藏药匙叶翼首草化学成分研究*
张隽荣,井宇星,赵佳文,王韦,高路,杨志,王京昆,江志勇
(1.民族药资源化学国家民委-教育部重点实验室、云南民族大学民族医药学院,昆明 650000;2.云南白药集团股份有限公司,昆明 650000)
匙叶翼首草(Pterocephalushooke)为川续断科(Dipsacaceae)翼花属Pterocephalus多年生植物。该属植物约有25种,主要分布在欧洲、亚洲及非洲热带,产自地中海区至亚洲中部和非洲热带。我国有匙叶翼首草(P.hookeri)和裂叶翼首草(P.bretschneideri)两种,主要分布在云南、四川、西藏、青海、甘肃等省区。匙叶翼首草亦为知名藏药,有报道其具有抗炎、镇静及促进骨损伤愈合等作用[1-2]。关于匙叶翼首草(P.hookeri)的化学成分研究国内外报道都较少,主要成分为三萜及其苷、环烯醚萜、黄酮等[3-5],其中主要以三萜皂苷类化合物报道为主。基于目前对匙叶翼首草(P.hookeri)的化学成分及生物活性研究报道很少,为了进一步明确该植物的化学成分,笔者对其90%乙醇提取物的正丁醇部分进行了化学成分研究,从中分离鉴定了21个化合物。通过质谱(MS),1D和2D NMR波谱数据分析,结合文献对照,将上述21个化合物分别鉴定为獐牙菜苷(sweroside,1)、木犀草素(luteolin,2)、3,4-二羟基肉桂酸甲酯[3,4- dihydroxycinnamic acid methyl ester,3]、songoroside A(oleanolic acid 3-O-β-D- xylopyranoside,4)、giganteaside D [oleanolic acid 3-O- α-L- rhamnopyranosyl(1→2)-β-D- xylopyranoside,5]、齐墩果酸-3-O-β-D-木吡喃糖基-(1→3)-α-L-鼠李吡喃糖基(1→2)-β-D-吡喃木糖苷[oleanolic acid- 3-O- β-D- xylopyranosyl-(1→3)- α-L- rhamnopyranosyl(1→2) -β -D- xylopyranoside,6]、hookeroside A [3-O- β-D- glucopyranosyl(1→4)- β-D- xylopyranosyl(l→3)-α-L- rhamnopyranosyl(1→2) -β -D-xylopyranosyl oleanolic acid 28-O-β-D- glucopyranosyl(l→6)-β-D-glucopy- ranoside,7]、 prosapogenin Ax(8)、hookeroside B [3-O-β-D- xylopyranosyl(1→4)-β-D- glucopyranosyl(l→4)- β-D- xylopyranosyl(1→3)- α-L- rhamnopyranosyl(1→2)-β-D- xylopyranosyloleanolic acid 28-O-β-D- glucopyranosyl(l→6)-β-D- glucopyranoside,9]、prosapogeninBx [oleanolic acid 3-O- β-D- xylopyranosyl(1→4)-β-D- glucopyranosyl(1→4)-β-D- xylopyranosyl(1→3) -α-L- rhamnopyranosyl(1→2)- β-D- xylopyranoside,10]、hookeroside C [3-O- α-L- rhamnopyranosyl-(1→2)-β-D- xylopyranosyl(1→4)-β-D- glucopyranosyl-(1→ 4)-β-D- xylopyranosyl(1→ 3)- α-L- rhamnopyranosyl(1→ 2)-β-D- xylopyranosyl oleanolic acid 28-O- β-D- glucopyranosyl(l→ 6)-β-D- glucopyranoside,11]、hookeroside D [oleanolic acid 3-O- α-L- rhamno- pyranosyl(1→2)-β-D- xylopyranosyl(1→ 4)-β-D- glucopyranosyl(1→ 4)-β-D-xylopyranosyl(1→ 3)- α-L- rhamnopyranosyl(1→ 2)-β-D- xylopyranoside,12]、马钱子苷(log- anin,14)、齐墩果酸(oleanolic acid,15)、β-谷甾醇(β-sitosterol,16)、sylvestroside I(17)、triplostoside A(18)、sylvestroside IV dimethyl acetal(19)、(+)-1-羟基松香醇4′-O-β-D-吡喃葡萄糖苷 [(+)-1-hydroxypinoresinol 4′-O-β-D- glucopyranoside,20]、(+)-1-羟基松香醇4″-O-β-D吡喃葡萄糖苷[(+)-1- hydroxypinoresinol 4″-O-β-D-glucopyranoside,21]。其中化合物20,21为首次从该属植物中分离得到,化合物1~3为首次从该种植物中分离得到。下一步拟开展上述化学成分的生物活性研究,为科学利用我国民族民间药用植物提供实验基础。
1 仪器与药材
1.1仪器 VG Auto Spec-3000型质谱仪;Bruker AM - 400型核磁共振仪(以TMS为内标测定);柱色谱硅胶(0.045~0.075 mm)、薄层色谱硅胶GF254(青岛美高化工有限公司);Sephadex LH-20(Pharmacia公司)。HPLC为Aglilent 1260半制备液相色谱仪(多波长紫外检测器)。
1.2药材 匙叶翼首草全株植物于2011年8月采集于云南香格里拉,经云南民族大学杨青松副教授鉴定为Pterocephalushookeri,标本保存于云南民族大学民族医药学院(TSYJ-201415)。
2 提取与分离
干燥匙叶翼首草全株植物6 kg,打磨成粉末,用8倍量90%乙醇提取9次,过滤,合并滤液减压浓缩,得到1.5 kg浸膏。将浸膏混悬于适量的水中,依次用石油醚、乙酸乙酯、正丁醇萃取,得到石油醚部分浸膏5 g、乙酸乙酯部分浸膏45 g,正丁醇部分浸膏800 g。正丁醇部分用0.075~0.150 mm硅胶1 kg拌样进行柱层析,洗脱梯度系统为三氯甲烷-甲醇-水(9:1:0→7:3:0.5),经薄层色谱(TLC)检测合并相同组分,得到5个组分,分别记为Fr.1~Fr.5。
2.1取Fr.2部分(25 g) 以甲醇-水(60:40→100:0)作为流动相,进行MCI柱层析,每100 mL一个馏分,经TLC检测后合并相同组分,分别标记为Fr.1-1、Fr.1-2、Fr.1-3、Fr.1-4四个组分,其中Fr.1-2、Fr.1-3经正相硅胶柱及Sephadex LH-20反复分离纯化得化合物2(28 mg)、3(50 mg)、16(15 mg)和化合物17(22 mg)。
2.2取Fr.3部分(40 g) 以0.045~0.075 mm硅胶60 g拌样,以三氯甲烷-甲醇-水(9:1:0→85:15:0.1→8:2:0.2)为流动相进行硅胶柱层析,每400 mL一个流分,经TLC检测合并相同组分,分别标记为Fr.3-1、Fr.3-2、Fr.3-3。其中Fr.3-2经MCI柱层析MeOH-H2O(20:80→100:0)梯度洗脱,经分离后得到3个组分,分别标记为:Fr.3-2-1(4.5 g)、Fr.3-2-2(11 g) 和Fr.3-2-3(4.5 g)。取Fr.3-2-2部分1.2 g经HPLC以45%甲醇作为流动相反复制备得到化合物1(28 mg),4(201 mg)和14(12 mg)。Fr.3-2-2采用制备HPLC(28%乙腈)反复分离得化合物5(98 mg),18(170 mg),19(54 mg),20(7 mg)。
2.3取Fr.4部分(15 g) 采用MCI柱层析甲醇-水(20:80-100:0)梯度洗脱,每100 mL一个流分,经TLC检测合并得Fr.4-1~Fr.4-4四个组分。各组分分别采用正相柱层析,结合反相制备HPLC分离得到化合物6(76 mg),7(23 mg),8(103 mg),9(55 mg),10(17 mg),11(25 mg),12(14 mg),13(9 mg),21(18 mg)和22(23 mg)。
3 结构鉴定
3.1化合物1白色无定型粉末。ESIMS(+): 358[M+Na]+;1H-NMR(CD3OD,400MHz) δ: 7.60(1H,s,H-3),5.57(1H,ddd,J=8.3,10.4,16.8Hz,H-8),5.33(1H,d,J=16.8 Hz,H-10a),5.29(1H,d,J=10.4 Hz,H-10b),5.14(1H,d,J=6.4 Hz,H-1),4.79(1H,d,J=7.8 Hz,H-1');13C-NMR(CD3OD,100MHz) δ: 97.9(C-1),153.9(C-3),106.0(C-4),28.4(C-5),25.8(C-6),69.7(C-7),133.3(C-8),43.8(C-9),120.8(C-10),168.5(C-11),99.7(C-1'),74.7(C-2'),77.8(C-3'),71.5(C-4'),78.3(C-5'),62.6(C-6')。以上波谱数据与文献[6]报道的一致,鉴定其为獐牙菜苷(sweroside)。
3.2化合物2黄色无定型粉末。ESIMS(-): 285[M-H]-;1H-NMR(C5D5N,400 MHz) δ: 13.81(1H,s,5-OH),7.91(1H,d,J=2.0 Hz,H-2'),7.55(1H,dd,J=8.0,2.0 Hz,H-6'),7.29(1H,d,J=8.4 Hz,H-5'),7.28(1H,s,H-3),6.74(1H,s,H-8),6.84(1H,s,H-6);13C-NMR(C5D5N,100 MHz) δ: 164.8(C-2),104.0(C-3),182.7(C-4),163.1(C-5),99.9(C-6),165.8(C-7),94.7(C-8),158.5(C-9),104.9(C-10),122.9(C-1'),114.6(C-2'),147.7(C-3'),151.7(C-4'),116.8(C-5'),119.5(C-6')。以上波谱数据与文献[7]报道的一致,鉴定其为木犀草素(luteolin)。
3.3化合物3白色无定型粉末。ESIMS(-): 193[M-H]-;1H- NMR(C5D5N,400MHz) δ: 7.54(1H,d,J=16.0Hz,H-8),7.03(1H,s,H-2),6.93(1H,d,J=8.4 Hz,H-6),6.77(1H,dd,J=8.4,2.4 Hz,H-5),6.26(1H,d,J=16.0 Hz,H-7),3.75(3H,s,H-10);13C-NMR(C5D5N,100 MHz) δ: 127.6(C-1),115.0(C-2),146.8(C-3),149.5(C-4),116.4(C-5),122.9(C-6),114.8(C-7),146.9(C-8),169.7(C-9),51.9(C-10)。以上波谱数据与文献[8]报道的一致,鉴定其为3,4-二羟基肉桂酸甲酯[3,4-dihydroxycinnamic acid methyl ester]。
3.4化合物4白色无定型粉末。ESIMS(+): 611[M+Na]+;1H-NMR(C5D5N,400 MHz) δ: 5.14(1H,brs,H -12),4.93(1H,d,J=7.6 Hz,xyl H-1),3.36(1H,m,H -3);13C-NMR(C5D5N,100 MHz) δ: 38.5(C-1),28.1(C-2),88.6(C-3),39.6(C-4),55.9(C-5),18.4(C-6),33.8(C-7),39.7(C-8),48.0(C-9),37.0(C-10),23.6(C-11),123.0(C-12),144.8(C-13),41.0(C-14),28.2(C-15),23.7(C-16),46.6(C-17),41.0(C-18),46.4(C-19),30.0(C-20),33.3(C-21),33.2(C-22),28.3(C-23),16.8(C-24 ),15.5(C -25),16.9(C-26),26.7(C-27),180.2(C-28),26.1(C-29),23.8(C-30)。以上波谱数据与文献[3,5]报道的一致,鉴定其为songoroside A(oleanolic acid 3-O-β-D-xylopyranoside)。
3.5化合物5白色无定型粉末。ESIMS(+): 757 [M+Na]+;1H-NMR(C5D5N,400 MHz) δ: 6.51(1H,brs,rha,H-1′),4.86(1H,d,J=7.2 Hz,xyl H-1),5.25(1H,brs,H -12),2.72(1H,dd,J=13.8,3.7 Hz,H-18),1.12,0.99,0.94,0.92,0.91,0.76,0.74(each3H,s);13C-NMR(C5D5N,100 MHz) δ: 38.5(C-1),27.1(C-2),88.0(C-3),39.1(C-4),56.9(C-5),18.4(C-6),32.8(C-7),39.2(C-8),48.0(C-9),37.3(C-10),24.0(C -11),123.0(C-12),145.0(C-13),42.2(C-14),28.3(C-15),24.1(C-16),46.6(C-17),42.5(C-18),47.0(C-19),31.0(C-20),34.3(C-21),33.5(C-22),28.0(C-23),17.3(C-24),15.5(C-25),17.2(C-26),26.7(C-27),180.0(C-28),24.1(C-29),24.0(C-30)。糖部分数据见表1。以上波谱数据与文献[3]报道的一致,鉴定其为giganteaside D[oleanolic acid 3-O-α-L-rhamnopyranosyl(1→2)-β-D-xylopyranoside]。
3.6化合物6白色无定型粉末。ESIMS(+): 889 [M+Na]+;1H-NMR(C5D5N,400MHz) δ: 6.52(1H,brs,rha H -1′),5.41(1H,d,J=7.6 Hz,xyl H-1),5.36(1H,brs,H -12),4.90(1H,d,J=7.2 Hz,xylH-1''),2.79(1H,dd,J=13.8,3.7 Hz,H-18),1.11,0.99,0.93,0.92,0.90,0.78,0.77(each3H,s);13C-NMR(C5D5N,100 MHz) δ: 38.5(C-1),26.1(C-2),88.6(C-3),39.0(C-4),55.9(C-5),18.4(C-6),33.8(C-7),39.7(C-8),48.1(C-9),37.6(C-10),23.0(C-11),122.3(C-12),144.0(C-13),42.0(C-14),28.2(C-15),23.5(C-16),47.6(C-17),42.5(C-18),47.4(C-19),31.0(C-20),34.2(C-21),33.2(C-22),28.7(C-23),17.8(C-24 ),15.5(C-25),17.5(C-26),26.0(C-27),180.1(C-28),23.1(C-29),23.3(C-30)。糖部分数据见表1。以上波谱数据与文献[3]报道的一致,鉴定其为齐墩果酸-3-O-β-D-木吡喃糖基-(1→3)-α-L-鼠李吡喃糖基(1→2)-β-D-吡喃木糖苷[oleanolic acid -3 -O-β-D-xylopyranosyl(1→ 3)- α-L- rhamnopyranosyl(1→2) -β-D-xylopyranoside]。
3.7化合物7白色无定型粉末。ESIMS(+): 1375 [M+Na]+;1H-NMR(C5D5N,400 MHz) δ: 6.14(1H,brs,rha H -1′),5.25(1H,brs,H -12),5.24(1H,d,J=7.6 Hz,xyl H -1),5.18(1H,d,J=6.8 Hz,xylH-1''),4.97(1H,dJ=7.8 Hz,glc H-1'''),4.95(2H,d,J=8.4 Hz,glc H-1',glc H-1″),2.81(1H,dd,J=13.5,3.8Hz,H-18),1.10,0.98,0.93,0.92,0.91,0.77,0.74(each3H,s);13C-NMR(C5D5N,100 MHz) δ: 38.0(C-1),26.1(C-2),88.6(C-3),39.6(C-4),56.6(C-5),18.4(C-6),33.8(C-7),39.0(C-8),48.0(C-9),37.1(C-10),23.2(C-11),122.6(C-12),144.8(C-13),42.0(C-14),28.2(C-15),23.8(C-16),46.0(C-17),42.0(C-18),47.2(C-19),31.0(C-20),34.3(C-21),33.2(C-22),28.3(C-23),17.8(C-24 ),15.4(C-25),17.0(C-26),26.4(C-27),175.8(C-28),23.1(C-29),23.7(C-30)。糖部分数据见表1。以上波谱数据与文献[3]报道的一致,鉴定其为hookeroside A[3-O- β-D- glucopyranosyl(1→4)- β-D- xylopyranosyl(l→3) -α-L- rhamnopyranosyl(1→2)- β-D- xylopyranosyloleanolic acid 28-O-β-D- glucopyranosyl(l→6) -β -D-glucopyranoside]。
3.8化合物8白色无定型粉末。ESIMS(+): 1051[M+Na]+;1H-NMR(C5D5N,400 MHz) δ: 6.22(1H,brs,rha H -1'),5.31(1H,d,J=8.0 Hz,xyl H -1),5.28(1H,d,J=7.8 Hz,xyl H-1''),5.26(1H,brs,H -12),5.01(1H,d,J=7.8 Hz,glc H -1'''),2.82(1H,dd,J=13.8,3.7 Hz,H -18),1.11,0.98,0.93,0.92,0.90,0.78,0.75(each3H,s);13C NMR(C5D5N,100 MHz) δ: 38.5(C-1),26.1(C-2),88.6(C-3),39.6(C-4),56.4(C -5),18.0(C-6),33.8(C-7),39.4(C-8),48.0(C-9),37.3(C-10),23.0(C-11),122.7(C-12),144.8(C-13),42.0(C-14),28.4(C-15),23.5(C-16),46.2(C-17),42.0(C-18),47.3(C-19),31.0(C-20),34.3(C-21),33.2(C-22),28.4(C-23),17.8(C-24 ),15.5(C-25),17.4(C-26),26.7(C-27),180.0(C-28),23.3(C-29),23.7(C-30)。糖部分数据见表1。以上波谱数据与文献[3]报道的一致,鉴定其为prosapogenin Ax。
3.9化合物9白色无定型粉末。ESIMS(+): 1507 [M+Na]+;1H-NMR(C5D5N,400 MHz) δ: 6.25(1H,brs,rha H -1'),4.81(1H,s,J=7.5 Hz,xyl H-1),5.35(1H,d,J=7.3 Hz,glc H-1'''),5.28(1H,brs,H-12),4.81(1H,d,J=7.6 Hz,xyl H-1''''),2.82(1H,dd,J=13.8,3.7 Hz,H -18),1.12,0.98,0.93,0.92,0.90,0.77,0.75(each3H,s);13C-NMR(C5D5N,100 MHz) δ: 38.5(C-1),26.2(C-2),88.4(C-3),39.6(C-4),56.9(C-5),18.2(C-6),33.8(C-7),39.5(C-8),48.8(C-9),37.1(C-10),23.4(C-11),122.4(C-12),144.4(C-13),42.1(C-14),28.2(C-15),23.4(C-16),45.6(C-17),42.2(C-18),47.0(C-19),31.4(C-20),34.0(C-21),33.5(C-22),28.3(C-23),17.7(C-24 ),15.5(C-25),17.5(C-26),26.7(C-27),176.1(C-28),23.0(C-29),23.6(C-30)。糖部分数据见表1。以上波谱数据与文献[3]报道的一致,鉴定其为hookeroside B [3-O-β-D- xylopyranosyl(l→ 4)-β-D- glucopyranosyl(l→4)-β-D- xylopyranosyl(l→3) -α -L- rhamnopyranosyl(1→2) -β-D- xylopyranosyloleanolic acid 28-O-β-D- glucopyranosyl(1→6) -β-D-glucopyranoside]。
3.10化合物10白色无定型粉末。ESIMS(+): 1183 [M+Na]+;1H-NMR(C5D5N,400 MHz) δ: 6.55(1H,brs,rha H-1'),6.27(1H,d,J=8.2 Hz,glc H-1'''),5.36(1H,d,J=7.8 Hz,xyl H-1),5.26(1H,brs,H-12),5.18(1H,d,J=7.4 Hz,xyl H-1''),5.01(1H,d,J=7.6 Hz xyl H-1''''),2.92(1H,dd,J=13.4,3.6 Hz,H -18),1.12,0.98,0.93,0.92,0.90,0.77,0.75(each3H,s);13C-NMR(C5D5N,100 MHz) δ: 38.3(C-1),26.2(C-2),88.6(C-3),39.6(C-4),56.1(C-5),18.0(C-6),33.8(C-7),39.6(C-8),48.1(C-9),37.2(C-10),23.4(C-11),122.1(C-12),144.8(C-13),42.2(C-14),28.2(C-15),23.7(C-16),46.2(C-17),42.0(C-18),47.4(C-19),31.1(C-20),34.3(C-21),33.2(C-22),28.3(C-23),17.4(C-24 ),15.0(C-25),17.7(C-26),26.5(C-27),180.3(C-28),23.5(C-29),23.8(C-30)。糖部分数据见表1。以上波谱数据与文献[3]报道的一致,鉴定其为prosapogeninBx [oleanolic acid 3-O- β-D- xylopyranosyl(1→4) -β-D- glucopyranosyl(1→4) -β -D- xylopyranosyl(1→3) -α -L- rhamnopyranosyl(1→2)- β-D- xylopyranoside]。
3.11化合物11白色无定型粉末。ESIMS(+): 1653 [M+Na]+;1H-NMR(C5D5N,400 MHz) δ: 6.58(1H,brs,rha H-1'),6.27(1H,d,J=8.2 Hz,glc H-1'''),6.26(1H,brs,rha H-1''''').5.31(1H,d,J=7.6 Hz,xyl H-1''),5.26(1H,brs,H-12),5.01(1H,d,J=8.2 Hz,xyl H-1''''),4.93(1H,d,J=8.6 Hz,glc H-1'''),2.80(1H,dd,J=13.1,3.6Hz,H-18),1.12,0.99,0.93,0.91,0.90,0.78,0.75(each3H,s);13C-NMR(C5D5N,100 MHz) δ: 38.1(C-1),26.1(C-2),88.6(C-3),39.6(C-4),56.9(C-5),18.0(C-6),33.4(C-7),39.7(C-8),48.0(C-9),37.1(C-10),23.6(C-11),122.5(C-12),144.0(C-13),42.4(C-14),28.2(C-15),23.7(C-16),46.4(C-17),42.0(C-18),47.5(C-19),31.0(C-20),34.3(C-21),33.4(C-22),28.3(C-23),17.8(C-24),15.5(C-25),17.9(C-26),26.5(C-27),175.9(C-28),23.0(C-29),23.5(C-30)。糖部分数据见表1。以上波谱数据与文献[3]报道的一致,鉴定其为hookeroside C [3-O-α-L-rhamnopyranosyl-(1→2)-β-D-xylopyranosyl(1→4)-β-D-glucopyranosyl-(1→4)-β-D-xylopyranosyl(1→3)-α-L-rhamnopyranosyl(1→2)-β-D-xylopyranosyloleanolic acid 28-O-β-D-glu- copyranosyl(l→6)-β-D-glucopyranoside]。
3.12化合物12白色无定型粉末。ESIMS(+): 1329 [M+Na]+;1H-NMR(C5D5N,400 MHz) δ: 6.55(1H,brs,rha,H-1'),6.27(1H,d,J=8.2 Hz,glc H-1'''),6.25(1H,brs,rha H-1'''''),5.31(1H,d,J=7.6 Hz,xyl H-1),5.26(1H,brs,H-12),5.18(1H,d,J=6.7 Hz,xyl H-1''),5.01(1H,d,J=8.2 Hz,xyl H-1''''),2.82(1H,dd,J=13.8,3.8 Hz,H-18),1.12,0.99,0.93,0.91,0.90,0.78,0.75(each3H,s);13C-NMR(C5D5N,100 MHz) δ: 38.4(C-1),26.1(C-2),88.6(C-3),39.6(C-4),56.8(C-5),18.4(C-6),33.8(C-7),39.7(C-8),48.0(C-9),37.1(C-10),23.5(C-11),122.4(C-12),144.5(C-13),42.0(C-14),28.2(C-15),23.9(C-16),46.6(C-17),42.0(C-18),47.4(C-19),31.0(C-20),34.3(C-21),33.2(C-22),28.3(C-23),17.5(C-24 ),15.5(C-25),17.9(C-26),26.6(C-27),180.1(C-28),23.1(C-29),23.7(C-30)。糖部分数据见表1。以上波谱数据与文献[3]报道的一致,鉴定其为hookeroside D [oleanolic acid 3-O-α-L-rhamnopyranosyl(1→2)-β-D-xylopyranosyl-(1→4)-β-D-glucopyranosyl-(1→4)-β-D-xylopyranosyl(1→3)-α-L-rhamnopyranosyl-(1→2)-β-D-xylopyranoside]。
3.13化合物14白色无定型粉末。ESIMS(+): 429 [M + K]+;1H-NMR(CD3OD,400 MHz) δ:7.38(1H,d,J=1.2 Hz ,H -3),5.27(1H,d,J=4.4 Hz,H-1),4.65(1H,d,J=7.6 Hz,H-1'),4.04(1H,m,H-7),1.09(1H,d,J=6.8 Hz,H-10);13C-NMR(CD3OD,100 MHz) δ: 97.7(C-1),152.1(C-3),114.0(C-4),32.1(C-5),42.7(C-6),74.7(C-7),42.1(C-8),46.5(C-9),13.4(C-10),169.5(C-11),51.6(OMe),100.0(C -1'),75.0(C-2'),78.0(C-3'),71.6(C-4'),78.3(C-5'),62.8(C-6')。以上波谱数据与文献[8-9]报道的一致,鉴定其为马钱子苷(loganin)。
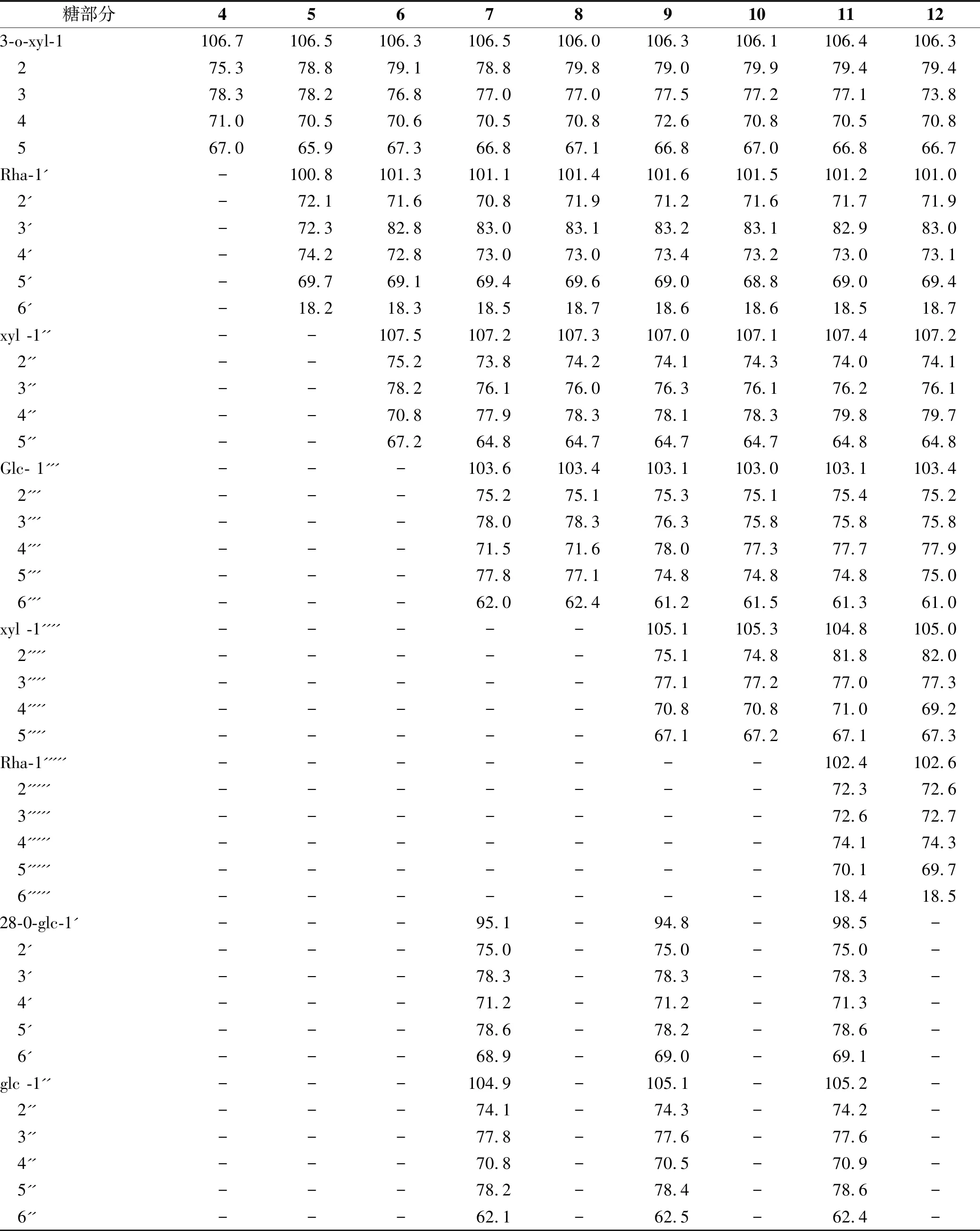
表1 化合物4~12 13C-NMR(C5D5N,100 MHz) 糖部分数据
3.14化合物15白色无定型粉末。ESIMS(+) : 456[ M]+;1H-NMR(C5D5N,400 MHz) δ: 5.26(1H,brs,H-12),2.82(1H,dd,J=13.8 ,3.6 Hz,H-18),1.11,0.99,0.93,0.92,0.91,0.77,0.74(each3H,s);13C-NMR(C5D5N,100 MHz) δ: 39.1(C-1),27.6(C-2),78.7(C-3),38.4(C-4),55.2(C-5),18.2(C-6),32.6(C-7),39.1(C-8),47.5(C-9),36.9(C-10),23.3(C-11),122.2(C-12),143.8(C-13),41.1(C-14),27.6(C-15),23.4(C-16),45.9(C-17),41.6(C-18),46.3(C-19),30.6(C-20),33.8(C-21),32.9(C-22),27.9(C-23),15.4(C-24),15.1(C-25),16.7(C-26),26.6(C-27),180.8(C-28),32.9(C-29),23.4(C-30)。以上波谱数据与文献[10-11]报道的一致,鉴定其为齐墩果酸(oleanolic acid)。
3.15化合物16无色针晶(三氯甲烷)。ESIMS(+):414[M]+;1H-NMR(CDCl3,400 MHz) δ: 5.35( 1H,brs,H-6),3.53( 1H,m,H-3);13C-NMR(100 MHz,CDCl3) δ: 37.5(C-1),31.8(C-2),71.9(C-3),46.0(C-4),140.9(C-5),121.9(C-6),31.9(C-7),32.1(C-8),50.3(C-9),36.4(C-10),28.4(C-11),39.9(C-12),42.5(C-13),56.9(C-14) ,26.3(C-15),29.2(C-16),56.0(C-17),12.0(C-18),19.3(C-19),32.1(C-20),19.7(C-21),33.8(C-22),23.7(C-23),36.6(C-24),19.6(C-25),12.2(C-26),22.6( C-27),19.9(C-28),18.9(C-29)。以上波谱数据与文献[4,10]报道的一致,鉴定其为β-谷甾醇(β-sitosterol)。
3.16化合物17白色无定型粉末。ESIMS(+): 771 [M+Na]+;1H-NMR(CD3OD,400MHz) δ: 7.49(1H,s,H-3),7.43(1H,s,H-3'),5.78(1H,ddd,J=8.3,10.4,16.8 Hz,H-8),5.56(1H,d,J=5.4 Hz,H-1),5.33(1H,d,J=16.8 Hz,H-10a),5.29(1H,d,J=10.4 Hz,H-10b),5.23(1H,m,H-7'),4.71(1H,d,J=7.6 Hz,H-1'''),4.67(1H,d,J=8.0 Hz,H-1''),4.64(1H,dd,J=5.6,5.9 Hz,H-7),1.07(3H,d,J=6.8 Hz,H-10');13C-NMR(CD3OD,100 MHz) δ: 98.0(C-1),153.7(C-3),112.1(C-4),31.3(C-5),34.1(C-6),61.4(C-7),136.0(C-8),45.6(C-9),119.5(C-10),168.7(C-11),97.7(C-1'),152.8(C-3'),113.3(C-4'),32.8(C-5'),40.4(C-6'),78.6(C-7'),41.2(C-8'),47.3(C-9'),14.0(C-10'),169.5(C-11')。糖部分数据:100.3(C-1''),74.8(C-2''),78.4(C-3''),71.7(C-4''),78.5(C-5''),62.9(C-6''),100.3(C-1'''),74.8(C-2'''),78.1(C-3'''),71.7(C-4'''),78.5(C-5'''),61.4(C-6''')。以上波谱数据与文献[6,12]报道的一致,鉴定其为sylvestroside I。
3.17化合物18白色无定型粉末。ESIMS(+): 815 [M+Na]+;1H-NMR(CD3OD,400 MHz) δ: 7.47(1H,s,H-3),7.45(1H,s,H-3'),5.78(1H,ddd,J=8.3,10.4,16.8Hz,H-8),5.56(1H,d,J=5.4 Hz,H-1),5.33(1H,d,J=16.8 Hz,H-10a),5.29(1H,d,J=10.4 Hz,H-10b),5.22(1H,m,H-7'),4.71(1H,d,J=8.0 Hz,H-1'''),4.69(1H,d,J=8.0 Hz,H-1''),4.54(1H,m,H-7),3.71(3H,s,CH3O-11'),1.09(3H,d,J=6.4 Hz,H-10');13C -NMR(CD3OD,100 MHz) δ: 97.8(C-1),153.2(C-3),111.9(C-4),29.5(C-5),33.3(C-6),104.2(C-7),135.8(C-8),45.3(C-9),119.8(C-10),168.2(C-11),53.6(OMe-7),52.7(OMe-7),97.4(C-1'),152.4(C-3'),113.2(C-4'),32.5(C-5'),40.3(C-6'),78.3(C-7'),40.9(C-8'),47.0(C-9'),13.7(C-10'),169.3(C-11'),51.7(OMe-11')。糖部分数据: 100.3(C-1''),74.6(C-2''),78.1(C-3''),71.5(C-4''),78.5(C-5''),62.7(C-6''),100.3(C-1'''),74.8(C-2'''),78.3(C-3'''),71.7(C-4'''),78.6(C-5'''),61.2(C-6''')。以上波谱数据与文献[12-13]报道的一致,鉴定其为triplostoside A。
3.18化合物19白色无定型粉末。ESIMS(+): 653 [M+Na]+;1H-NMR(CD3OD,400 MHz) δ: 7.49(1H,s,H-3),5.79(1H,ddd,J=8.3,10.4,16.8 Hz,H-8),5.56(1H,d,J=5.4 Hz,H-1),5.34(1H,d,J=16.8 Hz,H-10a),5.28(1H,d,J=10.4 Hz,H-10b),5.26(1H,m,H-7'),4.72(1H,d,J=7.6 Hz,H-1''),4.61(1H,m,H-7),3.76(3H,s,OMe-11'),3.31(6H,s,OMe×2-7);13C-NMR(CD3OD,100 MHz) δ: 97.9(C-1),153.5(C-3),111.8(C-4),29.6(C-5),33.3(C-6),104.2(C-7),135.8(C-8),45.3(C -9),119.8(C-10),168.1(C-11),53.5(OMe×2-7),70.8(C-1'),172.0(C-3'),52.8(C-4'),37.7(C-5'),39.1(C-6'),80.1(C-7'),42.4(C-8'),43.3(C-9'),13.5(C-10'),170.3(C-11'),53.2(OMe-11')。糖部分数据: 100.0(C-1''),74.6(C-2''),78.0(C-3''),71.5(C-4''),78,.4(C-5''),62.7(C-6'')。以上波谱数据与文献[12]报道的一致,鉴定其为sylvestroside IV dimethyl acetal。
3.19化合物20黄色无定型粉末。TLC紫外灯(波长254 nm)有强荧光,10%硫酸乙醇溶液显色为黑色,结合1H-NMR及13C-NMR数据推测其分子式为C26H32O12。ESI-MS:m/z559 [M+Na]+;1H-NMR(400 MHz,CD3OD):δ 7.15(1H,d,J=8.3 Hz,H-5″),7.11(1H,brd,J=1.3 Hz,H-2″),7.05(1H,brd,J=1.4Hz,H-2′),6.95(1H,dd,J=8.3 Hz,1.2 Hz,H-6″),6.87(1H,dd,J=8.1,1.5 Hz,H-6′),6.78(1H,d,J=8.1 Hz,H-5′),4.89(1H,d,J=7.0 Hz,H-1‴),4.84(1H,d,J=5.2 Hz,H-6),4.70(1H,s,H-2),4.46(1H,t,J=8.6 Hz,H-4a),4.06(1H,d,J=9.3 Hz,H-8a),3.87(1H,s,3″-OMe),3.85(3H,s,3′-OMe),3.04(1H,m,H-5);13C-NMR(100 MHz,CD3OD): δ 92.9(C-1),88.9(C-2),72.1(C-4),62.4(C-5),87.7(C-6),76.0(C-8),132.7(C-1′),113.3(C-2′),150.3(C-3′),147.6(C-4′),116.0(C-5′),121.3(C-6′),133.5(C-1″),111.2(C-2″),149.1(C-3″),147.4(C-4″),117.4(C-5″),120.5(C-6″),102.8(C-1‴),74.8(C-2‴),77.7(C-3‴),71.3(C-4‴),78.1(C-5‴),62.4(C-6‴)。上述波谱数据与文献[14]报道的一致,鉴定其为(+)-1-羟基松香醇4′-O-β-D-吡喃葡萄糖苷 [(+)-1-hydroxylpinoresinol4′-O-β-D-glucopyranoside]。
3.20化合物21黄色无定型粉末。TLC紫外灯(波长254 nm)有强荧光,10%硫酸乙醇溶液显色为黑色,结合1H-NMR及13C-NMR数据推测其分子式为C26H32O12。ESI-MS:m/z559 [M+Na]+;1H-NMR(400 MHz,CD3OD):δ 7.16(1H,d,J=8.3 Hz,H-5″),7.12(1H,brd,J=1.6 Hz,H-2″),7.04(1H,brd,J=1.5 Hz,H-2′),6.97(1H,dd,J=8.4,1.5 Hz,H-6″),6.85(1H,dd,J=8.1,1.6 Hz,H-6′),6.78(1H,d,J=8.1 Hz,H-5′),4.88(1H,overlapped,H-1‴),4.66(1H,s,H-2),4.47(1H,t,J=8.6 Hz,H-4a),4.05(1H,d,J=9.3 Hz,H-8a),3.87(1H,s,3″-OMe),3.86(3H,s,3′-OMe),3.04(1H,m,H-5);13C-NMR(100 MHz,CD3OD): δ 92.8(C-1),89.3(C-2),72.0(C-4),62.5(C-5),87.3(C-6),76.1(C-8),129.0(C-1′),112.7(C-2′),148.7(C-3′),147.5(C-4′),115.7(C-5′),121.5(C-6′),137.2(C-1″),111.8(C-2″),150.9(C-3″),147.5(C-4″),117.8(C-5″),120.2(C-6″),102.7(C-1‴),74.9(C-2‴),77.8(C-3‴),71.3(C-4‴),78.2(C-5‴),62.5(C-6‴)。上述波谱数据与文献[14]报道的一致,鉴定其为(+)-1-羟基松香醇4″-O-β-D-吡喃葡萄糖苷[(+)-1-hydroxypinoresinol 4″-O-β-D-glucopy- ranoside]。

