运动对棕色脂肪功能的影响及作用机制
付鹏宇,龚丽景,胡 扬
运动对棕色脂肪功能的影响及作用机制
付鹏宇1,龚丽景2,胡 扬2
1.北京体育大学 运动人体科学学院, 北京 100084; 2. 北京体育大学 中国运动与健康研究院, 北京 100084
研究综述了运动对棕色脂肪组织(brown adipose tissue,BAT)功能的影响及其发挥作用的可能通路机制。随着肥胖发病率的日益增加,由此引发的多种慢性疾病正严重威胁着人类的健康。近年来,BAT以其耗能产热能力而备受关注。随着研究的深入,发现活化的BAT具有对抗肥胖所致慢性炎症状态和促进糖脂代谢的功能,以上特性都使BAT成为对抗肥胖及代谢相关疾病的新靶点。运动作为减脂降重和预防慢病的有效手段,其发挥促进健康作用的机制可能与激活BAT有关。具体作用机制如下:1)运动发挥促进BAT产热功能可能与VEGF信号通路、PI3K-Akt信号通路、PPAR信号通路有关;2)发挥抗炎作用可能与ErbB信号通路、Jak-STAT信号通路、TGF-β信号通路、胰岛素信号通路有关;3)发挥促进糖脂代谢的作用可能与PPAR信号通路、AMPK信号通路、胰岛素信号通路有关。综上,运动可通过调控多条信号通路而发挥促进BAT产热、提高抗炎特性及调控糖脂代谢变化的作用。
运动;棕色脂肪;产热;抗炎;糖脂代谢
1 前言
棕色脂肪组织(brown adipose tissue,BAT)可通过非颤抖性产热调节体温,其多室的棕色脂肪细胞以含有大量线粒体和高表达解耦联蛋白1(uncoupling protein 1,UCP-1)等特异性基因为特征,在调节全身能量平衡中发挥关键作用,有助于控制肥胖及其相关疾病的发展[17,19,32]。BAT在调控代谢中也发挥着重要作用,表现在BAT氧化葡萄糖和脂质,进而调节血糖平衡并降低血脂;BAT还有抑制巨噬细胞炎性特征的能力,可抵抗肥胖所致慢性炎症状态[17]。此外,BAT调控多囊卵巢综合征等疾病的研究也逐渐被报道[6,17,24,44],提示需更加全面地审视BAT的功能。有研究证实,成人体内存在有活性BAT[40,43],且BAT的量和活性会受多种生理状态(如性别[11,21]、激素水平[22,42]、肥胖易感性[1,28]、饮食状况[35])和外界刺激(如寒冷[46]、低氧环境[3,7])的影响[15,30]。运动作为促进能量代谢的有效手段,被证明有激活BAT产热功能的作用[23,45],但有关运动对BAT其他功能的研究还少有报道。而对运动调控BAT功能的探索多集中于白色脂肪棕色化的通路研究,缺少运动直接调控BAT功能的基因和通路研究。综上所述,本研究将对运动调节BAT功能及其发挥作用的可能机制进行阐述。
2 运动对BAT功能的影响
2.1 运动促进BAT产热
BAT产热功能通过其线粒体内膜上的氧化和ATP生成过程解耦联实现[20]。线粒体发生和功能相关基因表达的增加以及募集能力的增强会提高BAT产热能力,如环氧化酶-2(cyclooxygenase-2,COX-2)过表达可通过COX-2-前列腺素(prostaglandin,PG)途径增加UCP-1含量和BAT产热,提高基础代谢率[48]。长期有氧运动可显著增加BAT质量、血流量和氧耗速率,增强线粒体生物发生、活性和呼吸功能[37,52];还可上调诸如UCP-1等BAT特异性基因的表达,增加与线粒体功能相关的2型脱碘酶(type 2 deiodinase,DIO2)、细胞色素氧化酶(cytochrome oxidase)等酶的活性[27]。
2.2 运动促进BAT的抗炎功能
4周的中等强度跑台训练干预后可以下调BAT中巨噬细胞表达基因1(macrophage expressed gene 1,Mpeg1)、单核细胞向巨噬细胞转化基因(monocyte to macrophage differentiation-associated,Mmd)、趋化因子配体28 [chemokine (C-C motif) ligand 28,Ccl28]、白介素-6受体α(interleukin 6 receptor alpha,Il6ra)、肿瘤坏死因子受体超家族成员1b(tumor necrosis factor receptor superfamily member 1b,Tnfrsf1b)和瘦素受体(leptin receptor,Lepr)等由肥胖所上调的炎症反应和促炎相关基因,并通过调节Janus激酶/信号转导和转录激活子(Janus kinases signal transducer and activator of transcription, Jak-STAT)、ErbB、转化生长因子-β(transforming growth factor-beta,TGF-β)和胰岛素(Insulin)信号通路而促进BAT发挥抵抗肥胖所致的慢性炎症状态的作用[5]。
运动调控BAT抗炎功能的可能机制在于:1)脂肪酸(fatty acid,FA)释放可刺激巨噬细胞的浸润[29],而运动可以提高BAT的β氧化能力,使FA在局部的释放速率减慢[54];2)运动所诱导的去甲肾上腺素等激素可在重塑BAT免疫细胞(B细胞)的表型中发挥重要作用[38];3)运动可以抑制BAT中促炎因子的表达[51];4)长期适宜强度的运动可以促进BAT中氧化系统和抗氧化系统平衡,改善炎症状态[10]。
2.3 运动促进BAT的糖脂代谢
运动刺激交感神经,诱发成熟的BAT细胞释放去甲肾上腺素,后者与β-肾上腺素能受体结合后,激活G-蛋白激活腺苷酸环化酶(adenylyl cyclase,AC),促进环磷酸腺苷(cyclic Adenosine monophosphate,cAMP)、蛋白激酶A(protein kinase A,PKA)和p38分裂原激活的蛋白激酶(mitogen activated protein kinases,MAPK)对脂多糖刺激酶(激素敏感脂肪酶、脂肪三酰基甘油脂肪酶和单酰基甘油脂肪酶)的激活作用;同时,运动引起的去甲肾上腺素水平上调可促进BAT细胞对葡萄糖的摄取,增加BAT的糖代谢能力。抗阻训练可激活PI3K/Akt/mTOR/PGC-1α/UCP-1信号通路,增加BAT中Glut4 mRNA和蛋白表达,促进BAT对葡萄糖的摄取,降低血糖,从而改善高脂膳食所致的机体糖耐量和胰岛素耐量降低,下调胰岛素抵抗大鼠的Lee’s指数和体重[2]。脂质组学研究发现,自由转轮运动可以显著下调BAT中甘油三酯(triglyceride,TG)的总丰度及磷脂代谢和脂肪酸生物合成相关基因的表达[33]。
3 运动干预调控BAT功能的可能机制
运动激活BAT可能由以下过程介导:1)BAT受交感神经系统的支配,运动可通过提高交感神经兴奋性而刺激BAT功能的增强[55];2)由于BAT与肌细胞同源,而运动可促进肌源性细胞因子——鸢尾素(Irisin)[18]及β氨基异丁酸(beta aminoisobutyric acid,BAIBA)[41]表达,且可通过调节Irisin(运动-PGC-1α-FNDC5- PPARα轴)和Sirt1信号途径[39]激活BAT;3)运动可通过对其他组织产生适应性改变而提高BAT活性,如运动可促进下丘脑脑源性的神经营养因子表达以增强线粒体活性和促进血管生成[8]。长期运动可增加小鼠肩胛间BAT前体细胞募集和增加BAT相对数量和活性[25,50]。综上研究,运动可通过激活BAT在促进产热、抑制肥胖所致的慢性炎症状态和增加糖脂代谢方面发挥着重要作用。本实验室前期通过mRNA表达谱芯片研究发现,运动可通过以下信号通路调控BAT的不同功能(表1)。
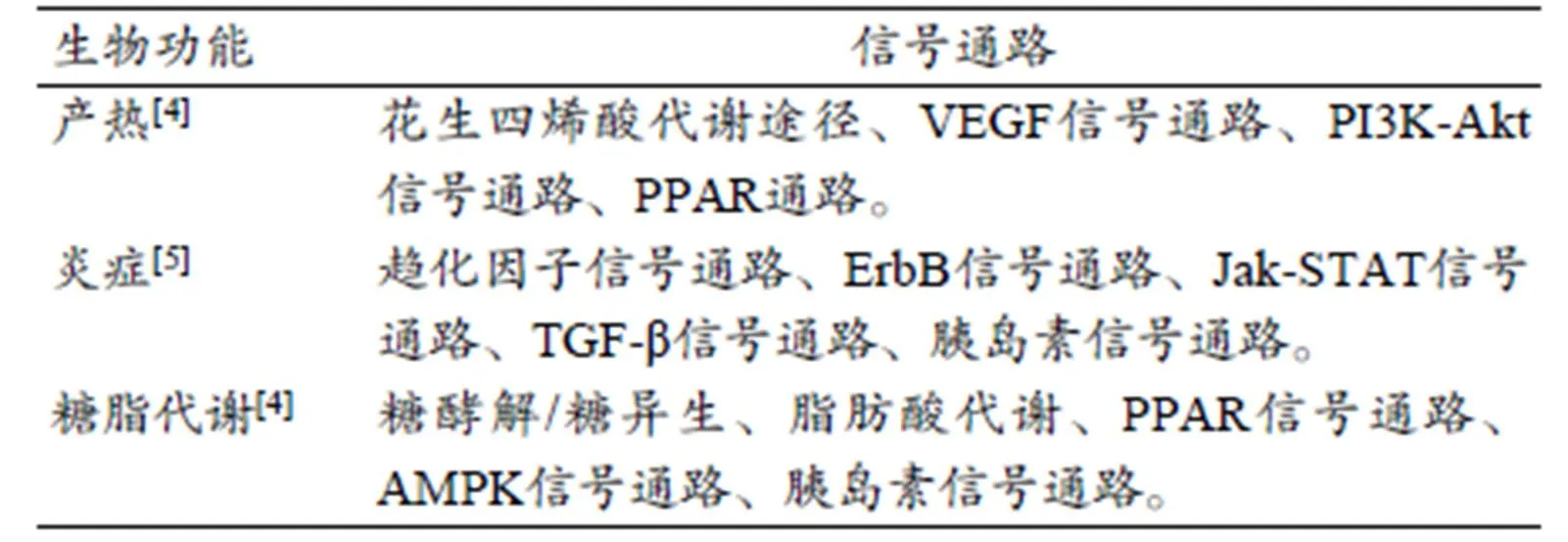
表1 运动调控BAT功能的信号通路
Table 1 Signaling Pathways for BAT Functions Regulating by Exercise
3.1 运动调控BAT产热功能相关通路
血管内皮生长因子B(vascular endothelial cell growth factor B,VEGF-B)大量表达于BAT,可通过调节脂肪酸转运蛋白(fatty acid transport proteins,FATPs)在血管内皮细胞表达,增加FA的摄取和代谢[31]。其下游的前列腺素E合酶(prostaglandin E synthase,ptges)和前列腺素I2合酶/环前列腺素(prostaglandin I2 synthase,ptgis)均为PG家族成员,可抑制白色脂肪细胞分化,增加BAT细胞氧耗。运动激活VEGF/COX-2/PG信号通路可提高BAT活性,而其下游的PG(I2)/Ptgir(prostaglandin receptor)/PPARγ信号通路可促进间充质干细胞(mesenchymal stem cell,MSC)向BAT细胞分化,有助于增加机体产热和能耗[13]。此外,运动还可通过激活VEGF通路下游的PI3K/Akt通路而激活哺乳动物雷帕霉素靶蛋白(mammalian target of rapamycin,mTOR),进而通过PPAR通路提高UCP-1的表达。有氧运动可通过上调上述这些通路而增强BAT的产热能力[4](图1)。
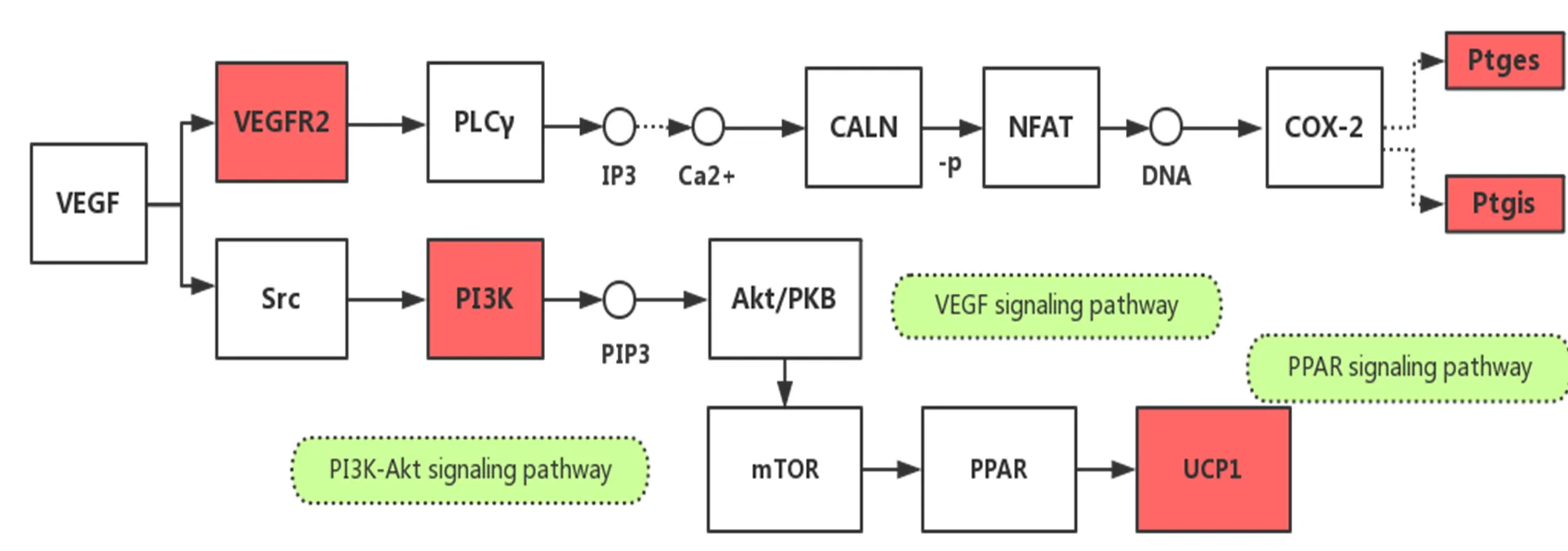
图1 运动通过VEGF、PPAR和PI3K-Akt信号通路调控BAT产热功能
Figure 1. Exercise Regulates Thermogenesis of BAT by VEGF, PPAR and PI3K-Akt Signaling Pathways
注:图中红色部分表示上调表达基因,实线箭头表示直接调控作用,虚线箭头表示间接调控作用,-p表示去磷酸化,下同。
3.2 运动调控BAT炎症相关通路
神经调节因子-1(Neuregulin1,NRG1)通过与表皮生长因子受体ErbB3/4结合,介导ErbB2受体活化,进一步激活细胞外调节蛋白激酶1/2(extracellular regulated protein kinases,ERK1/2)和/或PI3K(Akt)通路促进血管新生[26],以使促炎因子大量转运至循环系统,加剧机体代谢性病变的进程。促炎因子IL-6与其受体结合后,激活JAK激酶,通过JAK-STAT通路参与机体的炎症反应[47],细胞因子信号转导抑制因子-3(suppressor of cytokine signaling 3,SOCS-3)是JAK-STAT下游的基因,SOCS-3过表达可参与瘦素抵抗的发生。转化生长因子-β(transforming growth factor-β,TGF-β)在炎症介质(IL、NF-κB)及活性氧(reactive oxygen species,ROS)作用下被激活上调,发挥调控炎症细胞、参与免疫反应的作用[12]。Smad在TGF-β/Smad通路中作为负反馈调节信号[9],可单独发挥抑制巨噬细胞标记基因的作用。饱和FA作为一种炎症介质,可激活脂肪组织巨噬细胞中的NF-κB,提高炎症和胰岛素抵抗水平,破坏Insulin信号通路[36]。运动可通过上调BAT中Insulin信号通路和下调Jak-STAT、ErbB、TGF-β信号通路而发挥抗炎作用(图2)。
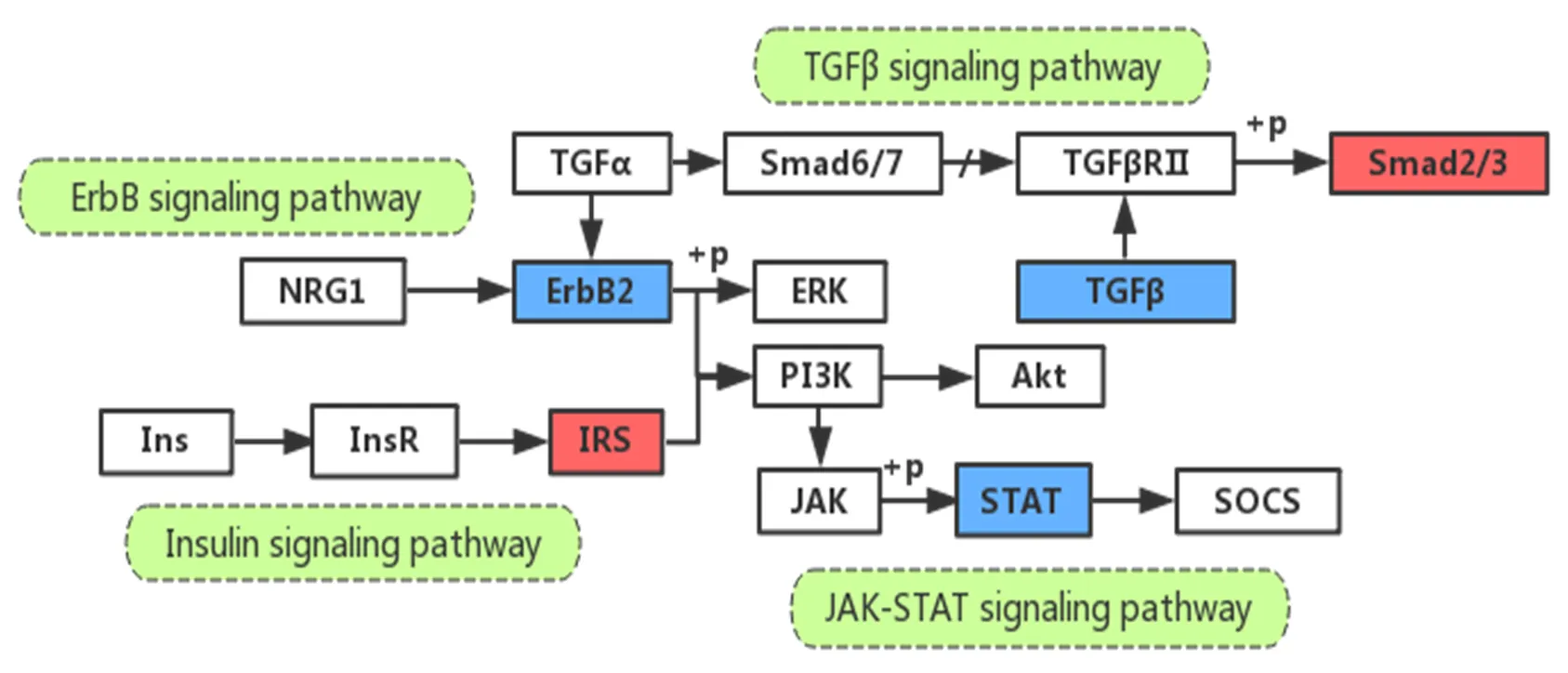
图2 运动通过ErbB、Jak-STAT、TGF-β和Insulin信号通路调控BAT抗炎功能
Figure 2. Exercise Regulates Anti-inflammatory Function of BAT by ErbB, Jak-STAT, TGF-β and INSULIN Signaling Pathways
注:图中蓝色部分表示下调表达基因;+p表示磷酸化;/表示抑制作用,下同。
3.3 运动调控BAT糖脂代谢功能相关通路
PPARγ对BAT功能的影响表现为调节其细胞分化成熟和产热,促进脂质分解,增加BAT的产热原料[14],而运动可促进脂肪酸结合蛋白5(fatty acid binding protein 5,Fabp5)-PPARβ/γ信号通路上调,促进脂代谢,维持能量平衡[16,53]。AMPK可促进BAT对FA的摄取和氧化,调节脂解作用并增加产热,抑制AMPK表达会阻止BAT细胞正常分化[34],运动可通过调控AMPK-Acaca/Fans途径促进BAT合成,Insulin通路在BAT维持葡萄糖稳态方面发挥着重要的作用,运动通过激活BAT中Insulin通路可显著提高葡萄糖清除能力和胰岛素敏感性[49],而使BAT更高效地获取葡萄糖用于产热。运动可通过上调PPAR、AMPK和Insulin信号通路提高BAT中糖脂代谢相关基因长链酰基辅酶A合成酶家族成员5(acyl-CoA synthetase long-chain family member 5,Acsl5)、脂肪酸合成酶(fatty acid synthase,Fasn)、乙酰辅酶A羧化酶α(acetyl-Coenzyme A carboxylase alpha,Acaca)、1,6-二磷酸果糖酶1(fructose bisphosphatase 1,Fbp1)的表达,而调控糖脂代谢能力[4](图3)。
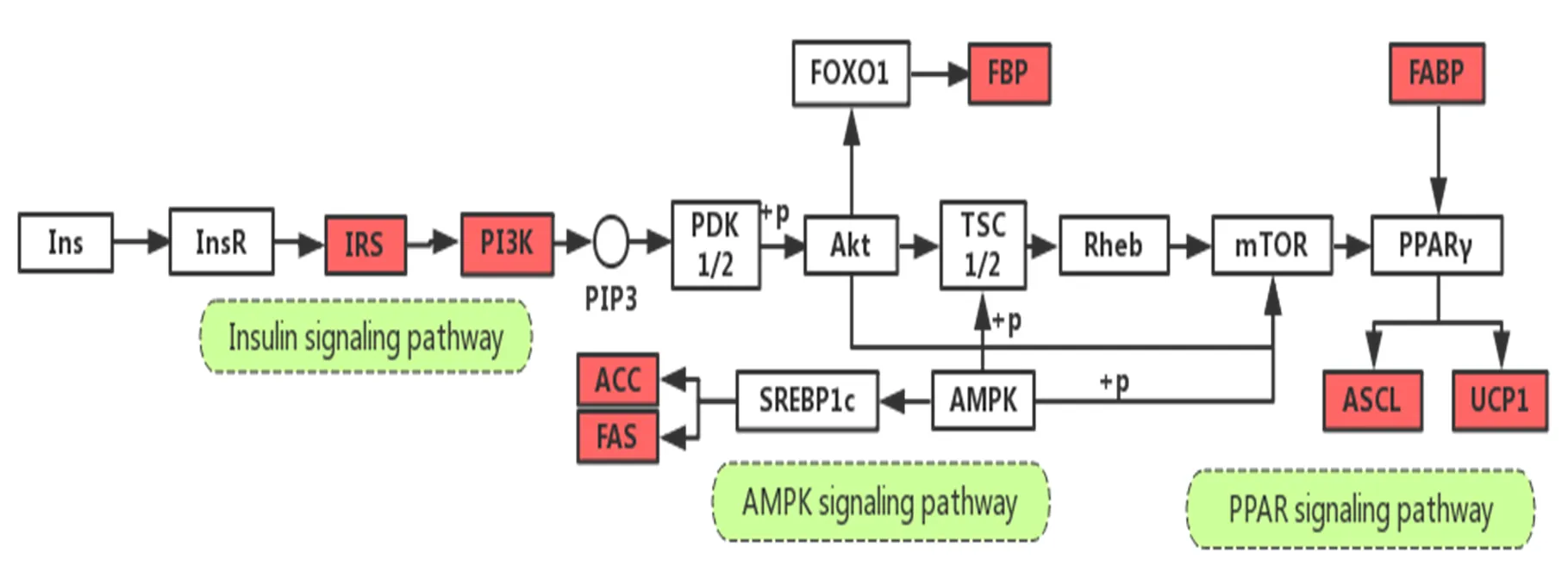
图3 运动通过PPAR、AMPK和Insulin信号通路调控BAT糖脂代谢功能
Figure 3. Exercise Regulates Glycolipid Metabolism Function of BAT by PPAR, AMPK and Insulin Signaling Pathways
4 小结与展望
研究BAT功能及运动适应机制,可为治疗肥胖及其所引发的慢性炎症状态、糖脂代谢紊乱提供新的研究靶点。但BAT的功能受多种因素的调控,不同的生理病理状态和不同的环境刺激都会对BAT产生不同的影响,需要进一步探究不同的运动形式及运动方案对BAT活性及功能的影响。
[1] 崔菊,陈爱群,庞婧,等.高脂饮食影响小鼠体内能量代谢的性别差异[J].山西医科大学学报,2017,48(7):658-664.
[2] 杜海平.抗阻力爬梯运动与MSTN抗体及联合干预调控棕色脂肪糖代谢及分子机制研究[D].西安:陕西师范大学,2016.
[3] 付鹏宇,龚丽景,段佳妍,等.低氧运动对肥胖小鼠脂肪UCP-1和PGC-1α表达的影响[J].中国运动医学杂志,2015,34(11):1070-1075.
[4] 付鹏宇,龚丽景,朱镕鑫,等.有氧运动对肥胖小鼠棕色脂肪mRNA表达谱的影响[J].北京体育大学学报,2016,39(9):50-56.
[5] 付鹏宇,龚丽景,朱镕鑫,等.有氧运动调控肥胖小鼠棕色脂肪组织炎症反应相关基因表达及通路的研究[J].中国运动医学杂志,2018,37(9):764-771.
[6] 傅晓华,徐维海,裘胜春,等.棕色脂肪组织及其与多囊卵巢综合征关系的研究进展[J].浙江大学学报(医学版),2017,46(3):315-320.
[7] 龚丽景,付鹏宇,朱镕鑫,等.低氧对肥胖小鼠棕色脂肪组织相关基因表达的影响及其机制[J].中国应用生理学杂志, 2018, 34(1): 88-92,100.
[8] 黄涛,李效凯.运动与脂肪组织棕色化研究进展[J].体育科学, 2016,36(7):71-78.
[9] 刘成敏,张成仁,王秀梅.Smads介导的TGF-β信号转导通路与肿瘤关系的研究进展[J].中华肿瘤防治杂志,2010,17(8):631-634.
[10] 李娟.C57BL/6J小鼠白色脂肪与棕色脂肪组织蛋白质组学研究[D].北京:北京协和医学院,2015.
[11] 唐永祥,何婷婷,朱泽华,等.18F-FDG PET/CT棕色脂肪摄取的影像学特点、影响及干预因素[J].中国医药指南,2017,15(19):161-162.
[12] 王今越,王小虹,冯维斗.运动训练抑制了TGFβ通路并缓解了D-半乳糖诱导衰老大鼠的肌肉流失[J].体育科学,2014,34(10):72-77.
[13] 徐灵均,廖信辉,张吉翔.环氧合酶-2与肥胖[J].生命的化学,2011,31(6):912-915.
[14] 严黄,颉欣妮,徐成,等.过氧化物酶体增殖物激活受体γ与适应性产热调节[J].国际药学研究杂志,2015,42(1):1-7.
[15] 袁慧琦,侯少贞.影响棕色脂肪组织产热活性因素的研究进展[J].动物医学进展,2016,37(10):98-103.
[16] ARMSTRONG E H, GOSWAMI D, GRIFFIN P R,. Structural basis for ligand regulation of the fatty acid-binding protein 5, peroxisome proliferator-activated receptor β/δ (FABP5-PPARβ/δ) signaling pathway[J]. J Biol Chem, 2014, 289(21): 14941-14954.
[17] BABOOTA R K, SARMA S M, BOPARAI R K,. Microarray based gene expression analysis of murine brown and subcutaneous adipose tissue: Significance with human[J]. PLoS one, 2015, 10 (5): e0127701.
[18] BOSTROM P, WU J, JEDRYCHOWSKI M P,. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis[J].Nature, 2012, 481(7382): 463-468.
[19] BUTTERFIELD G E, GATES J FLEMING S,. Increased energy intake minimizes weight loss in men at high altitude[J]. J Appl Physiol, 1992, 72(5): 1741-1748.
[20] CANNON B, NEDERGAARD J. Brown adipose tissue: function and physiological significance[J]. Physiol Rev, 2004, 84(1): 277-359.
[21] CHOI D K, MUKHERJEE R, YUN J W. Gender-dependent gene expressions in brown adipose tissue of lean and obese rats fed a high fat diet[J]. Biotechnol Bioproc E, 2012, 17(5):1080-1092.
[22] CYPESS A M, LEHMAN S, WILLIAMS G,. Identification and importance of brown adipose tissue in adult humans[J]. N Engl J Med, 2009, 360(15): 1509-1517.
[23] DE MATTEIS R, LUCERTINI F, GUESCINI M,. Exercise as a new physiological stimulus for brown adipose tissue activity[J]. Nutr Metab Cardiovas, 2013, 23(6): 582-590.
[24] DOWAL L, PARAMEAWARAN P, PHAT S,. Intrinsic properties of brown and white adipocytes have differential effects on macrophage inflammatory responses[J]. Mediat Inflamm, 2017, 2017(25): 9067049.
[25] DUNSTAN D. Diabetes: Exercise and T2DM-move muscles more often![J]. Nat Rev Endocrinol, 2011, 7(4): 189-190.
[26] FULLER S J, SIVARAJAH K, SUGDEN P H. ErbB receptors, their ligands, and the consequences of their activation and inhibition in the myocardium[J]. J Mol Cell Cardiol, 2008, 44(5): 831-854.
[27] IGNACIO D L, FORTUNATO R S, NETO R A,. Blunted response of pituitary type 1 and brown adipose tissue type 2 deiodinases to swimming training in ovariectomized rats[J]. Horm Metab Res, 2012, 44(11): 797-803.
[28] JOO J I, YUN J W. Gene expression profiling of adipose tissues in obesity susceptible and resistant rats under a high fat diet[J]. Cell Physiol Biochem, 2011, 27(3-4): 327-340.
[29] KOSTELI A, SUGARU E, HAEMMERLE G,. Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue[J]. J Clin Invest, 2010, 120(10): 3466-3479.
[30] LEHNIG A C, STANFORD K I. Exercise-induced adaptations to white and brown adipose tissue[J]. J Exp Biol, 2018, 221(Pt Suppl 1): jeb161570.
[31] LI X. VEGF-B: A thing of beauty[J]. Cell Res, 2010, 20(7): 741-744.
[32] LIPPL F J, NEUBAUER S, SCHIPFER S,. Hypobaric hypoxia causes body weight reduction in obese subjects[J]. Obesity, 2010, 18(4): 675–681.
[33] MAY F J, BAER L A, LEHNIG A C,. Lipidomic adaptations in white and brown adipose tissue in response to exercise demonstrates molecular species-specific remodeling[J]. Cell Rep, 2017, 18(6): 1558-1572.
[34] MICHELL T B, ROSS S E, BLACKBURN J T,. Functional balance training, with or without exercise sandals, for subjects with stable or unstable ankles[J]. J Athl Train, 2006, 41(4): 393-398.
[35] NAKAI Y, HASHIDA H, KADOTA K,. Up-regulation of genes related to the ubiquitin-proteasome system in the brown adipose tissue of 24-h-fasted rats[J]. Biosci Biotechnol Biochem, 2008, 72(1): 139-148.
[36] NGUYEN M T, FAVELYUKIS S, NGUYEN A K,. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways[J]. J Biol Chem, 2007, 282(48): 35279-35292.
[37] OH-ISHI S, KIZAKI T, TOSHINAI K,. Swimming training improves brown-adipose-tissue activity in young and old mice[J]. Mech Ageing Dev, 1996, 89(2): 67-78.
[38] PETERSON K R, FLAHERTY D K, HASTY A H. Obesity alters B Cell and macrophage populations in brown adipose tissue[J]. Obesity (Silver Spring), 2017, 25(11): 1881-1884.
[39] QIANG L, WANG L, KON N,. Brown remodeling of white adipose tissue by Sirt1-dependent deacetylation of PPARγ[J]. Cell, 2012, 150(3): 620-632.
[40] RASMUSSEN A T. The glandular status of brown multilocular adipose tissue [J]. Endocrinology, 1922, 6(6): 760-770.
[41] ROBERTS L D, BOSTROM P, O’SULLIVAN J F,. β-Aminoisobutyric acid induces browning of white fat and hepatic β-oxidation and is inversely correlated with cardiometabolic risk factors[J]. Cell Metab, 2014, 19(1): 96-108.
[42] RODRIGUEZ A M, MOMJO M, ROCA P,. Opposite actions of testosterone and progesterone on UCP1 mRNA expression in cultured brown adipocytes[J]. Cell Mol Life Sci, 2002, 59(10): 1714-1723.
[43] SACKS H, SYMONDS M E. Anatomical locations of human brown adipose tissue: functional relevance and implications in obesity and type 2 diabetes[J]. Diabetes, 2013, 62(6): 1783-1790.
[44] SEALE P, BJORK B, YANG W,. PRDM16 controls a brown fat/skeletal muscle switch[J]. Nature, 2008, 454(7207): 961-967.
[45] SEGAWA M, OH-ISHI S, KIZAKI T,. Effect of running training on brown adipose tissue activity in rats: a reevaluation[J]. Res Commun Mol Path, 1998, 100(1): 77-82.
[46] SHORE A M, KARAMITRI A, KEMP P,. Cold-induced changes in gene expression in brown adipose tissue, white adipose tissue and liver[J]. PLoS one, 2013, 8(7): e68933.
[47] SIVEEN K S, SIKKA S, SURANA R,. Targeting the STAT3 signaling pathway in cancer: Role of synthetic and natural inhibitors[J]. Biochim Biophys Acta, 2014, 1845(2): 136-154.
[48] VEGIOPOULOS A, MULLER-DECKER K, STRZODA D,. Cyclooxygenase-2 controls energy homeostasis in mice by de novo recruitment of brown adipocytes[J]. Science, 2010, 328 (5982): 1158-1161.
[49] WANG X, WAHL R. Responses of the insulin signaling pathways in the brown adipose tissue of rats following cold exposure[J]. PLoS one, 2014, 9(6): e99772.
[50] XU X, YING Z, CAI M,. Exercise ameliorates high-fat diet-induced metabolic and vascular dysfunction, and increases adipocyte progenitor cell population in brown adipose tissue[J]. Am J Physiol Regul Integr Comp Physiol, 2011, 300(5): R1115-1125.
[51] XU Z, XU X, ZHONG M,. Ambient particulate air pollution induces oxidative stress and alterations of mitochondria and gene expression in brown and white adipose tissues[J]. Part Fibre Toxicol, 2011, 8(1): 20.
[52] YOSHIOKA K, YOSHIDA T, WAKABAYASHI Y,. Effects of exercise training on brown adipose tissue thermogenesis in ovariectomized obese rats[J]. Endocrinol Jpn,1989,36(3):403-408.
[53] YU S, LEVI L, CASADESUS G,. Fatty acid-binding protein 5 (FABP5) regulates cognitive function both by decreasing anandamide levels and by activating the nuclear receptor peroxisome proliferator-activated receptor β/δ (PPARβ/δ) in the brain [J]. J Biol Chem, 2014, 289(18): 12748-12758.
[54] ZHOU Z, SHEN Y T, CHEN Z,. Cidea-deficient mice have lean phenotype and are resistant to obesity[J]. Nat Genet, 2003, 35(1): 49-56.
[55] ZOUHAL H, JACOB C, DELAMARCHE P,. Catecholami-nes and the effects of exercise, training and gender[J]. Sports Med, 2008, 38(5): 401-423.
The Effect of Exercise on Function of Brown Adipose Tissue and Its Mechanism
FU Peng-yu1,GONG Li-jing2,HU Yang2
1.Beijing Sport University, College of Sports Human Sciences, Beijing 100084, China; 2.Beijing Sport University, China Institute of Sport and Health Science, Beijing 100084, China.
This study elaborates the effect of exercise on the function of brown adipose tissue (BAT) and its possible pathway mechanism. The incidence of obesity is increasing and induce many chronic diseases, which is seriously threatening human health. In recent years, the thermogenesis and energy consumption characteristics of BAT has made it widely concerned. With the deepening of research, it was found that activated BAT also has the function of combating the chronic inflammatory state and promoting glycolipid metabolism caused by obesity. All of these make it a new target against obesity and metabolic related diseases. Exercise, as an effective means of reducing fat and weight and preventing chronic diseases, may play a role in promoting health by activating BAT. The specific mechanisms are as follows: Exercise promoting the thermogenesis function of BAT may be related to VEGF signaling pathway, PI3K-Akt signaling pathway and PPAR signaling pathway; The function of combating the chronic inflammatory state may be related to ErbB signaling pathway, Jak-STAT signaling pathway, TGF-beta signaling pathway and insulin signaling pathway; The function of regulating glycolipid metabolism may be related to PPAR signaling pathway, AMPK signaling pathway and insulin signaling pathway. In conclusion, exercise can promote thermogenesis of BAT, improve anti-inflammatory properties and regulate glycolipid metabolism by regulating multiple signaling pathways.
G804.7
A
1000-677X(2018)11-0092-06
10.16469/j.css.201811010
2017-11-07;
2018-10-29
中央高校基本科研业务费专项资金资助课题(2016YB037)。
付鹏宇,女,在读博士研究生,主要研究方向为运动生物化学, E-mail:1402884452@qq.com。
龚丽景,女,实验师,博士,主要研究方向为运动与脂肪代谢、低氧与胃肠道,E-mail:lijing.gong@bsu.edu.cn。

