices were placed in 2%TTC (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) at 37°C in the dark for 20 minutes and photographed. The percent cerebral infarct volume was calculated using Image Pro Plus software (Cheng et al., 2015).
Immunohistochemical staining for glutamate aspartate transporter (GLAST) and glial glutamate transporter-1(GLT-1)

Figure 1 Effect of catgut implantation at acupoints on infarct volume in rats with spasticity after stroke.
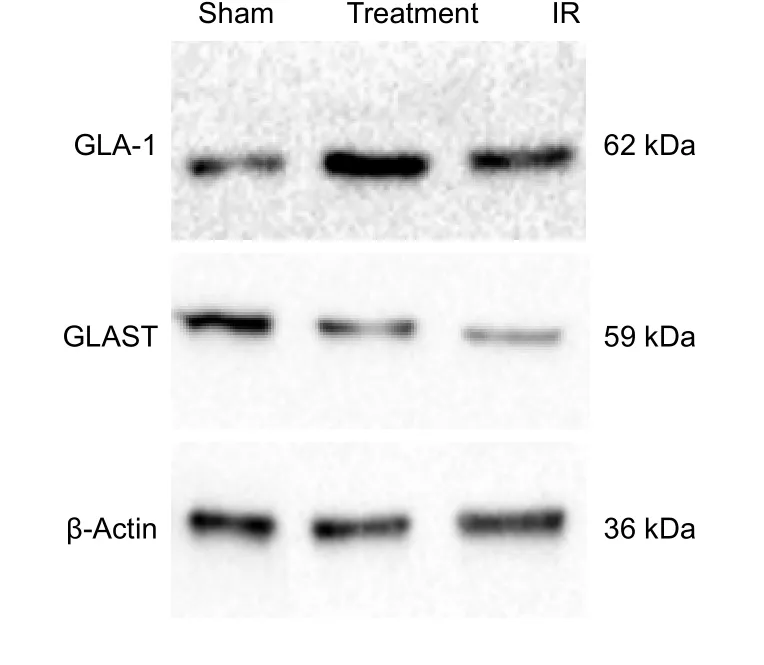
Figure 2 Effect of catgut implantation at acupoints on the protein expression levels of GLAST and GLA-1 in the injured brain of rats with spasticity after stroke, detected by western blot assay.

Figure 3 Effect of catgut implantation at acupoints on the expression of GLAST and GLA-1 in the injured brain of rats with spasticity after stroke (immunohistochemical staining).
After anesthesia, the rat was perfused through the heart with normal saline and 4% paraformaldehyde. The rat was decapitated and the brain was quickly removed and fixed in 4% paraformaldehyde for 48 hours. The tissue was then embedded in paraffin and sectioned into 5-µm-thick slices.The slices were dehydrated and incubated with rabbit anti-EAAT1 polyclonal antibody (1:500; GeneTex, Irvine, CA,USA; No. GTX37432) or rabbit anti-EAAT2 polyclonal antibody (1:500; GeneTex; No. GTX20262) at 4°C overnight,and then with goat anti-rabbit IgG (1:200; MultiSciences Biotech Co., Ltd., Hangzhou, China) at 37°C for 1 hour.After conjugating the primary antibody to the secondary antibody, the slices were visualized with 3,3′-diaminobenzidine(Beijing Solarbio Science & Technology Co., Ltd., Beijing,China), counterstained with hematoxylin (Beijing Solarbio Science & Technology Co., Ltd.), and mounted. The slides were photographed with a light microscope (Olympus,Tokyo, Japan) and analyzed using Image Pro Plus software(version 6.0; Media Cybernetics, Inc., Rockville, MD, USA)(Chang and Wang, 2003; Feng and Ji, 2017).
Western blot assay for GLAST and GLT-1
Protein concentrations were assessed with the bicinchoninic acid assay, and then, 20-µL aliquots of each sample, containing equal amounts of protein, were boiled for 5 minutes. After electrophoresis (120 V, 50 mA, 1.5 hours), the samples were transferred to a membrane, which was then blocked with 5% skim milk powder in TBST solution for approximately 1 hour, and incubated with rabbit anti-EAAT1 polyclonal antibody (1:200; GeneTex; No. GTX37432) or rabbit anti-EAAT2 polyclonal antibody (1:200; GeneTex; No.GTX20262) at 4°C overnight. The blot was then incubated with goat anti-rabbit IgG (H+L) conjugated to FITC (1:100)(Proteintech Group Inc., Chicago, USA) at 37°C for 1 hour.Developer solution was then added. Image Pro Plus software(version 6.0, Media Cybernetics) was used to analyze expression (Lai et al., 2012; Fu et al., 2014). The loading control was β-actin (β-actin antibody was from Proteintech Group, Inc.,Wuhan, China). Expression was calculated as the ratio of the optical density value of the target protein to that of β-actin.
Reverse transcription-polymerase chain reaction(RT-PCR) for GLAST and GLT-1
Brain tissue was taken out from rats and immediately placed in liquid nitrogen and stored at −20°C. Tissue samples were homogenized in TRIzol reagent (Thermo Fisher Scientific,Waltham, MA, USA), according to the manufacturer’s instructions. The RNA was dissolved in DEPC-treated water, and the purity and concentration were assessed. Real-time quantitative
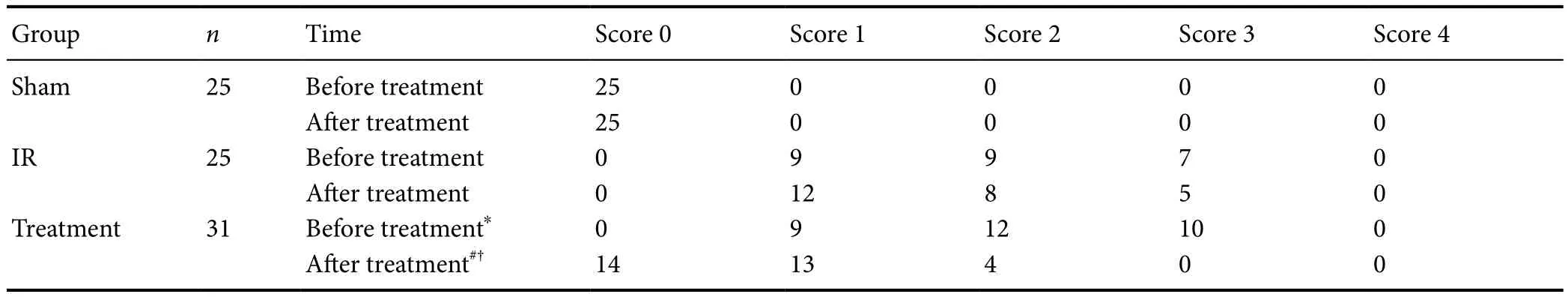
Table 1 Effect of catgut implantation at acupoints on the Zea Longa neurological deficit score in rats with spasticity after stroke
Data were analyzed using the Wilcoxon signed rank test. *P< 0.05,vs. sham group; #P< 0.05,vs. IR group; †P< 0.05,vs. before treatment. IR:Ischemia/reperfusion.PCR (RT-PCR) was performed using a kit according to the manufacturer’s instructions (Vazyme Biotech Co., Ltd., Nanjing, China), and relative mRNA expression was determined using the Ct value (Chen et al., 2008; Li et al., 2012).

Table 2 Effect of catgut implantation at acupoints on the Modified Ashworth Scale in rats with spasticity after stroke
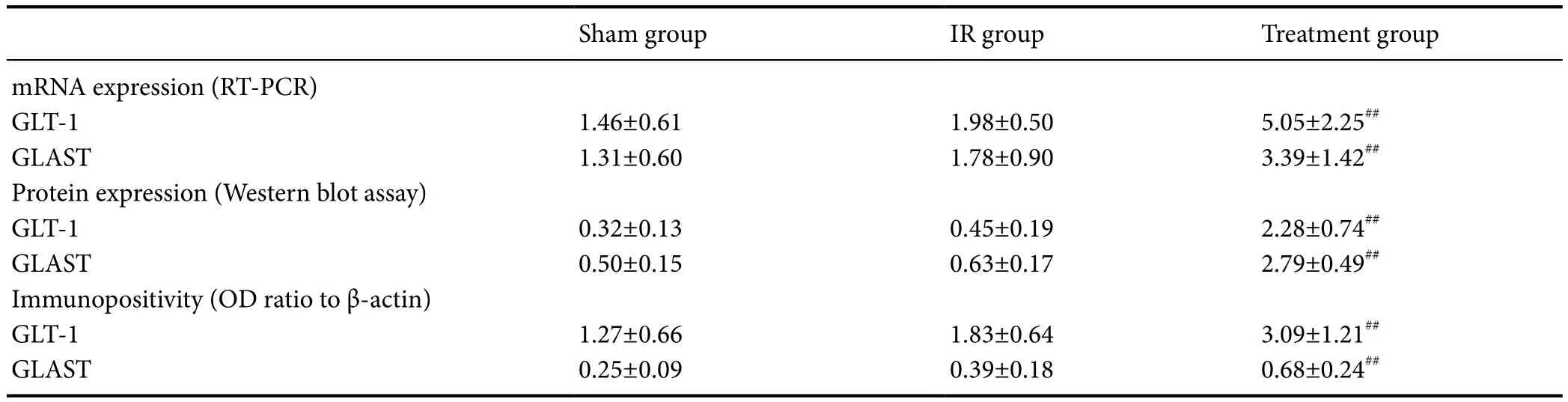
Table 3 Effect of catgut implantation at acupoints on the expression of GLAST and GLA-1 in the injured brain of rats with spasticity after stroke
Statistical analysis
All measurement data were expressed as the mean ± SD.Statistical analysis was performed with SPSS 17.0 software(IBM Corp., Armonk, NY, USA). The Wilcoxon signed rank test and independent-samplet-test were used.P< 0.05 was considered statistically significant.
Results
Quantitative analysis of experimental animals
No rats died in the sham group. Fifteen rats died, and 10 rats did not exhibit spasticity in the IR group. Eight rats died, and 11 rats did not exhibit spasticity in the treatment group. Rats in both the IR and treatment groups had Zea Longa neurological deficit scores of 1–3 and Modified Ashworth Scale scores of 1–4. Thus, the final analyses included 25 rats in the sham group, 25 in the IR group, and 31 in the treatment group. Ten rats each in the IR and treatment groups, and seven rats in the sham group were used for TTC staining. Six rats in the IR group, 10 rats in the treatment group and eight rats in the sham group were used for immunohistochemistry. Nine rats in the IR group, 11 rats in the treatment group, and 10 rats in the sham group were used for western blot assay and RT-PCR.
Catgut implantation at acupoints improved neurological function in rats with spasticity after stroke
The Zea Longa neurological deficit score was 0 in the sham group. Before treatment, the Zea Longa neurological deficit scores in the IR and treatment groups were not significantly different (P> 0.05), while they were significantly higher compared with the sham group (P< 0.05). After treatment,the neurological deficit in the treatment group significantly improved, compared with the IR group (P< 0.05) and before treatment (P< 0.05; Table 1).
Catgut implantation at acupoints improved muscle tone in rats with spasticity after stroke
The Modified Ashworth Scale score was 0 in the sham group. Muscle tone levels were not significantly different between the IR and treatment groups before treatment (P>0.05), but were significantly high compared with the sham group (P< 0.05). Muscle tone was significantly reduced in the treatment group compared with the IR group after treatment (P< 0.05) and before treatment (P< 0.05; Table 2).
Catgut implantation at acupoints decreased infarct volume in rats with spasticity after stroke
TTC staining showed that infarct volume was 0 in the sham group. Infarct volume was significantly decreased in the treatment group compared with the IR group after treatment (P< 0.05) and before treatment (P< 0.05; Figure 1).
Catgut implantation at acupoints elevated the expression of GLAST and GLA-1 in the injured brain of rats withspasticity after stroke
Immunohistochemical staining, western blot assay and RTPCR results demonstrated that the expression of GLAST and GLA-1 was elevated in the IR group compared with the sham group (P> 0.05). The expression of GLAST and GLA-1 was significantly elevated in the treatment group compared with the IR group (P< 0.05; Table 3, Figures 2 and 3).
Discussion
Spasm refers to involuntary muscle contraction, which manifests as increased muscle tension and a specific pattern of abnormal muscle coordination. According to traditional Chinese medicine, the occurrence of spasm after stroke is caused by damage to the governor vessel and a deficiency of qi and blood. The acupoints for catgut implantation lie along the meridian, which is not only the channel of qi and blood, but also a link between the exterior and interior and the viscera of the body, and is a major component of the body’s regulatory system. The governor vessel is in the middle of the spine, managing all theYangmeridians and entering into the brain. A damaged governor vessel leads to blood stasis in blood vessels, and theYang qidoes not disperse throughout the body, thereby resulting in spasm.
Catgut implantation at acupoints is an important part of traditional Chinese medicine and has a positive effect on physical dysfunction after stroke. Therefore,Dazhui(GV14),Jizhong(GV6) andHouhuilocated in governor vessels were selected to activateYang qiand relieve spasm. The production ofqiand blood by the spleen and stomach can be improved byGuanyuan(CV4) andZhongwan(CV12) in theRenchannel, which can stimulate theYang qiof the human body and relieve spasm (Jiang and Liu, 2009).
Limb spasm after stroke is caused by an impairment of the central inhibitory system and excitatory neurotransmitter release from central motor neurons, as well as increased excitability of α motor neurons, leading to stretch reflex hyperfunction (Yang et al., 2013). Zhang et al. (2009) showed that a reduction in glutamate in the brainstem can effectively relieve spasm. The glutamate transporter in the brainstem transports the accumulated glutamate to the cell, thereby terminating the excitation.
Previous studies have shown that cerebral ischemia and hypoxia-induced neuronal excitotoxicity results from impaired glutamate transporter activity and the accumulation of glutamate in the synaptic cleft (Barry et al., 2013; Sulkowski et al., 2014; Li et al., 2015). The removal of glutamate in the brainstem is mainly performed by GLAST and GLT-1 (Lagranha et al., 2014). GLAST is especially highly expressed in the cerebellum, with lower levels in the spinal cord and forebrain.GLT-1 is mainly expressed in the forebrain, hippocampus, cerebral cortex and striatum. Approximately 95% of glutamate is taken up by GLT-1 in the brain (Tani et al., 2014).
Because the accumulation of glutamate is a major reason for spasm after brain damage, we sought to investigate whether GLAST and GLT-1 expression are affected by catgut implantation at the acupoints. In our present study,infarct volume was reduced after suture implantation.GLAST and GLT-1 expression were increased by treatment following IR. GLAST and GLT-1 expression was higher in the IR group than in the sham group. Muscle tone was substantially reduced in the treatment group compared with the IR group. The muscle tone in the affected limb increased greatly 3 days after surgery, according to our preliminary experiments. Therefore, catgut implantation at the acupoints was carried out in the treatment group 3 days after MCAO,and GLAST and GLT-1 expression levels were assessed 7 days after treatment. GLAST and GLT-1 expression levels were not significantly different between the IR and sham groups, likely because the brain tissue was obtained 10 days after surgery, and therefore, the IR group had 10 days to recover from the operation. This amount of time was probably sufficient for GLAST and GLT-1 expression levels to recover to near-normal levels in the IR group.
In summary, limb spasm in rats after stroke was alleviated by catgut implantation at the acupoints, possibly because of the upregulation of GLAST and GLT-1. Our findings should be helpful for the development of clinical therapy for spasm after stroke. Further studies are needed to clarify the role of GLAST and GLA-1 in the pathogenesis of stroke.
Author contributions:XDF designed the study. RQL, MYW, JS and HLW performed experiments. MYW wrote the paper. CML gave technical or material support. FLL, JH, RCL and LM analyzed data. All authors approved the final version of the paper.
Conflicts of interest:We declare that we have no conflicts of interest.
Financial support:This study was supported by the National Natural Science Foundation of China, No.14202225, No. 81574042; the Traditional Chinese Medicine Leading Talent Funding Projects of Henan Province of China, No. 2000202; a grant from the Special Research Project on the Construction of the National Traditional Chinese MedicineClinical Research Base of the State Administration of Traditional Chinese Medicine of China, No. JDZX2015314. Funders had no involvement in the study design, data collection, analysis, and interpretation, paper writing, or decision to submit the paper for publication.
Institutional review board statement:The study protocol was approved by the Animal Ethics Committee of First Affiliated Hospital of Henan University of Traditional Chinese Medicine of China (Approval number:CAP2016(A)0010). The experimental procedure followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals(NIH Publications No. 8023, revised 1985).
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix,tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer review report:
Reviewer: Ryan Hirschi, University of Utah School of Medicine, USA.
Comments to authors: This is a novel manuscript investigating the effect of catgut implantation at acupoints on the expression of GLAST and GLT-1 in the brain of rats with spasticity after stroke. It presents data supporting improved spasticity after stroke in a rat model, which is exciting. The paper has numerous figures and tables which are of good quality and add significantly to the paper.
Barry MD, Bunday KL, Chen R, Perez MA (2013) Selective effects of baclofen on use-dependent modulation of GABAB inhibition after tetraplegia. J Neurosci 33:12898-12907.
Cao YJ, Cheng YB (2001) The improvement and exploration of the focal cerebral ischemia/reperfusion model in rats. Zhongguo Yingyong Shenglixue Zazhi 17:198-200.
Chang Q, Wang XL (2003) Effects of chiral 3-n-butylphthalide on apoptosis induced by transient focal cerebral ischemia in rats. Acta Pharmacol Sin 24:796-804.
Chen CM, Yang WZ, Wang CH, Shi SS, Chen JP, Huang Y, Cai DS (2008)Neuronal tolerance to hypoxia-ischemia through recombinant adeno-associated viral vectors expressing neuronal and inducible nitric oxide synthase An in vivo study. Neural Regen Res 3:157-161.
Chen DH (2009) The neuronal behavior and pain threshold of rats with muscle strain increased after cerebral apoplexy. Anhui Zhongyi Xueyuan Xuebao 28:44-46.
Cheng XQ, Li JR, Fang XK, Li YJ, Zhang W, Zhang Y, Lu GM (2015)A comparative study on the volume of cerebral infarction was measured by MRI and TTC staining in rat ischemic stroke model. Yixue Yingxue Xue Zazhi 25:1871-1873.
Cui CX, Kong LH, Zhou HJ (2010) Effect of acupoints catgut embedding therapy on the cortical VEGF mRNA expression in rats with focal cerebral ischemia-reperfusion injury. Hubei Zhongyi Zazhi 12:21-23.
Feng K, Ji LJ (2017) Effects of expression of NT-3, GAP-43, SYP-P38 and PKA in MCAO rat with Jianpi yizhi capsules. Zhonghua Zhongyiyao Zazhi 32:2923-2927.
Feng X, Yang S, Liu J, Huang J, Peng J, Lin J, Tao J, Chen L (2013a)Electroacupuncture ameliorates cognitive impairment through inhibition of NF-kappaB-mediated neuronal cell apoptosis in cerebral ischemia-reperfusion injured rats. Mol Med Rep 7:1516-1522.
Feng XD, Li RQ, Ren BB, Song XL (2013b) The effect of acupoint buried line on patients with upper limb spasm after stroke. Zhongguo Kangfu Yixue Zazhi 28:843-845.
Fu R, Fan YZ, Fan YC, Zhao HY (2014) Expression of arginyl-tRNA synthetase in rats with focal cerebral ischemia. J Huazhong Univ Sci Technolog Med Sci 34:172-175.
Gao ZQ, Yang J (2014) Research on the study of spasm hemiplegia after stroke. Zhongyiyao Linchuang Zazhi 26:201-203.Gong J, Zhang ZL (2010) The clinical observation of warm acupuncture therapy for stroke convulsion paralysis. Shizhen Guoyi Guoyao 21:1825-1826.
Gunduz A, Kumru H, Pascual-Leone A (2014) Outcomes in spasticity after repetitive transcranial magnetic and transcranial direct current stimulations. Neural Regen Res 9:712-718.
Hu JX, Chen XH (2016) Research progress of glutamate pathway and its regulation after cerebral ischemia. Fudan Xuebao: Yixue Ban 43:724-731.
Jiang JZ, Liu ZC (2009) Research progress on the clinical and mechanism of acupoint embedding therapy. Liaoning Zhongyiyao Daxue Xuebao 11:31-34.
Jiang W, Li LB, Yang M, Bi YZ, Hu KH, Zhang P, Shen YX, Yu Q (2011)Influence of acupuncture with exercise training on learning and memory functions, as well as microtubule-associated protein-2 and synaptophysin expression in the hippocampal CA3 region, in a rat model of cerebral infarction. Neural Regen Res 6:2124-2128.
Lagranha VL, Matte U, de Carvalho TG, Seminotti B, Pereira CC,
Koeller DM, Woontner M, Goodman SI, de Souza DO, Wajner M(2014) Increased glutamate receptor and transporter expression in the cerebral cortex and striatum of gcdh-/-mice: possible implications for the neuropathology of glutaric acidemia type I. PLoS One 9:e90477.Lai T, Li M, Zheng L, Song Y, Xu X, Guo Y, Zhang Y, Zhang Z, Mei Y(2012) Over-expression of VEGF in marrow stromal cells promotes angiogenesis in rats with cerebral infarction via the synergistic effects of VEGF and Ang-2. J Huazhong Univ Sci Technolog Med Sci 32:724-731.
Li M, Peng J, Song Y, Liang H, Mei Y, Fang Y (2012) Electro-acupuncture combined with transcranial magnetic stimulation improves learning and memory function of rats with cerebral infarction by inhibiting neuron cell apoptosis. J Huazhong Univ Sci Technolog Med Sci 32:746-749.
Li YH, Zhang CL, Zhang XY, Zhou HX, Meng LL (2015) Effects of mild induced hypothermia on hippocampal connexin 43 and glutamate transporter 1 expression following traumatic brain injury in rats. Mol Med Rep 11:1991-1996.
Li ZR (2007) Experimental Acupuncture. Beijing, China: China Press of Traditional Chinese Medicine.
Longa EZ, Weinstein PR, Carlson S, Cummins R (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20:84-91.
Qin L, Wei X, Liu L, Zhu H (2016) Effectiveness of Tai Chi on movement, emotion and quality of life in patients with stroke: a Meta-analysis. Zhongguo Zuzhi Gongcheng Yanjiu 20:297-303.
Seo JP, Lee MY, Kwon YH, Jang SH (2013) Delayed gait recovery in a stroke patient. Neural Regen Res 8:1514-1518.
Shi XM (2005) Clinical research on the treatment of 9005 cases of apoplexy with the acupuncture method of sharpening mind and inducing consciousness. Zhongyiyao Daobao 11:3-5.
Sulkowski G, Dąbrowska-Bouta B, Salińska E, Strużyńska L (2014)Modulation of glutamate transport and receptor binding by glutamate receptor antagonists in EAE rat brain. PLoS One 9:e113954.
Tani H, Dulla CG, Farzampour Z, Taylor-Weiner A, Huguenard JR,Reimer RJ (2014) A local glutamate-glutamine cycle sustains synaptic excitatory transmitter release. Neuron 81:888-900.
Uluç K, Miranpuri A, Kujoth GC, Aktüre E, Başkaya MK (2011) Focal cerebral ischemia model by endovascular suture occlusion of the middle cerebral artery in the rat. J Vis Exp:1978.
Yang SQ, Jin RJ, Zhu TM, Ma HY, Feng LJ, Mao XL (2013) The effects of expression of γ-aminobutyric acid interneuron rehabilitation training combined with electricity acupuncture to the limb spasm rats of stroke. Zhongguo Kangfu Yixue Zazhi 28:198-203.
Zhang L, Wang JC, Guo M, Wang CY, Yang ZH (2009) The observed of curative effect of myna and baclofen to the treatment of limb spasm. Zhongguo Zhongyi Jichu Yixue Zazhi 15:148-149.
Zhu Y, Yang YJ, Gu YH, Xie B, Jin HZ (2013) Efficiency of repetitive transcranial magnetic stimulation on rehabilitation of motor function in patients with stroke:A systematic review. Zhongguo Zuzhi Gongcheng Yanjiu 17:8758-8768.
- 中国神经再生研究(英文版)的其它文章
- Role of brain-derived neurotrophic factor during the regenerative response after traumatic brain injury in adult zebrafish
- Natural polyphenols effects on protein aggregates in Alzheimer’s and Parkinson’s prion-like diseases
- How random is the random forest ? Random forest algorithm on the service of structural imaging biomarkers for Alzheimer’s disease: from Alzheimer’s disease neuroimaging initiative (ADNI) database
- Protective effects of gonadal hormones on spinal motoneurons following spinal cord injury
- INVITED REVIEW
- Role of presynaptic calcium stores for neural network dysfunction in Alzheimer’s disease

