Injection of bone marrow mesenchymal stem cells by intravenous or intraperitoneal routes is a viable alternative to spinal cord injury treatment in mice
Bruna dos Santos Ramalho, Fernanda Martins de Almeida, Conrado Mendonça Sales, Silmara de Lima, Ana Maria Blanco Martinez
Laboratório de Neurodegeneração e Reparo, Departamento de Patologia – Faculdade de Medicina, HUCFF, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil
Introductio n
Spinal cord injury (SCI) is a devastating condition. Patients with SCI exhibit functional, sensory, and autonomic deficits due to neuronal and glial cell death, axonal degeneration,and demyelination. These deficits impact the patient’s quality of life and life expectancy (Ahuja et al., 2017), with great economic loss to society (Varma et al., 2013). There is an urgent need for effective treatments to attenuate these consequences.
In general, a central nervous system injury leads to nerve fiber transection and surrounding tissue damage. Dystrophic growth cones formed at the distal ends of damaged axons are exposed to a hostile glial microenvironment created by inhibitory molecules (Martinez et al., 2014). The presence of myelin inhibitory proteins and glial scar formation, usually accompanied by cavities filled with chondroitin sulfate proteoglycans, restricts axon growth (Mietto et al., 2015). Also,the expression of trophic factors is reduced in the lesion milieu, due to neuronal atrophy and cell death after axonal injury (Yiu and He, 2006; Wright et al., 2011).
Several experimental therapeutic strategies have been studied in the SCI field, and recent advances have led to the development of therapies that may act on the inhibitory microenvironment. Assorted lineages of stem cells are considered a good treatment for SCI (Liu et al., 2013; Zhou et al.,2013; Vismara et al., 2017). Previous studies by our group using pre-differentiated embryonic stem cells (Marques et al., 2010), human dental-pulp stem cells (de Almeida et al.,2011) and mesenchymal stem cells (de Almeida et al., 2015)in a mouse model of compressive SCI showed that these therapies led to similar favorable results. Among different stem cells, MSCs are strong candidates for transplantation,because they can be easily extracted and cultured, do not involve ethical issues, and have the ability to self-renew.In addition, MSCs show a high potential for multilineage differentiation (Azari et al., 2010; Dasari et al., 2014). It is currently accepted that MSCs have an immunomodulatory effect and exert a neuroprotective function through paracrine production of anti-apoptotic molecules and trophic factors, which promote neuronal plasticity and lead adult neural stem cells to differentiate toward the oligodendroglial lineage in the spared spinal cord (Tetzlaff et al., 2011; Jadasz et al., 2013; Dooley et al., 2016; Chen et al., 2018; Lindsay et al., 2017; Jadasz et al., 2018).
Although several studies have used different models of spinal cord injury, with various types of cell therapy, one of many unanswered questions is the best route of administration to benefit the patient. The protocols available for stem cell injections vary considerably in the literature on SCI. The administration route is highly important for clinical application, because injections directly into the injured spinal cord may cause further damage. MSCs show a tropism for the injury site (Spaeth et al., 2008), which supports the possibility of systemic injection. Therefore, we used two routes of administration of MSCs, intravenous and intraperitoneal, and evaluated the tissue and functional improvement following a compressive SCI.
Materials and Methods
Ethics statement
Surgical procedures and animal handling were carried out in accordance with the approved guidelines of the Committee on Animal Care of the Health Science Center of the Federal University of Rio de Janeiro. All experimental procedures were approved by the same committee (Protocol number DHEICB003). All animals used in this study were obtained from the Laboratory of Transgenic Animals of the Institute of Biophysics Carlos Chagas Filho (LAT/BioRio - Federal University of Rio de Janeiro).
MSCs culture
MSCs were harvested from the bone marrow of GFP-expressing C57BL/6 female and male mice, aged 8-12 weeks,weighing 20–25 g, as described previously (de Almeida et al.,2015). In summary, the bone marrow from the femurs and tibias of donor mice was flushed with Hank’s buffered saline solution (HBSS) and centrifuged at 700 ×gfor 5 minutes.Cells were seeded in 25-cm2culture flasks containing Dulbecco’s Modified Eagle’s medium (DMEM; Sigma-Aldrich,St. Louis, MO, USA) supplemented with 20% fetal bovine serum (FBS) and 100 U/mL penicillin and incubated for 3 days at 37°C in 5% CO2. Non-adherent cells were removed from the flasks, and adherent cells were washed once with HBSS, then fed with the supplemented medium; they were cultured and passaged three times, after which they were ready for use in the animals.
Spinal cord injury
Young adult female (8–10 weeks old) C57BL/6 mice (n=24), weighing 20–25 g, were anesthetized by intraperitoneal(i.p.) injection of a solution containing ketamine and xylazine (15 mg/kg and 100 mg/kg, respectively) and subjected to laminectomy followed by a compressive SCI, as described by Marques and colleagues (Marques et al., 2009). Extradural temporary closure of a 30-g vascular clip (Kent Scientific Corporation, INS 14120, USA) was performed around the exposed spinal cord, at T9level, for 1 minute, to cause the injury. Muscles and skin were sutured in layers. Animals were left to recover on a warm pad, received 1 mL of saline solution to compensate for dehydration and loss of blood,and were returned to their home cages with free access to food and water. Bladder emptying was performed manually twice a day until recovery of spontaneous urinary function.
Cell transplantation
if xed with 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4), then divided into two segments: one rostral and one caudal to the lesion center. Tissue samples were cryoprotected in gradually increasing concentrations of sucrose solution at 10%, 20%, 30% in PBS. The samples were left in 30% solution overnight, cold-embedded in OCT (Tissue Tek), and frozen. Serial cross sections (10 µm thick) were cut on a cryostat (Leica CM 1850, Wetzlar, Germany) and collected on gelatin-coated glass slides. To evaluate the presence of transplanted cells, spinal cord tissue was analyzed at two different time points, 7 days and 8 weeks after transplantation. The slides were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) alone, and GFP MSCs were tracked in the host tissue. All the other immunohistochemistry analyses were conducted 8 weeks after injection.The host tissue was stained immunohistochemically for glial fibrillary acidic protein (GFAP; an astrocyte marker), S-100(a Schwann cell marker), and Gal-C (an oligodendrocyte marker) to evaluate the fate of transplanted cells. The slides were incubated for 15 minutes in 0.06% potassium permanganate (to reduce tissue auto fluorescence), washed in PBS,incubated for 1 hour in a blocking solution (10% NGS in 0.3% PBS Triton) at room temperature, washed in 0.3% PBS Triton, and then incubated overnight in one of the following primary antibodies: rabbit anti-GFAP (1:100, Sigma-Aldrich); rabbit anti-S-100 (1:100, Sigma-Aldrich), or rabbit anti-Gal-C (1:100, Chemicon-Millipore Corporation, Temecula, CA, USA). Slides were then washed for 30 minutes and incubated for 2 hours with the appropriate secondary antibody (Alexa 546 goat anti-rabbit; 1:800; Sigma-Aldrich) followed by three PBS washes, counterstained with DAPI nuclear label, and coverslipped with Fluoromount(Sigma-Aldrich). Primary antibodies were omitted for the negative controls. Sections were analyzed under confocal microscopy (Disk scanning unit; Olympus, Tokyo, Japan).
To evaluate the levels of expression of trophic factors in spinal cord tissue, we performed immunohistochemistry(n= 3 per group) in the sections, as described above, for neurotrophin-3 (NT-3), neurotrophin-4 (NT-4), brain-de-
Cells were injected seven days after injury, because at this time, the lesion is characterized as subacute. Animals were randomly divided into four groups (n= 6 per group):DMEM i.p. and DMEM i.v., where mice received intraperitoneal or intravenous injections of DMEM (500 µL),respectively; and MSC i.p and MSC i.v., where mice received intraperitoneal or intravenous injections of 8 × 105MSCs in a final volume of 500 µL, respectively. The intravenous injection was madeviathe tail vein. An additional sham-operated group of mice (n= 6) underwent basic surgical procedures (anesthesia and laminectomy) but did not receive the compression injury.
Immunohistochemistry for trophic factors
The animals were anesthetized and perfused transcardially with 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). A length of spinal cord was dissected out and post-rived neurotrophic factor (BDNF) and nerve growth factor(NGF). Primary antibodies used were: goat anti-mouse NT-3 (1:100, Preprotech, INC., Rocky Hill, NJ, USA), goat anti-mouse NT-4 (1:100, PeproTech, Inc., Rocky Hill, NJ,USA), rabbit anti-human BDNF (1:100, PeproTech, Inc),or rabbit anti-human NGF (1:100, PeproTech, Inc). The secondary antibodies used were Alexa 546 goat anti-rabbit(1:800, Sigma-Aldrich) and Alexa 546 rabbit anti-goat (1:800,Sigma-Aldrich). The immunohistochemistry reactions of samples from the DMEM and MSC groups were performed together to allow direct comparison. All sections were photographed with the use of a 20× objective. The expressions of NT-3, NT-4, BDNF and NGF were quantified from these fluorescent images (Image-Pro Plus program, Rockville,MD, USA - version 6.0) by evaluating the ratio between the stained area and total field area.
White matter sparing analysis
White matter sparing was analyzed from serial sections that were collected in six parallel series of eight slides each,so that the first slide of each series contained six sections spaced 50 µm apart; a total of 2.4 mm of each tissue segment was sectioned. Tissue sections were stained with luxol fast blue (LFB; Harleco-Millipore Corporation, Temecula, CA,USA), which stains myelin. The spared white matter was calculated as the total cross-sectional area minus LFB nonstained area, which was reported as a percentage of total area; for this, we used the Image free software (Java – Oracle Corporation, Redwood, CA, USA).
Morphometric evaluation and ultrastructure analysis
Eight weeks after treatment, animals (n= 3 per group) were anesthetized with ketamine and xylazine as above, and perfused transcardially with 4% paraformaldehyde and 2% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4). The lesion epicenter (about 1 mm thick) was extracted and post- fixed by immersion at room temperature for 6 hours in a solution of 1% osmium tetroxide and 0.9% potassium ferrocyanide in 0.1 M cacodylate buffer. Samples were washed three times with 0.1 M phosphate buffer (pH 7.4), dehydrated in a graded acetone series (30%, 50%, 70%, 80%, 90% and 100%),embedded in resin (Embed-812, EMS), and polymerized for 48 hours at 60°C. Semithin (500 nm) and ultrathin (70 nm)sections from each group were cut on an RMC ultramicrotome. The semithin sections were stained with toluidine blue and imaged under a Zeiss microscope (Axioscop 2 Plus) using the Axiovision Program version 4.5 (Zeiss, Oberkochen,Germany). Our sample consisted of six images (1000× magnification) of a cross section of the spinal cord; and three images from the right side, showing the anterior, lateral,and posterior funiculi. The same procedure was repeated for the left side, as described previously (Marques et al., 2010).The number of myelinated nerve fibers was counted by two observers who were blinded to the experimental groups.Image free software (Java – Oracle Corporation) was used to quantify the number of fibers and to calculate the g-ratio values in all groups. The g-ratio was calculated by dividing
the inner axonal diameter by the outer fiber diameter. The results were expressed as ranges of 0.1–0.2, 0.2–0.3, 0.3–0.4,0.4–0.5, 0.5–0.6, 0.6–0.7, 0.7–0.8, 0.8–0.9, and 0.9–1.0. The lowest portions of the ranges were always included and the highest portions were excluded (e.g., the 0.2–0.3 range included 0.2 through 0.299, excluding 0.3). Ultrathin sections(60–80 µm) were collected on copper grids, stained with 5%uranyl acetate (30 minutes) and lead citrate (10 minutes),and imaged on a Zeiss 900 Transmission Electron Microscope (Zeiss, Oberkochen, Germany).
Behavioral tests
To assess the locomotor performance, we performed two tests: Global Mobility Test (Marques et al., 2009) and Basso Mouse Scale (Basso et al., 2006). The animals (n= 6 per group) were acclimated in the open field for 7 days before injury. Behavioral analyses were performed 1 day before injury, 1 day after injury, and then weekly up to 8 weeks after injection. The Global Mobility Test was assessed using a webcam (5 frames per second) to record animals for 1 minute in an open field, and the average speed (cm/s) of these animals was measured with Image Java free software.The Basso Mouse Scale is a 9-point scale of the animal’s locomotor ability, assessed by monitoring specific locomotion features such as ankle movement, paw position, weight support, plantar steps, hindlimb and forelimb coordination,and trunk stability. For this, the animals were placed in an open field and observed by two raters (blinded to the treatment) for 4 minutes to assess their performance using this scale.
Statistical analysis
All statistical analyses were performed in GraphPad Prism 4.0 (GraphPad software, San Diego, CA, USA) using oneway analysis of variance and Tukey’spost hoctest. Results were expressed as the mean ± SEM, andPvalues < 0.05 were considered significant.
RESULTS
MSCs migrated to the injury site, survived up to 8 weeks after transplantation, and did not express any glial markers
The survival of transplanted GFP+MSCs was evaluated 7 days and 8 weeks post-transplantation. Analysis of the samples by confocal microscopy revealed that both intravenously and intraperitoneally injected MSCs were present in the host tissue from 7 days (Figure 1A, B) up to 8 weeks (Figure 1C, D) after transplantation, suggesting that these cells were able to migrate to the injury site and survive in the new milieu. We also investigated whether these transplanted cells were able to differentiate in the host tissue. We performed immunostaining for several glial cell markers, GFAP (Figure 2A–D), Gal-C (Figure 2B–E) and S-100 (Figure 2C–F). Analyses of merged images showed that GFP+MSCs did not express any of the glial markers, indicating that MSCs injected by both routes did not differentiate into astrocytes,oligodendrocytes or Schwann cellsin vivo, and that all glial cells present in the spinal cord were host-derived.
MSC groups showed higher expression of trophic factors compared to DMEM groups, and the injection route did not affect these results
To evaluate the expression of trophic factors, we performed immunohistochemistry for BDNF, NGF, NT-3 and NT-4 in the spinal cord of mice from each experimental group. Eight weeks after cell transplantation, spinal cord tissue showed low immunoreactivity for all trophic factors, perhaps because they are soluble and diffuse rapidly within the tissue.Both groups that received the MSC injection showed a larger immunostained area than groups that received DMEM injection (P< 0.05 orP< 0.01), for all trophic factors analyzed(Figure 3A–D). However, there was no significant difference between the groups treated with MSC, indicating that the injection route did not affect the expression of trophic factors.
MSC groups showed better white matter preservation compared to DMEM groups, and there was no difference between the injection routes
Analysis of spinal cord sections stained with LFB revealed that both MSCs-treated groups (Figure 4B and D) showed better white matter preservation than those of the DMEM groups (Figure 4A and C). Quantitative analysis of LFB-stained sections showed higher values for percentage of spared white matter in MSC (51.8 ± 1.3% and 51.6 ± 1.8%in the MSC i.p. and MSC i.v. groups respectively) than DMEM (42.5 ± 0.9% and 43.7 ± 0.4% in the DMEM i.p. and DMEM i.v. groups respectively) treated mice in all the analyzed regions (P< 0.01), as illustrated in Figure 4F. However, there was no significant difference between the groups treated with MSCs, indicating that the injection route did not affect the preservation of white matter. Staining with LFB also revealed the normal white matter distribution in sham-operated animals (Figure 4E).
MSC therapy, by both routes, increased the total number of myelinated fibers and g-ratio values
Analysis of spinal cord morphology in semithin cross sections stained with toluidine blue in all groups showed different patterns of tissue organization. The mice that received DMEM showed a less-organized tissue structure, with dispersed groups of fibers; while the groups that received MSCs showed better tissue cytoarchitecture (Figure 5A and A’) and a larger number of myelinated fibers. However, all groups showed fibers undergoing degeneration (Figure 5A’). Quantitatively,the MSC treatment resulted in a significant increase in the number of myelinated fibers (969 ± 140 and 991 ± 141 for MSC i.p. and MSC i.v. groups, respectively) compared to the DMEM group (450 ± 82 and 441 ± 57 for DMEM i.p. and DMEM i.v. groups, respectively) (P< 0.05; Figure 5B). We also used semithin cross sections stained with toluidine blue to quantify the area of axons, myelin thickness and area of the fiber as additional parameters. As seen in Figure 5C–E, the mice that received MSCs showed significant improvements in the parameters studied compared to those that received DMEM, such as axon area (69.4 ± 9.3 µm2and 97.5 ± 8.2 µm2for MSC i.p. and MSC i.v. groups, respectively; and 37.8 ±5.4 µm2and 30.0 ± 1.2 µm2for DEMM i.p. and DMEM i.v.groups, respectively (P< 0.01), myelin area (114.8 ± 14.5 µm2and 139.8 ± 25.8 µm2for MSC i.p. and MSC i.v. groups, respectively; and 35.9 ± 3.9 µm2and 25.8 ± 4.0 µm2for DEMEM i.p. and DMEM i.v. groups, respectively (P< 0.05 orP< 0.01),and fiber area (184.3 ± 24.3 µm2and 237.3 ± 32.0 µm2for MSC i.p. and MSC i.v. groups, respectively; and 73.6 ± 6.3 µm2and 55.8 ± 3.7 µm2for DEMEM i.p. and DMEM i.v. groups, respectively (P< 0.05 orP< 0.01). Analyses of the g-ratio (Figure 5F;P< 0.01) showed that MSC animals had more fibers in the optimal range for spinal cord (0.7–0.8 range) (Chomiak and Hu, 2009). The ultrastructural analysis of spinal cords from DMEM and MSC groups revealed marked tissue disorganization, the presence of astrocyte processes, and few preserved fibers in the DMEM groups (Figure 6A and C); whereas the MSC groups showed better tissue preservation and the presence of regenerating axon sprouts, some of them in the process of myelination (Figure 6B and D). Both groups showed spared axons being remyelinated by oligodendrocytes.
Intraperitoneal and intravenous MSC injection enhanced the locomotor performance after SCI
To evaluate the effect of MSC transplantation on motor function, we performed the global mobility test (GMT) and monitored the BMS score up to 8 weeks after injection, in an open field. Analysis of GMT showed that the animals that received MSC (Figure 7B and D) transplants were able to walk for longer distances than the animals that received DMEM (Figure 7A and C). MSC-treated animals injected by both routes also achieved higher locomotor speed (5.5 ±1.0 cm/s for MSC i.p. and 5.9 ± 1.1 cm/s for MSC i.v.) than DMEM-treated animals (3.0 ± 1.3 cm/s for DMEM i.p. and 3.8 ± 1.2 cm/s for DMEM i.v.) (Figure 7E;P< 0.05). With respect to the BMS, the sham-operated animals showed normal scores during the entire assessment period. All injured animals showed paralysis (BMS score = 0) immediately after lesion, which continued for 1 or 2 days post-surgery. After that, the DMEM animals improved to an initial phase of recovery, reaching 2 as a maximum score in both routes of injection. The MSC-treated animals showed better results,reaching an intermediate phase of recovery, with a maximum score of 4 for MSC i.p., meaning that these animals were able to perform occasional plantar steps; and a maximum score of 5 for MSC i.v., meaning that these animals were able to perform frequent or constant plantar steps, with or without some coordination, which is a very good sign in terms of functional recovery. These animals began to improve their BMS scores at about 28 days after cell transplantation, and this locomotor improvement increased until 49 days after treatment. After that, the scores remained steady(Figure 7F;P< 0.05).
Discussion

Figure 1 Tracking of mesenchymal stem cells in the lesion epicenter of the spinal cord at 1 and 8 weeks after injection.

Figure 3 Immunoreactivities of trophic factors in the injured spinal cord at 8 weeks after spinal cord injury.
The development of treatments that can increase the regeneration of an injured spinal cord and reduce the side effects that occur after trauma is essential, because no treatment yet exists that can lead to full functional restoration. These poor clinical outcomes have motivated this pre-clinical study using systemic injection of mesenchymal stem cells as a therapeutic strategy. Our results showed that the MSCs administered by systemic routes were able to migrate to the injury site, promoting an increase in local expression of trophic factors and enhancing fiber sparing and/or regeneration, accompanied by substantial improvement in locomotor performance.
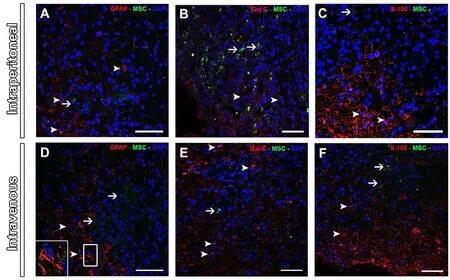
Figure 2 Expression of glial cell markers in the injured spinal cord at 8 weeks after MSC injection.

Figure 4 White matter sparing in the injured spinal cord at 8 weeks after cell transplantation.

Figure 5 Morphometry of myelinated nerve fibers in toluidine blue-stained semithin sections of the injured spinal cord.
Previous studies have established that MSC can be delivered to the injured spinal cord by several routes, such as intralesional (Zhou et al., 2013; de Almeida et al., 2015),lumbar puncture (Paul et al., 2009), and intravenous (Osaka et al., 2010; Kim et al., 2013a; Morita et al., 2016), among others. However, to the best of our knowledge, the intraperitoneal route has never been used to treat SCI. Injections directly into the parenchyma may further damage the tissue,a consequence to be avoided. Less invasive methods, such as intravenous or intraperitoneal injection, are a better choice for clinical use since they are safer and allow the use of multiple injections, a strategy tested with success in several experimental studies (Kim et al., 2013b; Richardson et al.,2014; Wei et al., 2014; Badner et al., 2016).
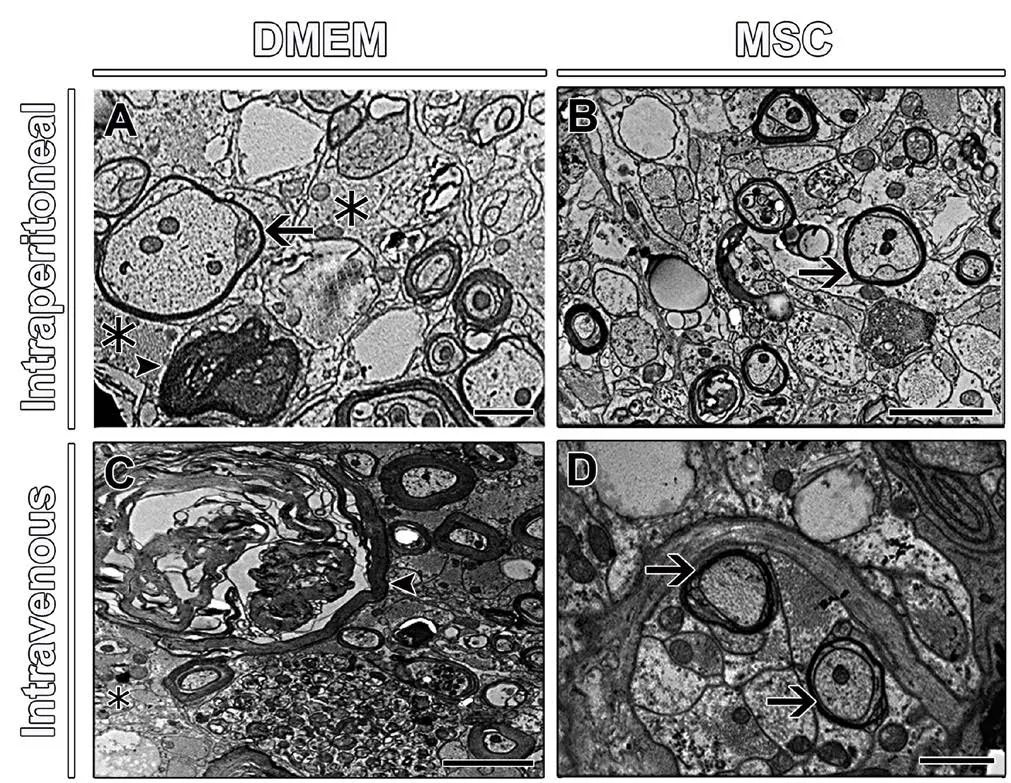
Figure 6 Ultrastructural observation of spinal cord parenchyma of mice.

Figure 7 Behavioral tests of mice.
The intraperitoneal route has been used before for MSCs injection in inflammatory diseases, with positive results.Yousefi and coworkers (Yousefiet al., 2013) have shown that intraperitoneal injection of MSCs was able to reduce the number of aggressor inflammatory cells in the brain and ameliorated the severity of clinical scores in mice with experimental autoimmune encephalomyelitis (EAE) (Yousefi et al., 2013). More recently, Kim et al. (2014) showed that intraperitoneal injection of MSCs was able to suppress peritoneal inflammation by restoring the mesothelial layer and decreasing complement activation in fungal or yeast peritonitis in rats. Also, Oh et al. (2014) showed that i.p. injection of MSCs almost completely prevented the development of experimental autoimmune uveitis (EAU) in mice by suppressing Th1/Th7 immune responses, and protected the retina from immune-mediated damage. The peritoneum is highly vascularized and therefore allows more cells to access the lymphatic and blood circulatory systems simultaneously(Wilson et al., 2010); afterwards, these cells are able to engraft to sites of tissue injury and inflammation. Our results showed that both routes of administration, intraperitoneal and intravenous, resulted in important improvements, with no significant statistical difference between them. Both the intravenous and intraperitoneal routes had no negative effects on the animals that received MSCs or DMEM injection.
An important issue regarding the use of cell therapy by systemic injection is the viability of the cells and their fate and differentiation after being transplanted. In this study, we located the GFP+MSCs at the injury site, in both routes, 7 days after injection, and these cells remained in the tissue for as long as 8 weeks after administration. These findings accord with other studies that identified the presence of cells injected intravenously, 4 and 6 weeks after transplantation (Osaka et al., 2010; Kang et al., 2012; Kim et al., 2013a). With respect to MSCin vivodifferentiation, we were unable to find any GFP+MSCs double-labeled for any neural lineage cell marker. However, previous studies by other groups (Zhou et al.,2013) found evidence that MSCs can differentiate into either neuronal or glial cells. Our present findings support the idea that these cells do not replace damaged spinal cord cells, but rather work through local paracrine effects.
The most obvious application for the use of MSCs is to recruit their trophic potential, because under normalin vitroconditions, these cells secrete a variety of trophic factors and cytokines (Majumdar et al., 1998). Although the labeling for trophic factors has been diffuse, as reported elsewhere even inin vitroexpression (de Almeida et al., 2015), we observed a larger marked area for all trophic factors analyzed in the groups treated with MSCs. Pisati et al. (2007) reported that when human mesenchymal stem cells are cultured with brain slices, they begin to express receptors for trophic factors,and secrete trophic factors such as NGF and NT-3. They also demonstrated thein vivoexpression of these neurotrophins 45 days after the stem cells were transplanted into the mouse brain. Sasaki et al. (2009) reported that the combination of BDNF and human MSCs is capable of promoting axonal sprouting, protection of corticospinal neurons, and bettering functional recovery after thoracic spinal cord injury in adult mice, suggesting that this neurotrophin can play an important role in nerve fiber regeneration. In addition, He et al. (2013) demonstrated that the functional improvement in rats with thoracic spinal cord injury after treatment with stem cells was related to BDNF expression. A recent study (Jadasz et al., 2018) showed thatin vitrotreatment of adult neural stem cells with adult rat bone marrow-derived mesenchymal stem cell-conditioned medium promoted the differentiation of oligodendrocytes. In agreement with these studies, our results showed higher expressions of BDNF, NGF, NT-3 and NT-4 in groups that received the MSC injection, which was accompanied by higher levels of white matter sparing and increased numbers of myelinated fibers. These results can be attributed to secretion of trophic factors by the injected cells, as described elsewhere (Osaka et al., 2010; Hawryluk et al., 2011;He et al., 2013; Kim et al., 2013a; de Almeida et al., 2015).
Together with the beneficial morphological results, we found better functional outcomes in our MSCs-treated animals. A larger number of myelinated fibers were in the optimal spinal cord g-ratio range (Chomiak and Hu, 2009). The g-ratio is a functional and structural index of optimal axonal myelination that is correlated with better conduction velocity.Animals that received the MSC transplant, by either route,showed better locomotor performance, with higher mean locomotor speeds in GMT and higher scores on the BMS scale,reaching the intermediate phase of functional recovery. These results are consistent with previous reports from our group of better locomotor performance by these two functional tests in embryonic stem cells locally transplanted into mice (Marques et al., 2010), human dental pulp stem cells locally transplanted into mice (de Almeida et al., 2011), and mesenchymal stem cells locally transplanted into mice (de Almeida et al., 2015)after compressive spinal cord injury. Our findings agree with those of other groups that also reported functional recovery after SCI and MSC transplantation (Cizkova et al., 2011; Kang et al., 2012; Kim et al., 2013a; Morita et al., 2016). These functional improvements are probably correlated with the better white matter preservation and axon myelination observed in the MSCs-treated animals.
In conclusion, we present evidence that systemic transplantation of MSCs, by either the intraperitoneal or intravenous route, has the potential to improve axonal myelination, white matter sparing, and motor function after SCI, through the local release of trophic factors. This study provides new insight into the benefits of systemic administration of stem cells and encourages the clinical application of this treatment.
Author contributions:BSR, FMA and AMBM designed the experiment,analyzed the data, and wrote the manuscript. BSR, FMA, CMS and SL performed the experiments. All authors have read and approved the final version of the paper.
Conflicts of interest:The authors declare no conflicts of interest.
Financial support:None.
Institutional review board statement:Surgical procedures and animal handling were carried out in accordance with the approved guidelines of the Committee on Animal Care of the Health Science Center of the Federal University of Rio de Janeiro. All experimental procedures were approved by the same committee (Protocol number DHEICB003).
Reporting statement:This study follows the Recommendations for the Conduct, Reporting, Editing and Publication of Scholarly Work in Medical Journals developed by the International Committee of Medical Journal Editors.
Biostatistics statement:The statistical methods of this study were reviewed by the researchers of Federal University of Rio de Janeiro/Brazil.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:He Huang, Xiangya School of Medicine, Central South University, China.
Ahuja CS, Wilson JR, Nori S, Kotter MR, Druschel C, Curt A, Fehlings MG(2017) Traumatic spinal cord injury. Nat Rev Dis Primers 3:17018.
Azari MF, Mathias L, Ozturk E, Cram DS, Boyd RL, Petratos S (2010) Mesenchymal stem cells for treatment of CNS injury. Curr Neuropharmacol 8:316-323.
Badner A, Vawda R, Laliberte A, Hong J, Mikhail M, Jose A, Dragas R,Fehlings M (2016) Early intravenous delivery of human brain stromal cells modulates systemic inflammation and leads to vasoprotection in traumatic spinal cord injury. Stem Cells Transl Med 5:991-1003.
Basso DM, Fisher LC, Anderson AJ, Jakeman LB, Mctigue DM, Popovich PG (2006) Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma 23:635-659.
Chen X, Wang S, Cao W (2018) Mesenchymal stem cell-mediated immunomodulation in cell therapy of neurodegenerative diseases. Cell Immunol 326:8-14.
Chomiak T, Hu B (2009) What is the optimal value of the g-ratio for myelinated fibers in the rat CNS? A theoretical approach. PLoS One 4:e7754.
Cizkova D, Novotna I, Slovinska L, Vanicky I, Jergova S, Rosocha J, Radonak J (2011) Repetitive intrathecal catheter delivery of bone marrow mesenchymal stromal cells improves functional recovery in a rat model of contusive spinal cord injury. J Neurotrauma 28:1951-1961.
Dasari VR, Veeravalli KK, Dinh DH (2014) Mesenchymal stem cells in the treatment of spinal cord injuries: a review. World J Stem Cells 6:120-133.
de Almeida FM, Marques SA, Ramalho Bdos S, Massoto TB, Martinez AM(2015) Chronic spinal cord lesions respond positively to tranplants of mesenchymal stem cells. Restor Neurol Neurosci 33:43-55.
de Almeida FM, Marques SA, Ramalho Bdos S, Rodrigues RF, Cadilhe DV,Furtado D, Kerkis I, Pereira LV, Rehen SK, Martinez AM (2011) Human dental pulp cells: a new source of cell therapy in a mouse model of compressive spinal cord injury. J Neurotrauma 28:1939-1949.
Dooley D, Lemmens E, Vangansewinkel T, Le Blon D, Hoornaert C, Ponsaerts P, Hendrix S (2016) Cell-based delivery of interleukin-13 directs alternative activation of macrophages resulting in improved functional outcome after spinal cord injury. Stem Cell Reports 7:1099-1115.
Hawryluk GW, Mothe A, Wang J, Wang S, Tator C, Fehlings MG (2011)An in vivo characterization of trophic factor production following neural precursor cell or bone marrow stromal cell transplantation for spinal cord injury. Stem Cells Dev 21:2222-2238.
He BL, Ba YC, Wang XY, Liu SJ, Liu GD, Ou S, Gu YL, Pan XH, Wang TH(2013) BDNF expression with functional improvement in transected spinal cord treated with neural stem cells in adult rats. Neuropeptides 47:1-7.Jadasz JJ, Kremer D, Göttle P, Tzekova N, Domke J, Rivera FJ, Adjaye J,Hartung HP, Aigner L, Küry P (2013) Mesenchymal stem cell conditioning promotes rat oligodendroglial cell maturation. PLoS One 8:e71814.
Jadasz JJ, Tepe L, Beyer F, Samper Agrelo I, Akkermann R, Spitzhorn LS,Silva ME, Oreffo RO, Hartung HP, Prigione A (2018) Human mesenchymal factors induce rat hippocampal- and human neural stem cell dependent oligodendrogenesis. Glia 66:145-160.
Kang ES, Ha KY, Kim YH (2012) Fate of transplanted bone marrow derived mesenchymal stem cells following spinal cord injury in rats by transplantation routes. J Korean Med Sci 27:586-593.
Kim JW, Ha KY, Molon JN, Kim YH (2013a) Bone marrow–derived mesenchymal stem cell transplantation for chronic spinal cord injury in rats:comparative study between intralesional and intravenous transplantation. Spine (Phila Pa 1976) 38:E1065-1074.
Kim SW, Kim SJ, Park SH, Yang HG, Kang MC, Choi YW, Kim SM, Jeun SS, Sung YC (2013b) Complete regression of metastatic renal cell carcinoma by multiple injections of engineered mesenchymal stem cells expressing dodecameric TRAIL and HSV-TK. Clin Cancer Res 19:415-427.
Lindsay SL, Toft A, Griffin J, MM Emraja A, Barnett SC, Riddell JS (2017)Human olfactory mesenchymal stromal cell transplants promote remyelination and earlier improvement in gait co-ordination after spinal cord injury. Glia 65:639-656.
Liu J, Chen J, Liu B, Yang C, Xie D, Zheng X, Xu S, Chen T, Wang L, Zhang Z (2013) Acellular spinal cord scaffold seeded with mesenchymal stem cells promotes long-distance axon regeneration and functional recovery in spinal cord injured rats. J Neurol Sci 325:127-136.
Majumdar MK, Thiede MA, Mosca JD, Moorman M, Gerson SL (1998)Phenotypic and functional comparison of cultures of marrow-derived mesenchymal stem cells (MSCs) and stromal cells. J Cell Physiol 176:57-66.
Marques SA, Garcez VF, Del Bel EA, Martinez AMB (2009) A simple, inexpensive and easily reproducible model of spinal cord injury in mice:morphological and functional assessment. J Neurosci Methods 177:183-193.
Marques SA, Almeida FM, Fernandes AM, dos Santos Souza C, Cadilhe DV, Rehen SK, Martinez AM (2010) Predifferentiated embryonic stem cells promote functional recovery after spinal cord compressive injury.Brain Res 1349:115-128.
Martinez AM, Goulart CO, Ramalho Bdos S, Oliveira JT, Almeida FM(2014) Neurotrauma and mesenchymal stem cells treatment: From experimental studies to clinical trials. World J Stem Cells 6:179-194.
Mietto BS, Mostacada K, Martinez AM (2015) Neurotrauma and inflammation: CNS and PNS responses. Mediators Inflamm 2015:251204.
Morita T, Sasaki M, Kataoka-Sasaki Y, Nakazaki M, Nagahama H, Oka S,Oshigiri T, Takebayashi T, Yamashita T, Kocsis JD (2016) Intravenous infusion of mesenchymal stem cells promotes functional recovery in a model of chronic spinal cord injury. Neuroscience 335:221-231.
Osaka M, Honmou O, Murakami T, Nonaka T, Houkin K, Hamada H,Kocsis JD (2010) Intravenous administration of mesenchymal stem cells derived from bone marrow after contusive spinal cord injury improves functional outcome. Brain Res 1343:226-235.
Paul C, Samdani AF, Betz RR, Fischer I, Neuhuber B (2009) Grafting of human bone marrow stromal cells into spinal cord injury: a comparison of delivery methods. Spine 34:328.
Richardson JD, Psaltis PJ, Frost L, Paton S, Carbone A, Bertaso AG, Nelson AJ, Wong DT, Worthley MI, Gronthos S (2014) Incremental benefits of repeated mesenchymal stromal cell administration compared with solitary intervention after myocardial infarction. Cytotherapy 16:460-470.
Sasaki M, Radtke C, Tan AM, Zhao P, Hamada H, Houkin K, Honmou O,Kocsis JD (2009) BDNF-hypersecreting human mesenchymal stem cells promote functional recovery, axonal sprouting, and protection of corticospinal neurons after spinal cord injury. J Neurosci 29:14932-14941.
Spaeth E, Klopp A, Dembinski J, Andreeff M, Marini F (2008) Inflammation and tumor microenvironments: defining the migratory itinerary of mesenchymal stem cells. Gene Ther 15:730-738.
Tetzlaff W, Okon EB, Karimi-Abdolrezaee S, Hill CE, Sparling JS, Plemel JR, Plunet WT, Tsai EC, Baptiste D, Smithson LJ (2011) A systematic review of cellular transplantation therapies for spinal cord injury. J Neurotrauma 28:1611-1682.
Varma AK, Das A, Wallace G, Barry J, Vertegel AA, Ray SK, Banik NL(2013) Spinal cord injury: a review of current therapy, future treatments,and basic science frontiers. Neurochem Res 38:895-905.
Vismara I, Papa S, Rossi F, Forloni G, Veglianese P (2017) Current options for cell therapy in spinal cord injury. Trends Mol Med 23:831-849.
Wei L, Zhang J, Xiao XB, Mai HX, Zheng K, Sun WL, Wang L, Liang F,Yang ZL, Liu Y, Wang YQ, Li ZF, Wang JN, Zhang WJ, You H (2014)Multiple injections of human umbilical cord-derived mesenchymal stromal cells through the tail vein improve microcirculation and the microenvironment in a rat model of radiation myelopathy. J Transl Med 12:246.
Wilson T, Stark C, Holmbom J, Rosling A, Kuusilehto A, Tirri T, Penttinen R, Ekholm E (2010) Fate of bone marrow-derived stromal cells after intraperitoneal infusion or implantation into femoral bone defects in the host animal. J Tissue Eng 2010:345806.
Wright KT, El Masri W, Osman A, Chowdhury J, Johnson WE (2011) Concise review: Bone marrow for the treatment of spinal cord injury: mechanisms and clinical applications. Stem Cells 29:169-178.
Yiu G, He Z (2006) Glial inhibition of CNS axon regeneration. Nat Rev Neurosci 7:617-27.
Yousefi F, Ebtekar M, Soleimani M, Soudi S, Hashemi SM (2013) Comparison of in vivo immunomodulatory effects of intravenous and intraperitoneal administration of adipose-tissue mesenchymal stem cells in experimental autoimmune encephalomyelitis (EAE). Int Immunopharmacol 17:608-616.
Zhou Z, Chen Y, Zhang H, Min S, Yu B, He B, Jin A (2013) Comparison of mesenchymal stromal cells from human bone marrow and adipose tissue for the treatment of spinal cord injury. Cytotherapy 15:434-448.
- 中国神经再生研究(英文版)的其它文章
- Role of brain-derived neurotrophic factor during the regenerative response after traumatic brain injury in adult zebrafish
- Natural polyphenols effects on protein aggregates in Alzheimer’s and Parkinson’s prion-like diseases
- How random is the random forest ? Random forest algorithm on the service of structural imaging biomarkers for Alzheimer’s disease: from Alzheimer’s disease neuroimaging initiative (ADNI) database
- Protective effects of gonadal hormones on spinal motoneurons following spinal cord injury
- INVITED REVIEW
- Role of presynaptic calcium stores for neural network dysfunction in Alzheimer’s disease

