Bioactive molecules in Siddha Polyherbal Nilavembu Kudineer alleviating symptoms of Dengue/Chikugunya
Rubeena Mattummal, Divya Kallingilkalathil Gopi, Sathiya Rajeshwaran Parameswaran, Sunil Kumar Koppala Narayana*
1 Siddha Central Research Institute, Central Council for Research in Siddha, Ministry of AYUSH, Govt. of India,Arumbakkam, Chennai, India.
Background
Many of the human diseases are cured by modern medicines which sometimes produces unfavorable reactions and toxic side effects. Plants, being a reservoir of medicinal compounds, help in preventing and curing ailments without serious adverse effects [1]. Compounds derived from living organisms, with their significant pharmacological activity, can compete with modern medicines [2]. Plants produce phytochemical constituents for defense against pathogen owing to their characteristic bioactivities. Therapeutic property of every plant is confined to the bioactive compounds present in it. Hence,the screening of these compounds is necessary for the standardization and validation of herbal drugs formed from it. Alkaloids, flavonoids, phenols, terpenoids,tannins and quinones etc. are the important classes of secondary metabolites in plants with significant pharmacological activities. Secondary metabolites derived from plants are reported to possess many important pharmacological characteristics such as anti-oxidant, anti-microbial, anti-allergic, hypoglycemic and anti-cancer properties [3].
Siddha is one of the ancient systems medicines,originating in ancient Tamilakam in south India and Sri Lanka with several polyherbal therapies which were formulated basing on Siddha principles. Siddha system of medicine uses the ancient beliefs and healing methodologies listed by the Siddhars (Siddha physicians)using plants, metals, minerals and various animal products. Diagnosis in this system includes assessing the equilibrium and derangement of the three humors of the body - Vaadham, Pittham and Kapam, the imbalance of which is believed to be the cause of various disease [4].
Dengue is the most prevalent arthropod-borne viral diseases in terms of morbidity and mortality in the recent decade which has re-emerged and remains endemic in more than 110 countries. Two fifths of the world populations (estimating around 100 million) Dengue fever infections, 2.1 million cases of Dengue hemorrhagic fever and 200 thousand deaths worldwide are caused by Dengue every year. Despite extremely high rates of Dengue for decades, Southeast Asia region still recorded an increase of 67% from 1985 - 1989 to 2002 - 2006 [5].Dengue appears in two forms, the classic and severe Dengue. Classic Dengue fever shows symptoms ranging from mild to high fever with retro-orbital pain, severe headaches, maculo-papular rashes, muscle and joint pain.The severe form, Dengue hemorrhagic fever and Dengue shock syndrome may present with abdominal bleeding,hemorrhage and circulatory failure, which is fatal without prompt and proper management [6]. There are four serologic types of Dengue virus (DENV), DENV-1, -2, -3 and -4. Chikugunya is also an arthropod-borne viral diseases with the classic symptoms of fever, joint pain,rash, etc, which is similar to that of Dengue. With the rapid expansion of Dengue and Chikugunya disease in most tropical and subtropical areas of the world, it is crucial to develop effective prevention and control measures, including antiviral drugs and vaccines against them [7].
Siddha medicines are prescribed either as single drug remedy or polyherbal/herb-mineral formulations.Nilavembu k udineer (NK) has been prescribed as a curative and preventive medicine against Dengue [8]. NK is a polyherbal formulation prepared by Andrographis paniculata (Brum.f.) Nees (whole plant), Chrysopogon zizanioides (L.) Roberty (root), Santalum album L. (heart wood), Trichosanthes cu cumerina L. (whole plant),Cyperus r otundus L. (rhizome), Zingiber o fficinale Roscoe (rhizome), Piper nigrum L. (fruit) and Mollugo cerviana (L.) Ser. (whole plant) in equal parts. Kudineer(decoction) is the common name given to the Siddha formulation in which the whole plant (s) or particular part of plant (s) is ground into coarse powder, called as Kudineer Cho ornam (coarse powder for preparation of decoction). It is then made into Kudineer by adding water and heated, so that the mixture of Kudineer Choornam and water reduces to 1/4th or 1/8th of its volume as mentioned in the literature. The dosage to be taken is 30 mL before food, three to four times a day. Lifetime of prepared Kudineer is 1 Samam (3 hours) [9]. The ingredients contain various bioactive compounds like andrographolide, β-vetivenene, α-zingiberene, α-copaene,cyperene, 2-monolinolenin, limonene, β-pinene,β-caryophyllene and α-santalol. The ingredients of the Kudineer possess anti-inflammatory, anti-microbial,analgesic, anti-oxidant, anti-viral, cytotoxic,hepatoprotective and anti-diabetic activities [10]. The anti-inflammatory, anti-viral, and analgesic effects of the various phytochemicals present in NK will help in suppressing and curing the clinical symptoms associated with Dengue. Since the studies about this Kudineer are very scanty, this review aims the documentation of the bioactive compounds and their characteristic pharmacological activities of NK ingredients to throw some light in support of action NK on Dengue.
Andrographis paniculata Burm.f. Nees
Andrographis pan iculata Burm.f. Nees, known as Nilavembu in Siddha, belongs to family Acanthaceae is used traditionally as a remedy against common cold,fever and inflammation, etc. The Indian Pharmacopoeia describes it as a major constituent of at least 26 ayurvedic formulations. In traditional Chinese medicine, it has a significant “cold property” which is used to relieve the body heat, and to drive out toxins from the body [11]. The medical use of Andrographis pa niculata against sore throat has been well known in Thailand [12]. In India, it is used to reduce griping, irregular bowel habits, and loss of appetite of children. Due to its “blood purifying”quality, it is suggested to make use in the treatment of leprosy, gonorrhea, scabies, boils and skin eruptions [13].The key bioactive component of Andrographis paniculata,is the major diterpenoidal constituent, and rographolide.The compound has been reported to have different pharmacological activities like anti-inflammatory [14],anti-cancer [15], anti-microbial [16] and hepatoprotective activities [17] (Table 1). Along with andrographolide the neoandrograpide also imparts the characteristic medicinal activities to the plant and acts as immunostimulant agents that are reported to have both antigen specific and non-specific immune responses [18]. Some studies proved the side effects of andrographolide in creating infertility [19] had led to the questioning of complete safety of NK.
Andrographis p aniculata is an annual herbaceous to arborescent plant with woody branched stem bearing simple, opposite, lanceolate leaves, pink-purple colored,two-lipped zygomorphic flowers and cylindrical to flattened capsules [20]. Beside NK, this plant is the ingredient of other Siddha formulation, Nilavembu camulam [21].
Chrysopogon zizanoids (L.) Roberty
Chrysopogon zizanioides (L.) Roberty is a perennial grass of Poaceae family known for its fragrance oils and medicinal properties. Vetiver oil is composed of more than 170 compounds that are mainly sesquiterpenes and their derivatives (Table 2). Because of the complex nature of the essential oil, it has not been studied intensively[39]. The drug is fibrous, wiry, long, cylindrical roots up to 2 mm in diameter, multi-branched, often attached with stout root stock, smooth or longitudinally grooved, color,light brown, odor strong aromatic, taste and slightly bitter[40]. Veti ver/Vilamiccam ver is used in many Siddha formulations, some of which includes Amirtataik kulikai,Incic choor anam, Maka V acanta kucumakram, Pitta Curak kudineer, Maka e lati kulikai, Nay uruvi nei,Parangi chakkai chooranam [21, 41].
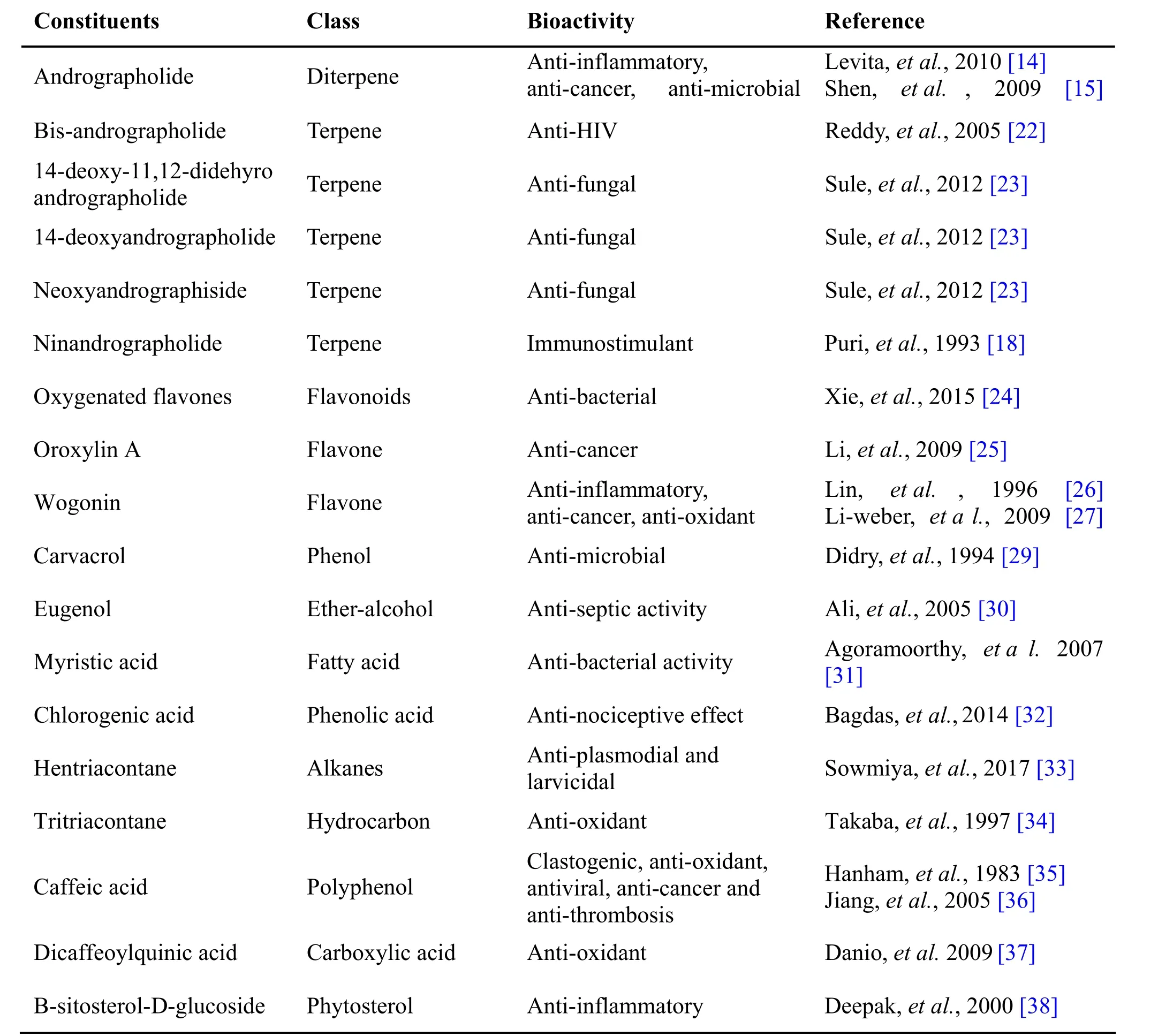
Table 1 Phytochemical constituents in Andrographis paniculata Burm.f. Nees
Cyperus rotundus L.
Cyperus r otundus L. belong to family Cyperaceae is a perennial sedge plant with flowering stem and linear leaves which form a sheath around stem. Rhizome of Cyperus r otundus L known as Koraik kizhangu, is used for the treatment of stomach, bowel disorders, and inflammatory diseases. Drug is a rhizome clothed with flexuous hair, outer surface dark brown, and white inside[47]. The drug is used as anti-inflammatory [48] and anti-malarial [49] (Table 3).
In Ayurveda the dried rhizome is used in dyspepsia/loss of appetite, indigestion, polydipsia/excessive thirst,irritable bowel syndrome, dyspnoea, cough, dysuria,vomiting, lacteal disorders, puerperal disorders, diarrhoea,rheumatoid arthritis and worm infestation [50]. In Siddha Cyperus rotundus is used in many preparations and some are Athimathura mathirai, Adathodai chooranam, Civatai chooranam, Cukku tailam, Ka pada Il akam, S anjeevi theenir. Chandraprakasa mathirai, Kapacurak kudineer,Thathupushti kul ikai, P arangi chakkai chooranam,Milagu thailam [21, 41].
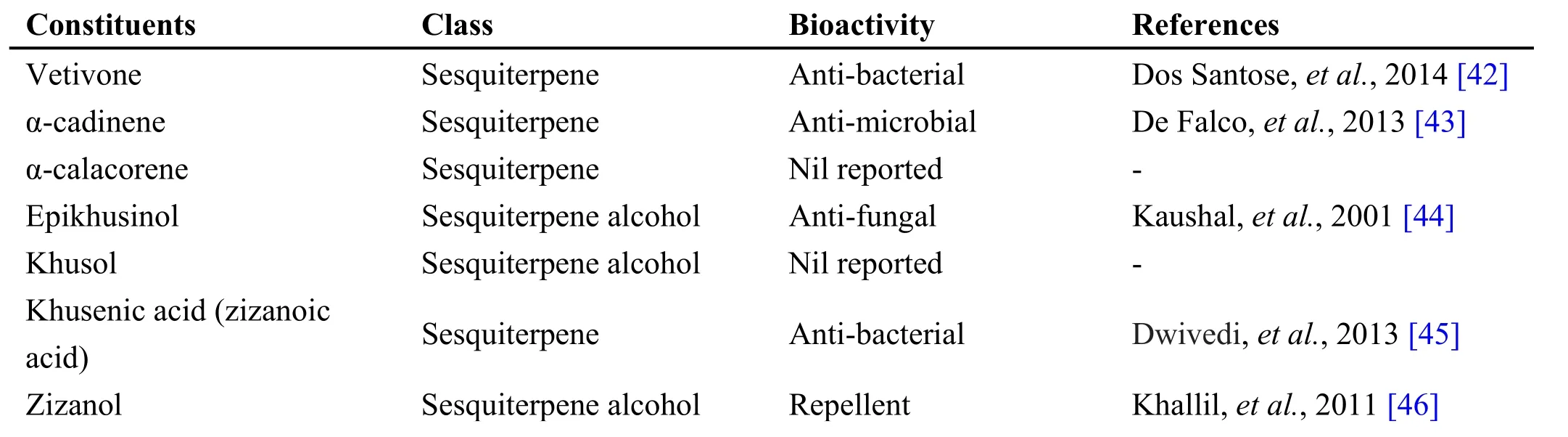
Table 2 Phytochemical constituents in Chrysopogon zizanoides (L.) Roberty
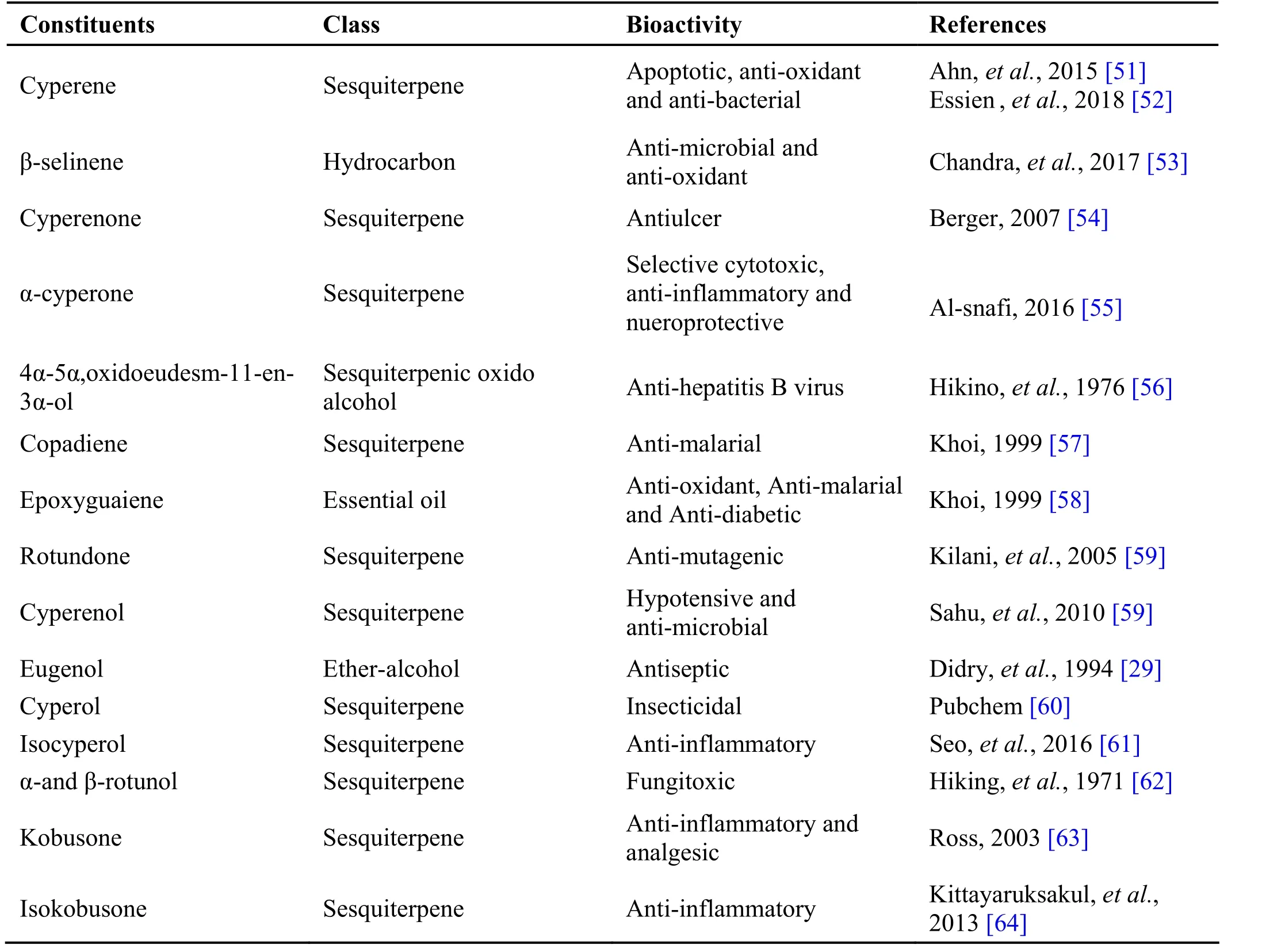
Table 3 Phytochemical constituents in Cyperus rotundus L.
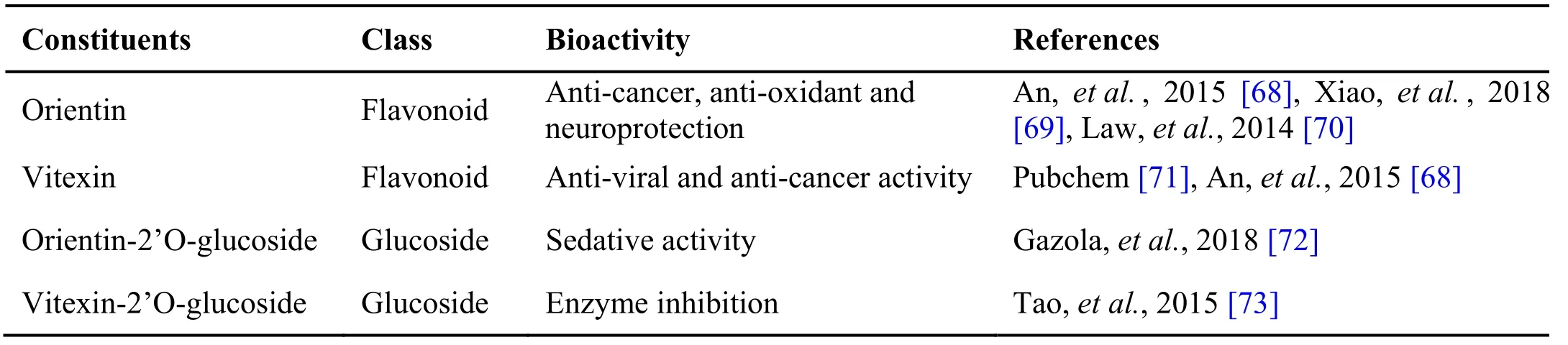
Table 4 Phytochemical constituents in Mollugo cerviana (L.) Ser.
Mollugo cerviana (L.) Ser.
Mollugo c erviana (L.) Ser. is commonly known as Parpadakam in Tamil from the family Molluginaceae. It is an erect slender annual herb with slender cylindrical stem bearing leaves in whorls; flowers numerous and having fruits as rounded capsules, used in Siddha,Ayurveda and folk medicine. The whole plant possesses medicinal properties. The drug is diuretic, anthelmintic,digestive and relieves constipation. It is bitter, cooling and constrictor [65] and used as laxative, stomachic,antiseptic, febrifuge and diaphoretic. It is found to be effective against burning sensation, burning eyes, gastric diseases and fever [20]. The whole plant is used in NK formulation. The pharmacological activities like anti-bacterial [66] and anti-inflammatory [67] actions of Mollugo cerviana have been reported in various studies(Table 4). The plant is also used to prepare Thonthasurak kutinir, a siddha formulation used to cure inflammation,fever and cough [41].
Piper nigrum L.
Piper nigrum L. is a perennial climbing vine of family Piperaceae having flowering woody stem with alternating leaves tapering towards the tip. Dried fruits of Piper nigrum are commonly used in gastrointestinal disorders.These little seeds with enormous health benefits have been an admirable natural remedy for treating various illnesses ranging from obesity to cancer. Fruit is an indehiscent one-seeded globose berry, ovoid to oblong,coarsely, deeply reticulately wrinkled grayish-black in colour, odour, aromatic, taste very pungent [74]. The drug is used as appetizer and is active against helminthiasis,colic [75, 76]. Piper nigrum is used in majority of Siddha formulations and some of them are Amukkara chooranam,Aruvatha mathirai, Iraca ganti meluku, Elatic chooranam,Noccit tailaam, Swasakutori chooranam, Milaku tailam,Vilvati tailam, etc [21, 41]. Piperine, isolated from Piper nigrum has anti-inflammatory, anti-nociceptive, and anti-arthritic property [76] (Table 5).
Santalum album L.
Cantanakkattai, the heart wood of santalum album L, is used as the ingredient drug in many formulations.Sandalwood oil has been widely used in folk medicine for treatment of common colds, bronchitis, skin disorders,heart ailments, general weakness and fever, etc. The heartwood occurs as solid heavy pieces of log or as chips varying in length and width and density. On transversely cut surface it is diffuse porous with medullary ray appearing as fine reddish line [101]. Santalum album L. is used to treat utricaria, diarrhoea and dysentery [102].Santalum album is used in the preparation of formulations such as Arakku tailam, Cintil nei, Cukku ta ilam, Il aku Cantanatit t ailam, Na ciroka Nacat ta ilam, Vallarai nei[21], Kirichannangaluku ennei, K umari t hailam and Mahavilvathi ilakam [41] (Table 6).
Trichosanthes cucumerina L.
Trichosanthes cucumeri na L., known as Peipudal in Siddha, is an annual climber widely used as a medicinal plant in different traditional medicine systems due to its various medicinal values. The fruit is usually consumed as a vegetable due to its good nutritional value [106]. The plant is rich in flavonoids, carotenoids and phenolic compounds which contributes to the antidiabetic,hepatoprotective, cytotoxic, anti -inflammatory, larvicidal effects of the plant. The fruit and roots of this plant contains bryonolic acid, bryononic acid,dihydrocucurbitacin B, chondrillasteryl glucoside and cucurbitacin B [107]. All these active constituents show antiviral characteristics and anti-malarial activity (Table 7). Trichosanthes cucumerina is a climber with palmately lobed leaves with unisexual flowers and ovoid-fusiform fruits [108]. Whole plant is used in the preparation of Cintil ne i, T honthakara kudineer and Manjal n oiku kudineer [21, 41].
Zingiber officinale Roscoe
Known as Chukku in Siddha, Zingiber officinale Roscoe is a perennial herb of family Zingiberaceae with thick rhizome which containing several bioactive constituents,possessing health promoting properties. The characteristic odor and flavor of ginger root come from a volatile oil composed of shogaol and gingerols which make up about 1-3% of the weight of fresh ginger [118]. The main pharmacological actions of compounds isolated include immuno-modulatory, anti-tumorigenic, anti-inflammatory,anti-apoptotic, anti-hyperglycemic, anti-lipidemic and anti-emetic activities [119] (Table 8). The major phytochemicals reported from essential oil of the rhizome are 6-shogaol, 6-gingerol and α-zingiberene. Zingiber officinale Roscoe is considered as a safe herbal medicine with only few and insignificant side effects. Dried drug consists of sympodially branched laterally compressed pieces of horizontal growing rhizome measuring 5 to 12 cm in length, 3 to 5 cm in height and 1 to 2 cm in thickness; the surface is marked with circular closely placed leaf scars, and small circular root scars at places,pale buff to brownish in colour with aromatic odour and pungent taste [74]. Zingiber of ficinale Roscoe has anti-inflammatory, hepatoprotective and antioxidant property in paracetamol induced animal model [120]. The drug is used in colic, haemorrhoids, diseases of throat and inflammation [74]. Dried Zingiber officinale Roscoe is a major ingredient in most of the Siddha preparations and some of them include Agathi ennei, Ka pada i lakam,Pooranathi ilakam, Milaku tailam, Cukku tailam, Nellikai ilakam, Kapa curak kudi neer, Talicati c uranam, Vilvati ilakam, etc [21, 41].
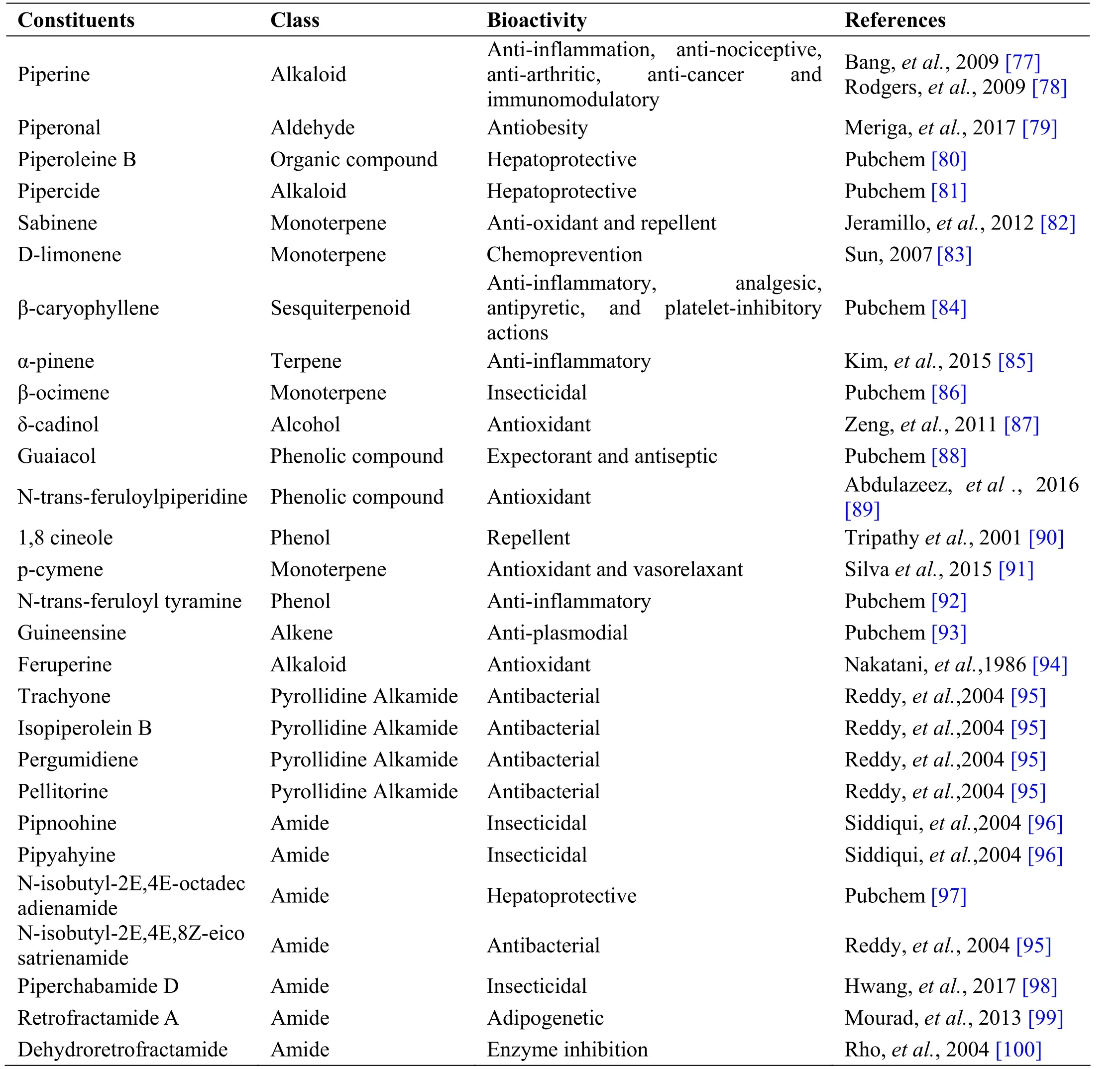
Table 5 Phytochemical constituents in Piper nigrum L.
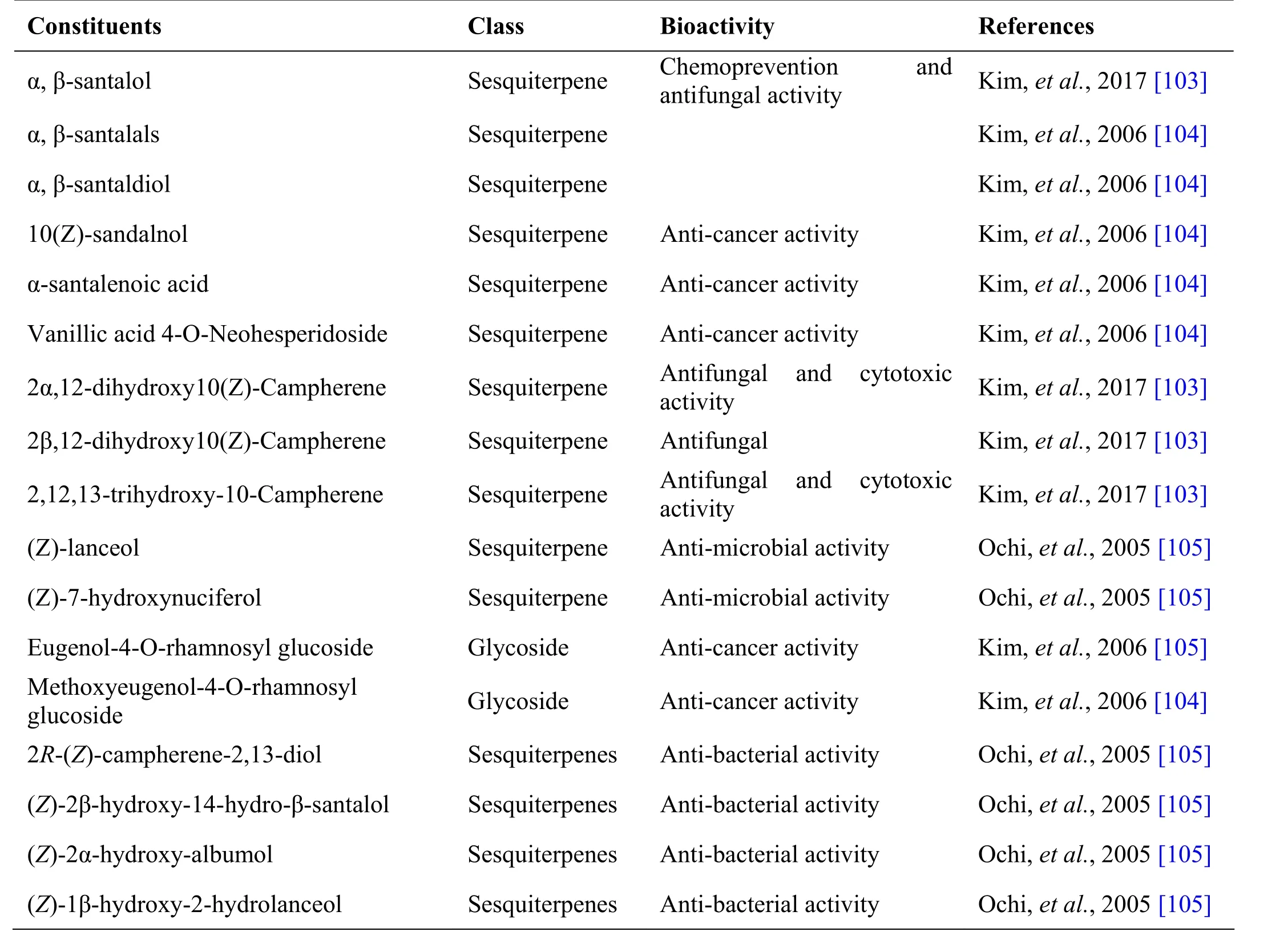
Table 6 Phytochemical constituents in Santalum album L.
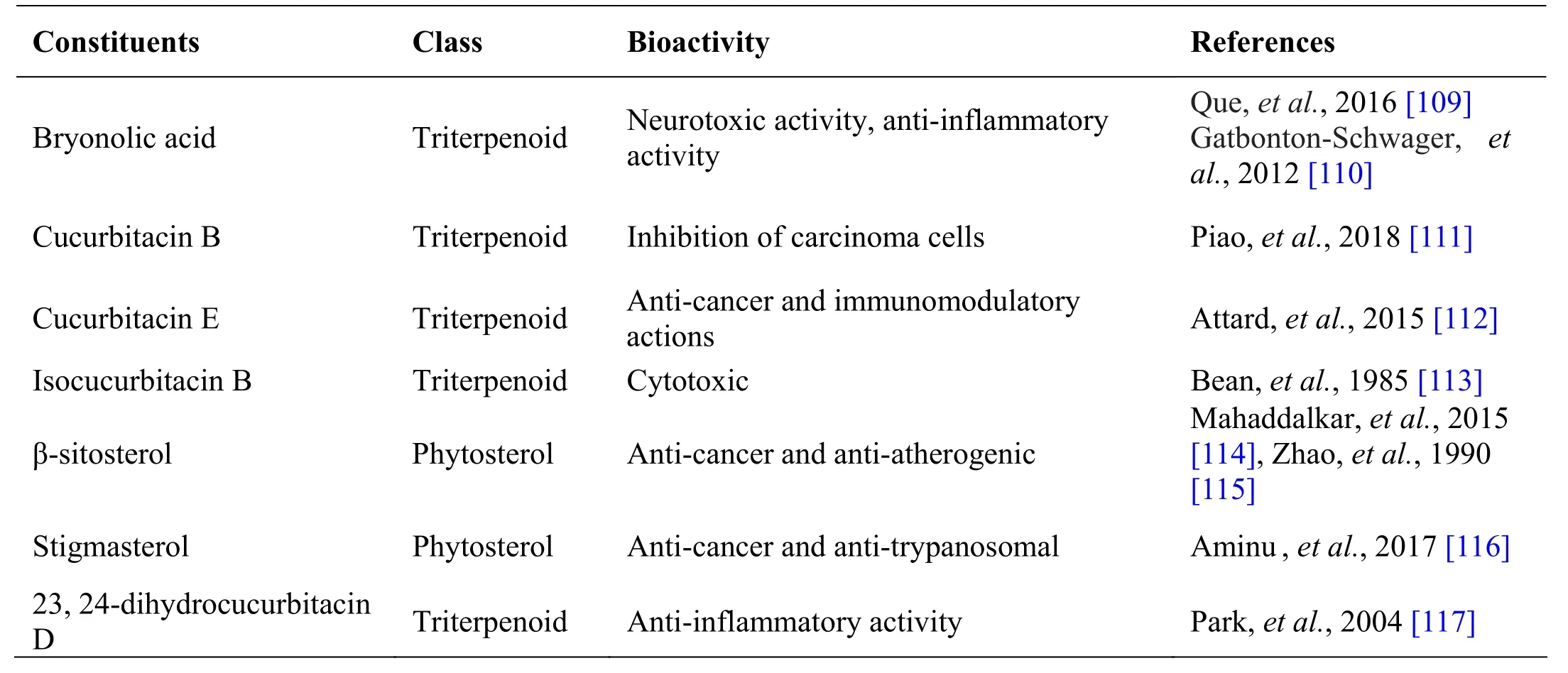
Table 7 Phytochemical constituents in Trichosanthes cucumerina L.

Table 8 Phytochemical constituents in Zingiber officinale Roscoe
Discussion
This review is an attempt to analyze the phytochemicals present in the ingredient drugs of NK so as to see what chemicals reported so far from the ingredients has actions beneficial for treating Dengue and Chikungunya.Essentially a Dengue/Chikungunya medicine must possess properties like analgesic, antipyretic,anti-inflammatory, and antiviral actions to alleviate the reported symptoms such as high fever, headaches, pain behind the eyes, joint and muscle pain, fatigue, nausea,vomiting, skin rash, mild bleeding from nose/gums, bruises,damage to lymph/blood vessels, liver enlargement, and circulatory system failure [142, 143].
Every polyherbal combination in Siddha is based on philosophy of Tridosham (three humours) and Panchamahabhutas (five basic elements) in consideration with the disease conditions. NK was formulated basing on ancient Siddha. But, not any specific bioactives necessarily are responsible for the therapeutic function of each drugs in modern perspective. As the drug is producing beneficial effects in cases of fevers,specifically Dengue and Chikungunya fevers, there is a need to see any connection between the symptoms and bioactivity of the molecules present in each ingredient. As all these eight ingredients are mixed and decoction made by boiling such individualistic action may not be so derogatory in deciding the action of NK. The synergistic action of all these bioactive compounds together may be imparting NK its characteristic medicinal property.
However, from this review, it is evident that these ingredients are used traditionally in the treatment of fever,inflammation, arthritis, gastric ulcer, jaundice and general debility condition while NK is also claimed to have antipyretic, anti-inflammatory, antiviral, and immunomodulatory actions [144]. The bioactives ingredients in combination might be providing relief from body pain, inflammation of the joints and enhancing immune mechanism suppressing the virus. The phytochemicals present in the ingredients also have antiviral and anti-microbial characteristics, which make it suitable medicine for the management of viral fevers. The combination of these ingredient drugs can be substantiated in modern terms on account of its immunostimulant action increasing the defense response in the body which will help an infected person to fight against the viral infection [143].
Potency of b ioactives of NK ingr edients to comb at symptoms of Dengue and Chikungunya
Andrographolide from Andrographis paniculata has been proved to possess anti-inflammatory and hepatoprotective activity, while ninandrographolide has shown immunostimulant property, Β-sitosterol-D-glucoside and wogonin also have anti-inflammatory activities (Table 1).Chrysopogon zizanioides has been reported to possess anti-bacterial and anti-fungal activities, while antiviral activity is yet to be established (Table 2). Cyperenone from Cyperus r otundus possess antiulcer activity;α-cyperone has anti-inflammatory and neuroprotective activity; kobusone has antinflammatory and analgesic activities; isokobuson and iso-cyperol also possess anti-inflammatory effects (Table 3). Mollugo cer viana possesses bioactives with good anti-oxidant potentials which is indirectly beneficial in combating many pathological conditions of body (Table 4). Piperoleine B from Piper nigrum has proved anti-inflammatory and hepatoprotective properties while pipercide has hepatoprotective action, while piperin showed immunostimulant and anti-inflammatory actions (Table 5). Santalum al bum has been reported to possess anti-bacterial and anti-fungal activities, while antiviral activity is yet to be established (Table 6). Cucurbitacin E from Trichosanthes c ucumerina possesses immunomodulatory actions (Table 7). 6-Gingerol, 6-shogaol,hexahydrocurcumin and nerolidol from Zingiber officinale have anti-inflammatory property (Table 8).While aforesaid are from isolated compounds. At extract level, some other properties beneficial in Dengue/Chikungunya like anti-fever (antipyretic) action of Andrographis p aniculata and Mollugo cer viana,anti-emetic property of Cyperus r otundus and Zingiber officinalis and antiviral property of Trichosanthes cucumerina have been proved.
Studies in lines with pr evention of Dengue using ingredients of NK
In a study carried out by Tang et al. [7] for the preliminary screening study for anti-Dengue agent,methanolic extract of Andrographis paniculata was able to maintain most of the normal cell morphologies without causing much cytopathic effect to the DENV-1 infected cells. Thus it possesses high potential to be an anti-Dengue agent, particularly towards DENV-1 serotype. Neoandrographolide, one of the principal diterpene lactones, isolated from a medicinal herb Andrographis p aniculata possesses significant anti-inflammatory effects [145]. The name given to the formulation stands to this ingredient which substantiates the naming.
The aqueous extract of Zingiber officinale may play an important role in the regulation of plasma leakage in Dengue virus infection and decrease the chances of severe Dengue complications by inhibiting the activity and expression of MMP-2 and MMP-9 while upregulating the expression of TIMP-1 and TIMP-2. Therefore, it can help in the development of new anti-Dengue agents [146].
Setback for Nilavembukudinner
Andrographolide a major phyto chemical present in Andrographis p aniculata has been proven to affect spermatogenesis in rats by preventing cytokinesis of the dividing spermatogenic cell lines with appearances of sertoli cell damage and a spermatotoxic effect [19]. The study pointed to a male reproductive toxic effect of a therapeutic use of andrographolide and confirmed the possible prospective use of andrographolide as a male contraceptive. The anti-spermatogenic abilities as well as ovulation preventive effect of the Andrographis paniculata reported may be the main reasons which lead to the controversy of safety of NK as drug. Hence the in-depth studies with standard clinical trials are required to clearly understand the effectiveness of this important formulation and to evade out the misconceptions generated so far. Trichosanthes c ucumerina has been shown to induce liver damage in rats [147]. Very little publication on scientific validations of NK in peer reviewed journals, lack of distinct standard operating procedure for its preparation, deliberative depressing misinformation will be a threat to this valuable traditional medicine.
Conclusion
Bio-actives reported from NK ingredients have shown efficacy for all major symptom of Dengue/Chikungunya.Anti-pyretic, anti-inflammatory, analgesic and immunostimulant effects have been attributed to more than one ingredient in NK. Though these bioactive molecules are present in individual drugs, there might be changes occurring in the structure of these molecules during preparation of Kudineer by mixing and heating all these eight plants with water. Hence more studies have to be undertaken for the scientific evaluation of mode of action of combination of these molecules on fevers like Dengue and Chikungunya. Scientific validation and standardization of the drug is also necessary to maintain its high degree of quality in the global herbal market.
Acknowledgement
The authors extend their heartfelt thanks to Director General, Central Council for Research in Siddha, Chennai for the support.
1. Fennell CW, Lindsey KL, McGaw LJ, et al.Assessing African medicinal plants for efficacy and safety: pharmacological screening and toxicology. J Ethnopharmacol 2004, 94: 205-217.
2. Anjana S, Thoppil JE. Cytological, phytochemical,pharmacological and in vitro regeneration studies on Pogostemon Deccanensis (Panigrahi) Press(Lamiaceae). Doctoral dissertation, University of Calicut 2016: 4.
3. Borneo R, León AE, Aguirre A, et al. Antioxidant capacity of medicinal plants from the Province of Có rdoba (Argentina) and their in vitro testing in a model food system. Food Chem 2009, 112: 664-670.
4. Siddha MM. Murugesan M. Indian medicine,Medicinal Plants Division, Homeopathy 1936:652-653.
5. Gibbons RV. Dengue conundrums. Int J Antimicrob Agents 2010, 365: 36-39.
6. Gubler DJ. Dengue and Dengue hemorrhagic fever.Clin Microbiol Rev 1998, 11: 480-496.
7. Tang LI, Ling AP, Koh RY, et al. Screening of anti-Dengue activity in methanolic extracts of medicinal plants. BMC Complement Altern Med 2012, 12: 3.
8. https://www.nhp.gov.in/pitha-suram-Dengue-fever-_mtl accessed on 28th July 2018.
9. Mahadevan H, Palraj V. Literature review on Siddha herbal formulations (Kudineer) available for the management of Dengue. Int J Pharmacol 2016, 5:96.
10. Anbarasu K, Manisenthil KK, Ramachandran S.Antipyretic, anti-inflammatory and analgesic properties of nilavembu kudineer choornam: a classical preparation used in the treatment of chikungunya fever. Asian Pac J Trop Med 2011, 4:819-823.
11. Deng WL. Preliminary studies on the pharmacology of the Andrographis product dihydroandrographolide sodium succinate. New Lett Chin Herb Med 1978, 8:26-28.
12. Poolsup N, Suthisisang C, Prathanturarug S, et al.Andrographis paniculata in the symptomatic treatment of uncomplicated upper respiratory tract infection: systematic review of randomized controlled trails. J Clin Pharm Ther 2004, 29: 37-45.
13. Kumar S, Patil HS, Sharma P, et al. Andrographolide inhibits osteopontin expression and breast tumor growth through down regulation of P-13 kinase/Akt signaling pathway. Curr Mol Med 2012, 12:952-966.
14. Levita J, Nawawi AA, Mutalib A, et al.Andrographolide: a review of its anti-inflammatory activity via inhibition of NF-kappaB activation from computational chemistry aspects. Int J Pharm 2010,6: 569-576.
15. Shen K, Liu T, Xu C, et al. Andrographolide inhibits hepatoma cells growth and affects the expression of cell cycle related proteins. Yao Xue Xue Bao 2009,44: 973-979.
16. Singha PK, Roy S, Dey S. Antimicrobial activity of Andrographis paniculata. Fitoterapia 2003, 74:692-694.
17. Rana AC, Avadhoot Y. Hepatoprotective effects of Andrograhphis paniculata against carbon tetrachloride-induced liver damage. Arch Pharm Res 1991, 14: 93-95.
18. Puri A, Saxena R, Saxena RP, et al.Immunostimulant agents from Andrographis paniculata. J Natu Prod 1993, 56: 995-999.
19. Akbarsha MA, Murugaian P. Aspects of the male reproductive toxicity/male antifertility property of andrographolide in albino rats: effect on the testis and the cauda epididymidal spermatozoa. Phytother Res 2000, 14: 432-435.
20. Indian Council of Medical Research. Quality Standards of Indian Medicinal Plants, ICMR, New Delhi 2010, 8: 55-56, 255-263, 348.
21. The Siddha formulary of India Part I, The controller of Publications, Delhi 2011: 185, 174, 178, 193.
22. Reddy VL, Reddy SM, Ravikanth V, et al. A new bis-andrographolide ether from Andrographis paniculata Nees and evaluation of anti-HIV activity.Nat Prod Res 2005, 19: 223-230.
23. Sule A, Ahmed QU, Latip J, et a l. Antifungal activity of Andrographis paniculata extracts and active principles against skin pathogenic fungal strains in vitro. Pharm Biol 2012, 50: 850-856.
24. Xie Y, Yang W, Tan F. Antibacterial activities of flavonoids: structure-activity relationship and mechanism. Curr Med Chem 2015, 22: 132-149.
25. Li HN, Nie FF, Liu W, et al. Apoptosis induction of oroxylin A in human cervical cancer HeLa cell line in vitro and in vivo. Toxicology 2009, 257: 80-85.
26. Lin CC, Shieh DE. The anti-inflammatory activity of Scutellaria rivularis extracts and its active components, baicalin, baicalein and wogonin. Ame J Chin Med 1996, 24: 31-36.
27. M LW. New therapeutic aspects of flavones: the anticancer properties of Scutellaria and its main active constituents Wogonin, Baicalein and Baicalin.Cancer Treat Rev 2009, 35: 57-68.
28. Shieh DE, Liu LT, Lin CC. Antioxidant and free radical scavenging effects of baicalein, baicalin and wogonin. Anticancer Res 2000, 20: 2861-2865.
29. Didry N, Dubreuil L, Pinkas M. Activity of thymol,carvacrol, cinnamaldehyde and eugenol on oral bacteria. Pharm Acta Helv 1994, 69: 25-28.
30. Ali SM, Khan AA, Ahmed I, et al. Antimicrobial activities of Eugenol and Cinnamaldehyde against the human gastric pathogen Helicobacter pylori. Ann Clin Microbiol Antimicrob 2005, 4: 20.
31. Agoramoorthy G, Chandrasekaran M, Venkatesalu V,et al. Antibacterial and antifungal activities of fatty acid methyl esters of the blind-your-eye mangrove from India. Braz J Microbiol 2007, 38: 739-742.
32. Bagdas D, Ozboluk HY, Cinkilic N, et al.Antinociceptive effect of chlorogenic acid in rats with painful diabetic neuropathy. J Med Food 2014,17: 730-732.
33. Sowmiya R, Balasubramani G, Deepak P, et al.Clastogenic activity of caffeic acid and its relationship to hydrogen peroxide generated during autooxidation. Mutat Res 1983, 116: 333-339.
34. Takaba K, Hirose M, Yoshida Y, et al. Effects of n-tritriacontane-16, 18-dione, curcumin,chlorophyllin, dihydroguaiaretic acid, tannic acid and phytic acid on the initiation stage in a rat multi-organ carcinogenesis model. Cancer Lett 1997,113: 39-46.
35. Hanham AF, Dunn BP, Stich HF. Clastogenic activity of caffeic acid and its relationship to hydrogen peroxide generated during autooxidation.Mutat Res 1983, 116: 333-339.
36. Jiang RW, Lau KM, Hon PM, et al. Chemistry and biological activities of caffeic acid derivatives from Salvia miltiorrhiza. Curr Med Chem 2005, 12:237-246.
37. Danino O, Gottlieb HE, Grossman S, et al.Antioxidant activity of 1, 3-dicaffeoylquinic acid isolated from Inula viscosa. Food Res Int 2009, 42:1273-1280.
38. Deepak M, Handa SS. Antiinflammatory activity and chemical composition of extracts of Verbena officinalis. Phytother Res 2000, 14: 463-465.
39. Kim HJ, Chen F, Wang X, et al. Evaluation of antioxidant activity of vetiver (Vetiveria zizanioides L.) oil and identification of its antioxidant constituents. J Agric Food Chem 2005, 53:7691-7695.
40. Indian Council of Medical Research. Quality Standards of Indian Medicinal Plants Vol 4, ICMR,New Delhi 2006, 252.
41. Siddha formulary of India Part II, The controller of Publications, Delhi 2011, 296, 299, 309, 312.
42. Dos Santos DS, Oberger JV, Niero R, et al. Seasonal phytochemical study and antimicrobial potential of Vetiveria zizanioides roots. Acta Pharm 2014, 64:495-501.
43. De Falco E, Mancini E, Roscigno G, et al. Chemical composition and biological activity of essential oils of Origanum vulgare L. subsp. vulgare L. under different growth conditions. Molecules 2013, 18:14948-14960.
44. Kaushal S. Detection and chemical transformations of a few terpenoids. Doctoral dissertation, Punjab Agricultural University, Ludhiana.
45. Dwivedi GR, Gupta S, Roy S, et al. Tricyclic sesquiterpenes from Vetiveria zizanoides (L.) Nash as antimycobacterial agents. Chem Biol Drug Des 2013, 82: 587-594.
46. Khalil MA, Ayoub SM. Analysis of the essential oil of Vetiveria nigritana (Benth.) Stapf root growing in Sudan. J Med Plants Res 2011, 5: 7006-7010.
47. Quality Standards of Indian Medicinal Plants, Vol. 1,ICMR, New Delhi, 2003, 89-94.
48. Gupta MB, Palit TK, Singh N, et al.Pharmacological studies to isolate the active constituents from Cyperus rotundus possessing anti-inflammatory, anti-pyretic and analgesic activities. Indian J Med Res 1971, 59: 76-82.
49. Thebtaranonth C, Thebtaranonth Y,Wanauppathamkul S, et al . Antimalarial sesquiterpenes from tubers of Cyperus rotundus:structure of 10, 12-peroxycalamenene, a sesquiterpene endoperoxide. Phytochemistry 1995,40: 125-128.
50. Ayurvedic Pharmacopoeia of India. Part I. New Delhi: government of India, ministry of health and family welfare, department of Indian systems of medicine and homeopathy 2001, 115-117.
51. Ahn JH, Lee TW, Kim KH, et a l. 6-Acetoxy Cyperene, a Patchoulane‐type Sesquiterpene isolated from Cyperus rotundus Rhizomes induces caspase‐dependent apoptosis in human ovarian cancer cells. Phytother Res 2015, 29: 1330-1338.
52. Essien EE, Thomas PS, Ascrizzi R, et al. Senna occidentalis (L.) Link and Senna hirsuta (L.) HS Irwin &Barneby: constituents of fruit essential oils and antimicrobial activity. Nat Prod Res 2018: 1-4.
53. Chandra M, Prakash O, Kumar R, et al.β-Selinene-Rich essential oils from the parts of Callicarpa macrophylla and their antioxidant and pharmacological activities. Medicines 2017, 4: 52.
54. Berger RG. Flavours and fragrances: chemistry,bioprocessing and sustainability. Springer Science &Business Media 2007.
55. Al-Snafi AE. A review on Cyperus rotundus A potential medicinal plant. J Pharm 2016, 6: 32-48.
56. Hikino H, Aota K. 4α, 5α-Oxidoeudesm-11-en-3α-ol,sesquiterpenoid of Cyperus rotundus. Phytochem 1976, 15: 1265-1266.
57. Khoi NK, Cyperus L, In: de Padua, et a l. Plant resources of South-East Asia No. 12(1): Medicinal and poisonous plants 1. Backhuys Publisher, The Netherland 1999: 222-229.
58. Kilani S, Abdelwahed A, Chraief I, et al. Chemical composition, antibacterial and antimutagenic activities of essential oil from (Tunisian) Cyperus rotundus. J Essent Oil Res 2005, 17: 695-700.
59. Sahu S, Singh J, Kumar S. New terpenoid from the rhizomes of Cyperus Scariosus. Int J Chem Eng Appl 2010, 1: 25.
60. https://pubchem.ncbi.nlm.nih.gov/bioassay/1098456#. accesed on: 26th July 2018.
61. Seo YJ, Jeong M, Lee KT, et al. Isocyperol, isolated from the rhizomes of Cyperus rotundus, inhibits LPS-induced inflammatory responses via suppression of the NF-κB and STAT3 pathways and ROS stress in LPS-stimulated RAW 264.7 cells. Int J Immunopharmacol 2016, 38: 61-69.
62. Hiking H, Aota K, Kuwano D, et al. Structure and absolute configuration of α-rotunol and β-rotunol,sesquiterpenoids of Cyperus rotundus. Tetrahedron 1971, 27: 4831-4836.
63. Ross IA. Cyperus rotundus. In Medicinal Plants of the World. Humana Press, Totowa NJ. 2003,209-226.
64. Kittayaruksakul S, Zhao W, Xu M, et a l.Identification of three novel natural product compounds that activate PXR and CAR and inhibit inflammation. Pharm res 2013, 30: 2199-2208.
65. Jyothi B, Sudarsanam G, Sitaram B. Pharmacognosy of a local market sample of Parpataka Mollugo cerviana (L.) Ser Phcog J 2010, 2: 233-239.
66. Pavithra PS, Janani VS, Charumathi KH, et al.Antibacterial activity of plants used in Indian herbal medicine. Int J Green Pharm 2010, 4: 22-28.
67. Sadique J, Chandra T, Thenmozhi V, et al. The anti-inflammatory activity of Enicostemma littorale and Mollugo cerviana. Biochem Med Metabc Biol 1987, 37: 167-176.
68. An F, Wang S, Tian Q, et al. Effects of orientin and vitexin from Trollius chinensis on the growth and apoptosis of esophageal cancer EC-109 cells. Oncol Lett 2015, 10: 2627-2633.
69. Xiao Q, Piao R, Wang H, et al. Orientin-mediated Nrf2/HO-1 signal alleviates H2O2-induced oxidative damage via induction of JNK and PI3K/AKT activation. Int J Biol Macromol 2018, Online.
70. Law BN, Ling AP, Koh RY, et al. Neuroprotective effects of orientin on hydrogen peroxide-induced apoptosis in SH-SY5Y cells. Mol Med Rep 2014, 9:947-954.
71. https://pubchem.ncbi.nlm.nih.gov/bioassay/366284#sid=103582302 accessed on 26th July 2018.
72. Gazola AC, Costa GM, Zucolotto SM, et al. The sedative activity of flavonoids from Passiflora quadrangularis is mediated through the GABAergic pathway. Biomed Pharmacother 2018, 100: 388-393.
73. Tao Y, Cai H, Li W, et a l. Ultrafiltration coupled with high-performance liquid chromatography and quadrupole-time-of-flight mass spectrometry for screening lipase binders from different extracts of Dendrobium officinale. Anal Bioanal Chem 2015,407: 6081-6093.
74. Indian Council of Medical Research. Quality Standards of Indian Medicinal Plants, Vol. 8, ICMR,New Delhi 2010, 255-263, 348.
75. Government of India, Ministry of Health and Family Welfare, Department of Indian Systems of Medicine and Homeopathy. The Ayurvedic Pharmacopoeia of India. Part I, vol. III 1st edn. New Delhi, 2001,115-117.
76. Sharma PV. Susruta-Samhita (With englishtranslation of text and Dalhana’s commentary along with critical notes), vol. I.Chaukhambha Visvabharathi, Oriental Publishers and Distributors 1999, 331-335, 358-363.
77. Bang JS, Oh da H, Choi HM, et al.Anti-inflammatory and antiarthritic effects of piperine in human interleukin1 beta-stimulated fibroblast-like synoviocytes and in rat arthritis models. Arthritis Res Ther 2009, 11: R49.
78. Rodgers G, Doucette CD, Spurrell DR, et al.Immunomodulatory effects of piperine on dendritic cell function 2009: 50-36.
79. Meriga B, Parim B, Chunduri VR, et al. Antiobesity potential of Piperonal: promising modulation of body composition, lipid profiles and obesogenic marker expression in HFD-induced obese rats. Nutr Metab (Lond) 2017, 14: 72.
80. https://pubchem.ncbi.nlm.nih.gov/bioassay/453021#sid=103571769 accessed on 26th July 2018.
81. https://pubchem.ncbi.nlm.nih.gov/bioassay/453021#sid=103572066 accessed on 26th July 2018
82. Jaramillo Colorado BE, Martelo IP, Duarte E.Antioxidant and repellent activities of the essential oil from Colombian Triphasia trifolia (Burm. f.) P.Wilson. J Agric Food Chem 2012, 60: 6364-6368.
83. Sun J. D-Limonene: safety and clinical applications.Alter Med Rev 2007, 12: 259.
84. https://pubchem.ncbi.nlm.nih.gov/compound/528151 5#section=Pharmacology-andBiochemistry accessed on 26th July 2018.
85. Kim DS, Lee HJ, Jeon YD, et al. Alpha-pinene exhibits anti-inflammatory activity through the suppression of MAPKs and the NF-κB pathway in mouse peritoneal macrophages. Am J Chin Med 2015, 43: 731-742.
86. https://pubchem.ncbi.nlm.nih.gov/bioassay/1111942#sid=194155058 accessed on 26th July 2018.
87. Zeng LB, Zhang ZR, Luo ZH, et al. Antioxidant activity and chemical constituents of essential oil and extracts of Rhizoma homalomenae. Food Chem 2011, 125: 456-463.
88. https://pubchem.ncbi.nlm.nih.gov/compound/460#se ction=Drug-and-Medication-Information accessed on 26th July 2018.
89. Abdulazeez MA, Sani I, James BD, et al. Black Pepper (Piper nigrum L.) Oils. Essential Oils Food Preservation, Flavor and Safety 2016, 277-285.
90. Tripathi AK, Prajapati V, Aggarwal KK, et al.Toxicity, feeding deterrence, and effect of activity of 1, 8-cineole from Artemisia annua on progeny production of Tribolium castanaeum (Coleoptera:Tenebrionidae). J Econ Entomol 2001, 94: 979-983.
91. Silva M, Ribeiro FP, Medeiros MA, et al. The vasorelaxant effect of p-Cymene in rat aorta involves potassium channels. Sci World J 2015,2015: 458080.
92. https://pubchem.ncbi.nlm.nih.gov/bioassay/684430#sid=103485676 accessed on 26th July 2018.
93. https://pubchem.ncbi.nlm.nih.gov/bioassay/467874#sid=103572022 accessed on 26th July 2018.
94. Nakatani N, Inatani R, Ohta H, et al. Chemical constituents of peppers (Piper spp.) and application to food preservation: naturally occurring antioxidative compounds. Environ Health Perspect 1986, 67: 135.
95. Reddy SV, Srinivas PV, Praveen B, et al.Antibacterial constituents from the berries of Piper nigrum. Phytomedicine 2004, 11: 697-700.
96. Siddiqui BS, Gulzar T, Mahmood A, et al. New insecticidal amides from petroleum ether extract of dried Piper nigrum L. whole fruits. Chem Pharm Bull 2004, 52: 1349-1352.
97. National Center for Biotechnology Information.PubChemBioAssay Database; AID=453016,https://pubchem.ncbi.nlm.nih.gov/bioassay/453016(accessed July 25, 2018).
98. Hwang KS, Kim YK, Park KW, et al. Piperolein B and piperchabamide D isolated from black pepper(Piper nigrum L.) as larvicidal compounds against the diamondback moth (Plutella xylostella). Pest Manag Sci 2017, 73: 1564-1567.
99. Mourad AA, Nakamura S, Ueno T, et a l.Adipogenetic effects of retrofractamide A derivatives in 3T3-L1 cells. Bioorg Med Chem Lett 2013, 23: 4813-4816.
100. Rho MC, Lee SW, Park HR, et al. ACAT inhibition of alkamides identified in the fruits of Piper nigrum.Phytochemistry 2007, 68: 899-903.
101. Standards of Indian Medicinal Plants, Vol 6, ICMR,New Delhi 2008, 242.
102. Sharma PV. Clinical Uses of Medicinal Plants, 1st edn. Varanasi: Chaukhambha Visvabharati (Orient Publishers and Distributors) 1996, 138-139.
103. Kim TH, Ito H, Hatano T, et al. New antitumor sesquiterpenoids from Santalum album of Indian origin. Tetrahedron 2006, 62: 6981-6989.
104. Kim TH, Hatano T, Okamoto K, et al. Antifungal and Ichthyotoxic Sesquiterpenoids from Santalum album Heartwood. Molecules 2017, 22: 1139.
105. Ochi T, Shibata H, Higuti T. Anti-Helicobacter p ylori compounds from Santalum album. J Natu Prod 2005, 68: 819-824.
106. Datta SK. Fatty acid composition in developing seeds of Trichosanthes cucumerina L. Biological Memoirs 1987, 13: 69-72.
107. Jiratchariyakul W, Frahm AW. Cucurbitacin B and dihydrocucurbilacin B from Trichosanthes cucumerina. J Pharm Sci 1992, 19: 12.
108. http://keralaplants.in/keralaplantsdetails.aspx?id=Tri chosanthes_cucumerina (accessed July 25, 2018).
109. Que J, Ye M, Zhang Y, et a l. Bryonolic acid, a triterpenoid, protect against N-methyl-d-aspartate-induced neurotoxicity in PC12 cells. Molecules 2016, 21: 418.
110. Gatbonton-Schwager TN, Letterio JJ, Tochtrop GP.Bryonolic acid transcriptional control of anti-inflammatory and antioxidant genes in macrophages in vitro and in vivo. J Natu Prod 2012,75: 591-598.
111. Piao XM, Gao F, Zhu JX, et al. Cucurbitacin B inhibits tumor angiogenesis by triggering the mitochondrial signaling pathway in endothelial cells.Inter J Molecular Med 2018, 42: 1018-1025.
112. Attard E, Martinoli MG. Cucurbitacin E, an experimental lead triterpenoid with anticancer,immunomodulatory and novel effects against degenerative diseases. A mini-review. Curr Top Med Chem 2015, 15: 1708-1713.
113. Bean MF, Antoun M, Abramson D, et al.Cucurbitacin B and isocucurbitacin B: cytotoxic components of Helicteres isora. J Natu Prod 1985,48: 500.
114. Mahaddalkar T, Suri C, Naik PK, et al. Biochemical characterization and molecular dynamic simulation of β-sitosterol as a tubulin-binding anticancer agent.Eur J Pharmacol 2015, 760: 154-162.
115. Zhao J, Zhang CY, Xu DM, et a l. The antiatherogenic effects of components isolated from pollen typhae. Thromb Res 1990, 57: 957-966.
116. Aminu R, Umar IA, Rahman MA, et al. Stigmasterol retards the proliferation and pathological features of Trypanosoma congolense infection in rats and inhibits trypanosomal sialidase in vitro and in silico.Biomed Pharmacother 2017, 89: 482-489.
117. Park CS, Lim H, Han KJ, et al. Inhibition of nitric oxide generation by 23, 24-dihydrocucurbitacin D in mouse peritoneal macrophages. J Pharmacol Exp Ther 2004, 309: 705-710.
118. Yamahara J, Huang Q, Li Y, et al. Gastrointestinal motility enhancing effect of ginger and its active constituents. Chem Pharm Bull 1990, 38: 430-431.
119. Ali BH, Blunden G, Tanira MO, et al. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): a review of recent research. Food Chem Toxicol 2008,46: 409-420.
120. Kumar G, Karthik L, Rao KB. A review on pharmacological and phytochemical properties of Zingiber officinale Roscoe (Zingiberaceae). J Pharm Res 2011, 2963-2966.
121. Li F, Nitteranon V, Tang X, et al. In vitro antioxidant and anti-inflammatory activities of 1-dehydro-[6]-gingerdione, 6-shogaol,6-dehydroshogaol and hexahydrocurcumin. Food Chem 2012, 135: 332-337.
122. Zhu Y, Warin RF, Soroka DN, et al. Metabolites of ginger component [6]-shogaol remain bioactive in cancer cells and have low toxicity in normal cells:chemical synthesis and biological evaluation. PLoS one 2013, 8: 54677.
123. Bak MJ, Ok S, Jun M, et al. 6-shogaol-rich extract from ginger up-regulates the antioxidant defense systems in cells and mice. Molecules 2012, 17:8037-8055.
124. Weng CJ, Wu CF, Huang HW, et al. Anti‐invasion effects of 6‐shogaol and 6‐gingerol, two active components in ginger, on human hepatocarcinoma cells. Mol Nutr Food Res 2010, 54: 1618-1627.
125. Ezebuo FC, Lukong CB, Uzochukwu IC, et a l. In silico investigations revealed four potential colon cancer drugs from phytochemicals in Zingiber officinale. Int J Phytomed 2016, 8: 435-443.
126. Utegenova GA, Pallister KB, Kushnarenko SV, et al.Chemical composition and antibacterial activity of essential oils from Ferula L.. Molecules 2018, 23: pii:E1679.
127. Aras A, Iqbal MJ, Naqvi SK, et al. Anticancer activity of essential oils: targeting of protein networks in cancer cells. Asian Pac J Cancer Prev 2014, 15: 8047-8050.
128. El-Baroty GS, Abd HH, El-Bakyl RS, et al. Sale Characterization of antioxidant and antimicrobial compounds of cinnamon and ginger essential oils.Afr J Biochem Res 2010, 4: 167-174.
129. Yeo SK, Ali AY, Hayward OA, et al. β‐Bisabolene,a Sesquiterpene from the Essential Oil Extract of Opoponax (Commiphora guidottii), Exhibits Cytotoxicity in Breast Cancer Cell Lines. Phytother Res 2016, 30: 418-425.
130. Endo K, Kanno E, Oshima Y. Structures of antifungal diarylheptenones, gingerenones A, B, C and isogingerenone B, isolated from the rhizomes of Zingiber officinale. Phytochemistry 1990, 29:797-799.
131. National Center for Biotechnology Information.Pubchem Compound Database;CID=11369949,https://pubchem.ncbi.nlm.nih.gov/co mpound/11369949 (accessed July 24, 2018).
132. Yoshikawa M, Yamaguchi S, Kunimi K, et al.Stomachic principles in ginger. III. An anti-ulcer principle, 6-gingesulfonic acid, and three monoacyldigalactosylglycerols, gingerglycolipids A,B, and C, from Zingiberis Rhizoma originating in Taiwan. Chem Pharm Bull 1994, 42: 1226-1230.
133. National Center for Biotechnology Information.Pubchem Compound Database;CID=94378,https://pubchem.ncbi.nlm.nih.gov/comp ound/94378 (accessed July 24, 2018).
134. Rioja A, Pizzey AR, Marson CM, et al. Preferential induction of apoptosis of leukaemic cells by farnesol.FEBS Letters 2000, 467: 291-295.
135. Kim KY. Anti-inflammatory and ECM gene expression modulations of β-eudesmol via NF-κB signaling pathway in normal human dermal fibroblasts. Biomed Dermatol 2018, 2: 3.
136. https://pubchem.ncbi.nlm.nih.gov/bioassay/735306#sid=103194231 (accessed July 24, 2018).
137. https://pubchem.ncbi.nlm.nih.gov/bioassay/1111786#sid=194157758 (accessed July 24, 2018).
138. Silva AC, Lopes PM, Azevedo MM, et al. Biological activities of a-pinene and β-pinene enantiomers.Molecules 2012, 17: 6305-6316.
139. https://pubchem.ncbi.nlm.nih.gov/bioassay/332912#sid=103581593 (accessed July 24, 2018).
140. Elson CE, Maltzman TH, Boston JL, et al.Anti-carcinogenic activity of d-limonene during the initiation and promotion/progression stages of DMBA-induced rat mammary carcinogenesis.Carcinogenesis 1988, 9: 331-332.
141. Lorenzetti BB, Souza GE, Sarti SJ, et al. Myrcene mimics the peripheral analgesic activity of lemongrass tea. J Ethnopharmacol 1991, 34: 43-48.
142. https://www.webmd.com/a-to-z-guides/Dengue-feve r-reference#2 (accessed on 19/07/2018).
143. https://www.webmd.com/a-to-z-guides/what-is-chik ungunya#1-2 (accessed on 20/07/2018).
144. https://www.ayurtimes.com/nilavembu-kudineer-nila vembu-kashayam/ (accessed on 7/06/2018).
145. Liu J, Wang ZT, Ji LL. In vivo and in vitro anti-inflammatory activities of neoandrographolide.Am J Chin Med 2007, 35: 317-328.
146. Sharma BK, Klinzing DC, Ramos JD. Zingiber officinale Roscoe Aqueous extract modulates matrix metallo proteinases and tissue inhibitors of metalloproteinases expressions in Dengue virus infected Cells: Implications for prevention of vascular permeability. Trop J Pharm Res 2015, 14:1371-1381.
147. Kumar SS, Kumar BR, Mohan GK.Hepatoprotective effect of Trichosanthes cucumerina Var cucumerina L. on carbon tetrachloride induced liver damage in rats. J Ethnopharmacol 2009, 123:347-350.
 Traditional Medicine Research2018年5期
Traditional Medicine Research2018年5期
- Traditional Medicine Research的其它文章
- Is Thunder-Fire moxibustion effective in the treatment of adenomyosis combined with infertility?
- Can hip abduction reducetherisk of femoral head necrosisdeteriorated into osteoarthritis?A 3D finiteelement analysis
- Clinical efficacy of combined skin acupuncture and external washing therapy of Zhuang nationality medicine in the treatment of chronic eczema
- Evaluating theclinical efficacy of Thunder-Firemoxibustion combined with ovulation monitoring in thetreatment of adenomyosiscombined with infertility
- Rat model for study of the essence of spleen deficiency and dampness in Chinese medicine
- Theconcept of vaccinesfirst came from Chinesemedicine
