Biogeochemical conversion of sulfur species in saline lakes of Steppe Altai*
BORZENKO Svetlana V. KOLPAKOVA Marina N. SHVARTSEV Stepan L. ISUPOV Vitaly P.
1Institute of Natural Resources,Ecology and Cryology,Siberian Branch of Russian Academy of Sciences,Chita 672049,Russia
2Sobolev Institute of Geology and Mineralogy,Siberian Branch of Russian Academy of Sciences,Novosibirsk 630090,Russia
3National Research Tomsk Polytechnic University,Tomsk 634050,Russia
4Tomsk Department of the Tro fi muk Institute of Petroleum-Gas Geology and Geophysics,Siberian Branch of the Russian Academy of Sciences,Tomsk 634050,Russia
5Institute of Solid State Chemistry and Mechanochemistry,Siberian Branch of Russian Academy of Sciences,Novosibirsk 630090,Russia
AbstractThe aim of the present research is to identify the main mechanisms of sulfur behavior in saline lakes in the course of time and followed transformations in their chemical composition. The inf l uence of water on chemical composition of biochemical processes involved in decomposition of organic matter was determined by the study of behavior of reduced forms of sulfur in lakes. The determination of reduced forms of sulfur was carried out by successive transfer of each form of sulfur to hydrogen sulfi de followed by photometric measurements. The other chemical components were determined by standard methods (atomic absorption, potentiometric method, titration method and others). The salt lakes of the Altai steppe were studied in summer season 2013-2015. Analysis of the chemical composition of the saline lakes of Altai Krai has shown that carbonate-, hydrocarbonate- and chloride ions dominate among anions; sodium is main cation; sulfates are found in subordinate amounts. Reduced forms of sulfur occur everywhere: hydrogen and hydrosulfi de sulfurprevail in the bottom sediments; its derivative—elemental—prevails in the lakes water. The second important species in water of soda lakes is hydrosulfi de sulfurand in chloride lakes is thiosulfate sulfurThe lag in the accumulation of sulfates in soda lakes in comparison to chloride lakes can be explained by their bacterial reduction, followed by the formation and deposition ofiron sulfi des in sediments. In chloride lakes gypsum is a predominantly barrier for sulfates.
Keyword:saline lake; sulfate reduction; gypsum; Altai
1 INTRODUCTION
The high mobility of sulfur in natural conditions and its ability to change the valence depending on the geochemical environment causes formation ofits multiple intermediates compounds. The bacterial sulfate reduction process plays a central role in its transformation that is why the formation of reduced sulfur species on the surface of the Earth is connected with the activity of sulfate-reducing bacteria (Skryabin,1983). It is believed that polysulfi de-, thiosulfate ions,and elemental sulfur are beneficial sources of energy for sulfate and sulfate-reducing bacteria that use biosynthetic pathways, the fi nal destination of which are hydrogen sulfi de and hydrogen sulfi de ion (Sorokin et al., 2011; Nuyanzina-Boldareva et al., 2016, etc.). A significant part of the latter reacts predominantly with dissolved iron forming sulfi de minerals (hydrotroilite,pyrite, marcasite, and others) in bottom sediments.The intensity of sulfate reduction can reach several tens of mg/(L∙d) and be significantly ahead of the oxidation speed of produced hydrogen sulfi de(Kolosov et al, 2010; Borzenko and Zamana, 2011;Borzenko et al., 2014).
Sulfur cycle in lakes is closely connected with the biogenic elements and with carbon initially (Bernard,1979; Girguis et al., 2005). A striking example of the manifestation of this connection are salt lakes with the presence of microbial mats inhabited by numerous colonies of various bacteria in which chemo- and photosynthesis processes are carried out by sulfuroxidizing bacteria (Bazarova, 2009; Matyugina and Belkova, 2015, etc.). Modern methods proved that the active processes of anaerobic sulphate reduction and methanogenesis occur in bacterial mats (Yannash,1989). While studying the salt lakes of Altai Krai we noted extensive development of microbial mats, they often cover not only a coastal part, but also the entire area of small lakes (the Lake Petukhovo 2). According to some researchers (Krumbein, 1983; Namsaraev and Namsaraev, 2007; Zavarzin, 2007, etc.), most of the organic matter synthesized in these fouling spend on sulfate reduction that is why bacterial mats can be regarded as an additional source of reduced sulfur in lakes.
In the arid climate many chemical elements and ions (sulfates and chlorides) are concentrated in continental inland lakes due to water evaporation.Their initial contents should proportionately increase up to the stage of the gypsum formation (Shvartsev,2008; Zheng, 2014). However, numerous studies have shown that in the absence of an additional source of sulfates their accumulation comparable to concentrations of chlorides does not occur (Zamana and Borzenko, 2010; Shvartsev et al., 2014).Therefore, the main aim of present study is to identify a spatial distribution of the various sulfur species between water and bottom sediments of different saline lakes that determine their behavior in brine and salt lakes of the Altai steppe, as well as to suppose mechanisms and factors that control these processes.
2 RESEARCH METHOD
This article presents the results of chemical research of main elements and biogenic composition of some saline lakes in Southern Altai (in 2013-2015)(Tables 1, 2).
The collected samples of water were fi ltered and loaded into plastic bottles (0.5 L) for analysis of major components. The sampling bottles were previously washed with nitric acid and rinsed with distilled water.Lake waters were analyzed in the fi eld conditions (pH,Eh, electric conductivity, temperature), as well as in the Fundamental Research Laboratory of Hydrogeochemistry of Education and Research Centre“Water” (Tomsk Polytechnic University, Russia).
The mean value and accuracy was calculated for each method according to (ISC, 2012). Chemical analysis of water samples was carried out by conventional methods (Novikov et al., 1990; Fomin,1995). The concentrations of calcium and magnesium were determined by atomic absorption method in nitrous acetylene fl ame on a spectrophotometer SOLAAR 6M. Flame-emission method was used for determination of sodium and calcium. Potentiometric method with the application ofion-selective electrodes was used to determine pH, Eh, O2, Cl-. Titration was used to determine the content ofSulfate ion, in the form of barium sulfate, was determined by turbidimetric method. Hydrogen sulfi de H2S (hydrosulfi de HS-) and elemental sulfur S0were precipitated from 100 mL of water sample by zinc acetate on the “blue ribbon” fi lters. Thiosulfateswith sul fi teswere isolated with silver nitrate from the remainder of the fi ltrate (Zerkle et al.,2010). Photometric method determined all forms of sulfur with preliminary, sequential transfer them to hydrogen sulfi de (Volkov and Zhabina, 1990). At fi rst hydrogen sulfi de was distilled from precipitate in an argon environment, then elemental sulfur was extracted and reduced with chromium chloride II to hydrogen sulfi de in the acidic environment.Considering complexity of thiosulfatesand sul fi tesdivision, they were determined together(second precipitation) and were expressed in terms of thiosulfates (thiosulfate sulfur).
Our method allows determining the concentration of the total S0in water, including the truly dissolved sulfur, suspended sulfur, colloidal sulfur and elemental sulfur in the composition of the polysul fi de ionsIt should be noted that polysul fi de ion sulfurwas determined by the analyzing of hydrogen sulfi de and hydrosulfi de sulfur (Busev and Simonova, 1975).
Hydrogen sulfi de was analyzed by treating a weighed quantity of bottom sediments with diluted hydrochloric acid, then the mixture was heated and evolved hydrogen sulfi de was distilled in a tank filled with zinc acetate. After stripping of hydrogen sulfi de,elemental sulfur and thiosulfate were determined jointly by reducing them with chromium chloride II to hydrogen sulfi de with photometric end-point, as in the case with water. The detection limit of the sulfur determination method is 5 mg/L.
Determination of dissolved organic carbon TOC was carried out on a carbon analyzer LiquidTOC of Elementar (Germany) with infrared radiation detectorby high-temperature catalytic oxidation method of carbon compounds with decomposition to CO2at a temperature of 800°C. Accuracy rate of this method is less than 28%, when the TOC content is from 1 to 5 mg/L and 14% in case of TOC content 50-250 mg/L.
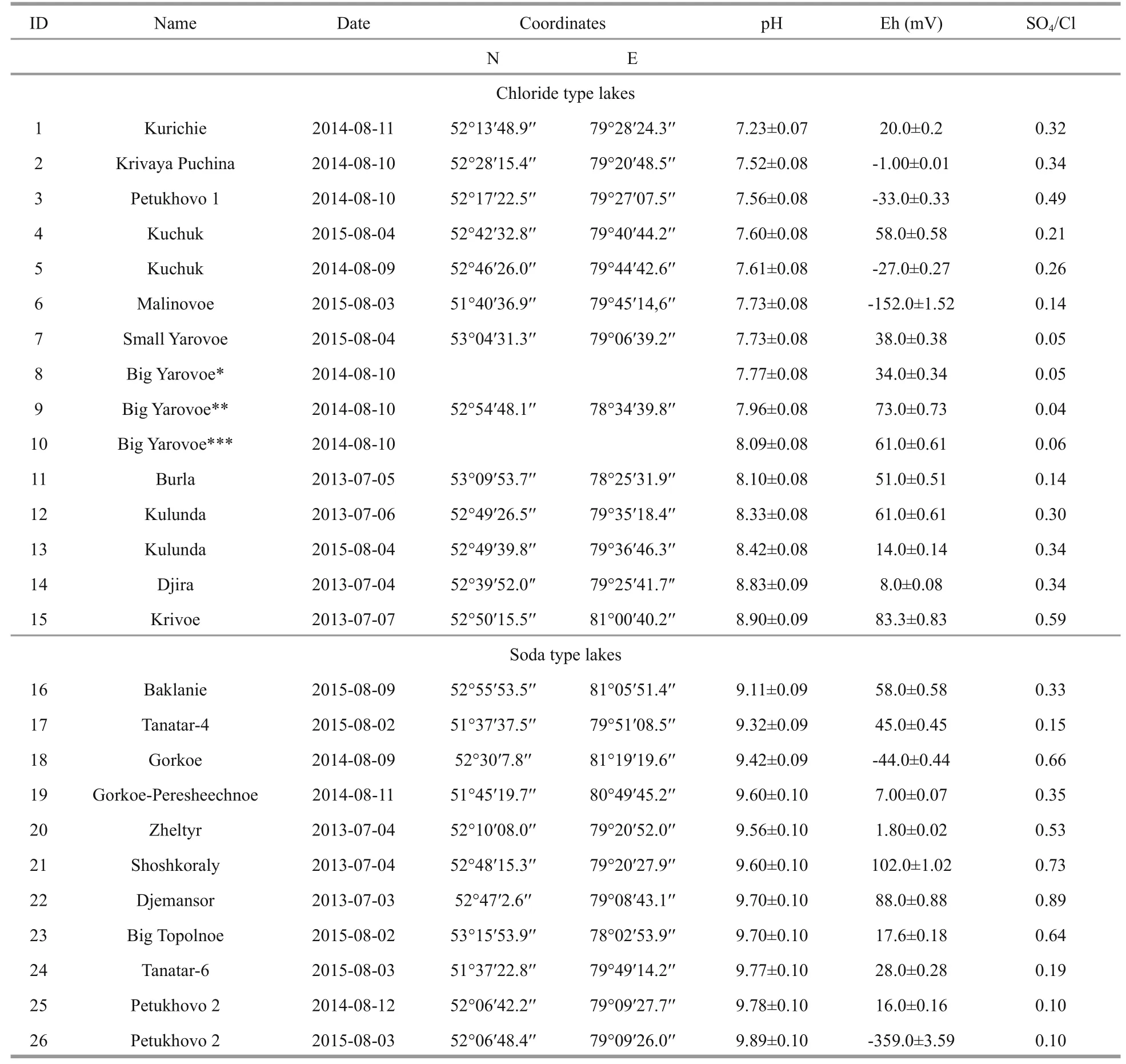
Table 1 Location of the lakes and their main chemical characteristics
The simulation of equilibria in the “water-minerals”system was carried out using physical-chemical code on programme PHREEQC (Parkhurst and Appelo,2013). This algorithm is based on the principle of chemical thermodynamic constants allowing estimating the degree of water saturation with respect to different sulfur-containing minerals, so to determine the ability of water to dissolve or precipitate solid phases in equilibrium with diverse species in solution.
3 RESULT AND DISCUSSION
Most lakes are located in the Kulunda steppe,which occupies the south-eastern part of the West Siberian Plain (Fig.1). From the west it is bounded by the valley of the river Irtysh, from the east by Priobskiy ridge, in the north boundary passes south of the lake Chany, and in the south it is determined by the river Aley. In the landscape there are two areas: the plain,which occupies the central, western and northern parts with the maximum absolute height of 150 m and the rolling area with relatively high margins in the south and in the east of the Kulunda basin (up to 300 m). The Kulunda basin consists mainly of sandysediments with the thickness of up to 1 300 m (Atlas of the Altai Territory, 1991). The climate is continental,with an average January temperature of -18°C, in July+21°C. Annual rainfall varies between 250-350 mm. Terraced terrain, which is clearly manifested during the motion from watershed to the numerous lakes, is characteristic for the Kulunda steppe.

Table 2 Chemical composition of water in lakes of steppe Altai (N according to Table 1) (g/L)
Regional groundwater involved in the salt lakes’water cycle is mainly of phreatic type, rarely subartesian. Groundwater depth in the watersheds is 10-15 m, and significantly decreases from 0.5 to 1.0 m in the direction of the lakes. The mineralization,on the contrary, increases in this direction from 1 to 5.3 g/L (Komlev, 2010). By our data (Kolpakova et al., 2016), he chemical composition of the water is mainly calcium and hydrocarbonate; sulfates dominate in anionic composition with an increase in salinity and pH. The cationic part is represented by a variable mixed calcium-magnesium-sodium composition (in ascending order).
All studied lakes are inland waters with different morphometric characteristics, bathyorography,sources of water-salt diet; a water bowl diameter is from 8-10 km (the Big Yarovoye Lake, the Burlinskoye Lake, etc.) to 35 km (the Kulunda Lake).Evaporation capacity is the main part of their supply balance, the value of this balance from the water surface is 350-600 mm in open water period. In dry years their surface area significantly reduces(Kolpakova et al., 2016).
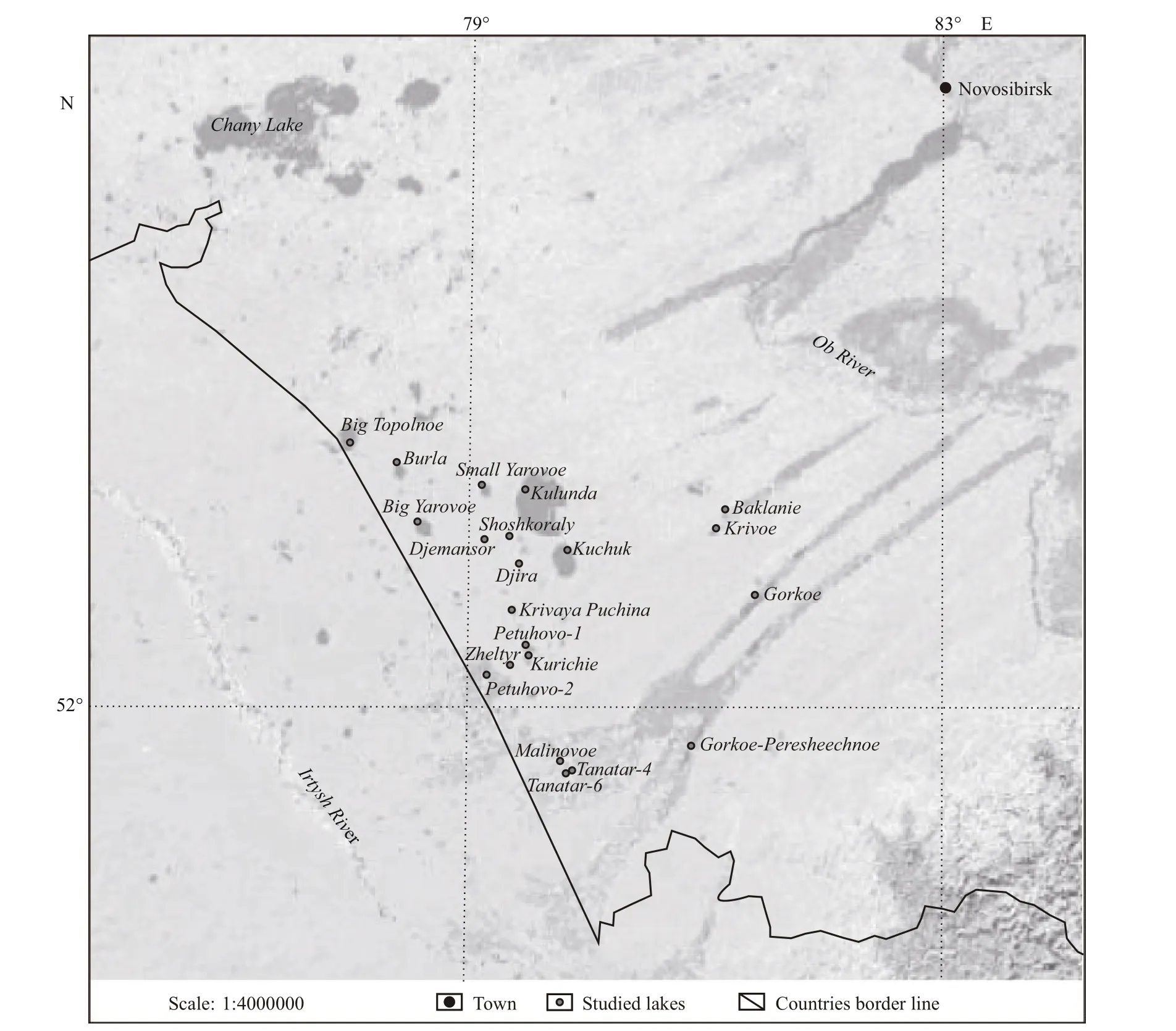
Fig.1 Location map of studied mineral lakes of steppe Altai
According to the classifi cation by Kurnakova-Valyashko (Valyashko, 1962), the Altai steppe lakes are chloride- (11) and soda- (10) types. Mineralization of lakes ranges from 1-2 to 590 g/L. Lower salinity is characteristic for soda lakes (Na-HCO3), and Na-Cl-HCO3types (from 1-2 to 100 g/L). Salinity of chloride lakes ranges from 40 to 590 g/L, and an average is 60 g/L. The main feature of soda lakes is high values of pH with maximum in the lake Petukhovo 2 (pH 9.78), while an average is 9.57. In chloride lakes an average pH is 8.02. Sodium prevails in these lakes(46-100 eq.%), its concentration ranges from 0.2 to 130 g/L. The content of magnesium and calcium is significantly lower. In some lakes calcium content is below the detection limit (Big Topolnoe), but the concentrations of calcium and magnesium grows with increasing salinity.
As can be seen from the data (Fig.2), in some lakes chloride ion becomes the predominant component on the early stages of the water concentration. The amount of carbonate and bicarbonate ions in soda lakes can reach 51 eq.%, with an average near 25 eq.%.Sulfate concentration also varies widely from a minimum set value in the soda lake Gorkoye-Peresheechnoe (0.08 g/L) to the maximum value 100 g/L in chloride lakes (Petukhovo 2). All lakes contain sulfates in subordinate amounts (about 2-28 eq.%).
The value of genetic SO4/Cl ratio indicates the loss of sulfates (Valyashko, 1962), and these values are significantly less than 1. By evaporative water concentration only, chloride and sulfate ions (up to the stage of gypsum formation) should be accumulated in approximately equal proportions, so SO4/Cl ratio must be equal to 1.
The lack in the sulfates accumulation can be explained by the presence of microbiological and geochemical barriers, such as sulfate reduction and sedimentation of sulfur-containing minerals.
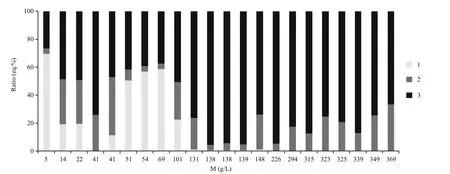
Fig.2 The ratio of basic anions (eq.%) depending on the mineralization (M) of mineral lakes1:

Table 3 Contents of reduced forms of sulfur in the salt lakes of Altai Krai

Fig.3 The ratio between the contents of reduced forms of sulfur and S0(a) and ratio between the contentsand S0(b)
The presence of hydrogen sulfi de and its derivatives(thiosulfate and elemental sulfur) suggests an active process of sulfate reduction (Table 3). Hydrogen sulfi de in the sediments occur in most lakes, the maximum content is determined in the lake Zheltyr(797.8 mg per 100 g of bottom sediments), with an average value-206.6 mg/100 g. Compounds of a higher valenceand S0accounts for up to 26% ofchloride lakes(Malinovoye, Kulunda and Small Yarovoye) have relatively high concentrations.
Hydrogen sulfi de is present in waters of nearly half of the studied lakes. Its concentration rarely exceeds fi rst hundreds of micrograms per liter, a relatively high value of hydrogen sulfi de is observed in the soda lake Tanatar 4 (890 μg/L). Hydrogen sulfi de derivatives (thiosulfate and elemental sulfur) are found more often; in concentrations from the detection limit to the maximum set values ofin the lake Tanatar 4 (223 μg/L), S0in the lake Kurichie(840 μg/L). In the amount of reduced forms of sulfur the Tanatar-4 Lake (1 194 μg/L) and the Kurichie Lake (884 μg/L) have relatively high values. A regular decrease of hydrogen sulfi de concentration and an increase of more oxidized derivatives from the sediment to the water column is observed in the spatial distribution of the reduced of sulfur species. In some cases (Tanatar-4, Big Yarovoye) hydrogen sulfi de content is higher in the surface layers than in the deeper layers of water or sediments. It is explained by the bacterial mats (algae) development on the water surface, their active decomposition leads to a further enrichment of water with hydrogen sulfi de and its derivatives. The most productive sulfate reduction takes place in soda lakes; average content of S2-is 287 mg per 100 g of sediment, in chloride lakes this value is considerably lower (150 mg). In waters of chloride lakes hydrogen sulfi de (H2S+HS-)calculated as S2-varies from detection limit to 67 μg/L(Petukhovo1). In soda lakes, where hydrogen sulfi de is predominantly in the form of HS-, the variation range is much wider: from zero (the Baklanie Lake)to the maximum set values in the lake Tanatar 4. In both geochemical lake types in ΣSred.content the predominant form is S0, the second one in soda lakes is S2-, and in chloride lakes is S0S4+(Fig.3a).
The reactions of bacterial reduction of sulphate ion and oxidation of hydrogen sulfi de to sulfate ion are complex. They can be represented like this:andrespectively. Numerous studies (Miao et al., 2012; Poser et al., 2013; Xu et al.,2013) have shown that it is a multistage process,wherein S0is formed solely in oxidation reactions chain. The close nature of the distributions of S0S4+to S2-(correlation coeffcient r is 0.84) can be explained by the dual nature of thiosulfates; hypothetically, they are an intermediate product of sulfate reduction to hydrogen sulfi de or the oxidation of the latter. Perhaps,in our case, they are preferentially products of reduction processes. Isotopic analysis could clarify their nature, but it is not possible at the level of fi xed concentration. Thiosulfates are also formed by the disproportionation reaction of polythionates (Busev and Simonova, 1975). Under the neutral-alkaline conditions polythionates are unstable and decompose to thiosulfates. The inverse relationship betweenand S0(ris -0.24) manifested in the soda lakes indicates the possibility of their formation according to the reactionin this case the presence ofˉ in redox reactions of sulfur is necessary. The migration of sulfur in the form oftogether with S0in substantial amounts is impossible,so there is no need (in this case) in the separation and analysis of samples on sulfi te- and polythionate ions.

Fig.4 Dependence of the contents ΣSredon TOC (a) and dependence of the contents S2-on M (b)The numbers are according to Table 1.
The presence of elemental sulfur in the water is a clear indication that under these conditions hydrogen sulfi de is oxidized by dissolved oxygen, and (or)involving bacteria. Sulfur concentrations vary greatly,with the maximum in the chloride lake Kurichie and the soda lake Zheltyr, with an average 82.4 μg/L in the soda lake and 174.3 μg/L in the chloride lake. S0content is often higher than its solubility threshold(6 μg/L), and that implies the various forms ofits location in the water. The presence of sulfur in the form of tiny suspended particles, condensing on the surface of the bacterial purple fouling, was observed in the soda lakes Petukhovo 2, Tanatar 4, etc. The presence of sulfur in the form of polysulfi des is confi rmed by concerted distribution of S0and S2-in soda lakes (Fig.3b).
The appearance of S0along with S2-in the lakes shows simultaneously occurring and differently directed processes of hydrogen sulfi de oxidation and sulfate reduction. The relationship between hydrogen sulfi de and its products of partial oxidation are determined by many factors: the content of sulfates and oxygen dissolved in water, relevant microf l ora,and the kinetic parameters of each stage of the process in these natural environments. The prevalence of elemental sulfur in the water can be explained by kinetics of the oxidation process, because oxidation rate of hydrogen sulfi de until S0is greater than the rate of the subsequent stages (Neretin et al., 1996).One of the ways of S0formation is the reaction of hydrogen sulfi de with iron oxide, which fi nal products are iron sulfi des (Skryabin, 1983), and they are found in the composition of black hydrogen sulfi de bottom sediments.
The amount of organic carbon TOC and water salinity are some of the factors which limit the process of sulfate reduction. The reduced sulfur is intensively formed in the area of high content of TOC (Fig.4a). In soda lakes the growth of water salinity is accompanied by an increase in the concentration of hydrogen sulfi de, on the contrary, in chloride lakes high salinity limits the formation of hydrogen sulfi de (Fig.4b).

Fig.5 SO4/Cl dependence on the contents of ΣSredThe line trend for the soda lakes.
As to the intensity of the process, the maximum rate of sulfate reduction (1 426 μmol/(dm3·d)) was observed in the lake Gorkoye (Foti et al., 2008) with a salinity of 14.4 g/L and a TOC content of 57.8 mg/L.Two main groups of bacteria Haloalkaliphilic:Desulfovibrionales and Desulfobacteraceae are dominant in these salt lakes (Sorokin et al., 2010,2011). According to some researches (Foti et al.,2008), number of bacteria involved in sulfate reduction exceeds 100 cells/mL. Most active sulfate genesis is seen here with elemental sulfur (Sorokin et al., 2004; Foti et al., 2007).
Effect of sulfate reduction on sulfate sulfur content is clearly seen through the ratio of ΣSred.to SO4/Cl(Fig.5). In the lakes with a relatively high content of(Tanatar-4, Zheltyr etc.) losses of sulfates are bigger. Low ΣSredconcentration and high value of SO4/Cl (Lake Djemansor) indicate the absence of microbiological barriers for sulfate reduction, so their contents are comparable with chlorides.
Thermodynamic calculation of water equilibrium with sulfur-containing minerals showed water undersaturated with respect to main sulfate minerals(gypsumanhydrite CaSO4, and epsomite(Table 4), because of low calcium and magnesium contents in lakes which isnot enough for such minerals formation even in lakes with high concentration of sulfate ions. In the soda lakes calcium is not accumulated due to the formation of secondary minerals: calcite CaCO3, dolomite CaMg(CO3)2and magnesite MgCO3, in some cases gaylussite Na2Ca(CO3)2.

Table 4 Minerals saturation index (lg SI), according to the results of the chemical data in Tables 1, 2
In chloride lakes restrictions relate more to magnesium. At the same time water equilibrium in some lakes is attained with barite BaSO4and celestite SrSO4and in other lakes with mackinawite FeS and pyrrhotite FeS. Reduced sulfur is a geochemical barrier for many elements with variable valence(manganese, iron, vanadium, molybdenum), so they precipitate in the form of the corresponding sulfi des(Yushkin, 1968). With relatively low content of oxygen in water and presence of hydrogen sulfi de, the formation of elemental sulfur is thermodynamically favorable, the saturation index of elemental sulfur is higher for soda lakes water (average lg SIsoda=5.5 and 3.1).
4 CONCLUSION
In general, it should be noted that in salt lakes of steppe Altai hydrogen sulfi de formation processes occur in accordance with normal (theoretical) course of biochemical processes, i.e. the content of reduced forms of sulfur increases by decreasing of sulfates in waters. The presence of hydrogen sulfi de is observed in the bottom sediments and in the surface water layer. Obviously, by this rate of bacterial production of hydrogen sulfi de, it does not have time to be chemically oxidized and it is present in the oxygencontaining water in reliably determined quantities.While there is the evidence of the oxidation of hydrogen sulfi de, a part of sulfates is irreversibly reduced to hydrogen sulfi de, further it combines with iron and then sulfates precipitate from the solution in the form of various iron sulfi des. High water salinity restricts the intensity of sulfate reduction in chloride lakes. As a result, organic matter accumulates in large amounts. The absence of relationship between the contents of organic matters and S2-in soda lakes indicates that organic matter produces and decomposes actively. The thermodynamic calculations show, that losses of sulfur can be possible as the result of formation of sulfate mineralsbarite, celestite and elemental sulfur. It is obvious that bacterial reduction of sulfates and secondary mineralization determine the behavior of sulfur in the lakes of steppe Altai.
 Journal of Oceanology and Limnology2018年3期
Journal of Oceanology and Limnology2018年3期
- Journal of Oceanology and Limnology的其它文章
- Response of the North Pacific Oscillation to global warming in the models of the Intergovernmental Panel on Climate Change Fourth Assessment Report*
- Effect of mesoscale wind stress-SST coupling on the Kuroshio extension jet*
- Surface diurnal warming in the East China Sea derived from satellite remote sensing*
- Cross-shelf transport induced by coastal trapped waves along the coast of East China Sea*
- Observations of near-inertial waves induced by parametric subharmonic instability*
- Seasonal variation and modal content ofinternal tides in the northern South China Sea*
