Chinese guidelines for diagnosis and treatment of esophageal carcinoma 2018 (English version)
National Health Commission of the People’s Republic of China
Contents
1. Overview
2. Diagnosis and treatment process for esophageal cancer
3. Principles of diagnosis and treatment
3.1 Clinical diagnosis
3.1.1 Risk factors and high-risk group
3.1.2 Clinical manifestations
3.2 Examinations
3.2.1 Lab tests
3.2.2 Tumor marker
3.2.3 Images
3.2.4 Endoscopy
3.2.5 Other tests
3.3 Diagnosis
3.3.1 Clinical diagnosis
3.3.2 Pathological diagnosis
3.4 Differential diagnosis
3.4.1 Other malignancies of esophagus
3.4.2 Benign tumors or tumor-like lesions of esophagus 3.4.3 Benign esophageal diseases
3.5 Pathological classification and staging
3.5.1 Segment of esophagus
3.5.2 Macroscopic type of esophageal carcinoma(Appendix A)
3.5.3 Pathological subtype and classification
3.5.4 Types of specimen and principle of specimen fixation
3.5.5 Principle of tissue dissection and description
3.5.6 Content and principle of pathological report
4. Standard treatment of esophageal carcinoma
4.1 Principle of treatment
4.2 Surgery
4.2.1 Principle of surgery
4.2.2 Indication of surgery
4.2.3 Contraindication of surgery
4.2.4 Follow-up
4.3 Radiotherapy
4.3.1 Indications for radiotherapy
4.3.2 Assessment of radiotherapy
4.3.3 Principle of radiotherapy
4.3.4 Dose
4.3.5 Normal tissue tolerance dose-limits
4.3.6 Concurrent chemotherapy regimens and dosing
4.3.7 Radiotherapy associated complications
4.3.8 Follow-up after radiotherapy
4.4 Medical management
4.4.1 Indications of chemotherapy for esophageal cancer
4.4.2 Assessment before chemotherapy
4.4.3 Regimens and dosing
4.4.4 Assessment of therapeutic effect
4.4.5 Prophylaxis and treatment of chemotherapy associated complications
4.4.6 Follow-up after chemotherapy
就主梁上部桥面板支架体系安装来看,工作人员首先要根据叠合梁的长、宽数据采用适当的钢管竖杆及横杆。在钢管的底部使用I14完成应力点的分散,减缓钢管底部的压力。钢管顶部和水平杆需要安装可调式托架以实现支架体系的稳定。其顶托的高度则通过拉线的手段进行更正调节,当这项工作完成后,开始进行钢管的架设,之后开展方木与模板的安装。关于模板与主梁翼板接触部分,要参考现场的测量数据进行。
4.4.7 Progress in molecular targeted therapy and immunotherapy
4.4.8 Supportive and palliative treatment
4.5 Principle of early-stage cancer/precancerous disease of esophagus and endoscopic treatment
4.5.1 High-risk group of esophageal cancer
4.5.2 Methods of screening
4.5.3 Preoperative assessment of endoscopic treatment on early stage esophageal cancer
4.5.4 Endoscopic therapy for early-stage esophageal carcinoma
4.6 Treatment model for esophageal cancer
4.6.1 Stage I (T1N0M0)
4.6.2 Stage Ib, stage II and part of stage IIIa(T1b-3N0M0, T1-2N1M0)
4.6.3 Stage III (T3N1M0, T4N0-1M0)
4.6.4 Stage IV (any T, any N, M1, N3 or T4b)
4.7 Traditional Chinese Medicine
1. Overview
Despite its wide variations in China, the case fatality rate of esophageal cancer is still high. In 2012, an estimation of 455,800 people would be diagnosed with esophageal cancer and 400,200 people would eventually die of their disease worldwide. Esophageal cancer is the 4th most common cause of cancer deaths in China, although its incidence somehow decreased during the recent years. According to Chen et al.’s report in 2017, the new cases of esophageal cancer in China reached 277,000 and 206,000 people died of this disease. The crude incidence rate of esophageal cancer in China is 20.35/100,000, including 15.03/100.000 in urban population and 30.73/100,000 in rural population while the crude death rate is 15.17/100,000 nationwide,including 14.41/100,000 in urban population and 21.05/100,000 in rural population. The incidence of esophageal cancer in China is more common in males and in rural population. High-prevalence areas mainly locate around the Taihang Mountain (e.g. provinces such as Henan, Hebei, Shanxi, Anhui, some areas of Shandong province such as: Taian, Jining, Heze, and the north of Jiangsu province). In the highest prevalence areas like Yangcheng of Shanxi province, Yangzhong of Jiangsu province and Cixian of Shanxi province, the crude incidence rate even reaches 109.5/100,000, 109.3/100,000 and 103.5/100,000, respectively (2003). Other highprevalence areas are associated with the migration of people from the middle regions of China, including Nanchong and Yanting of Sichuan province, Shantou of Guangdong province and Fuzhou of Fujian province, etc.The incidence rate and death rate of esophageal cancer rank the 6th and the 4th in all malignancies, respectively.Therefore, esophageal cancer has long been one of the most threatening malignant tumors. Screening for the high-risk groups and for people living in the highprevalence areas, early diagnosis and treatment are important to the improvement of the prognosis and living quality of patients with esophageal cancer. It effectively helps alleviate the medical cost of the people and government. Moreover, standardized diagnosis and treatment are also effective methods to improve the prognosis to benefit patients with mid-late esophageal cancer. Screening, early detection and standardized diagnosis and treatment should be important tasks for the hospitals and physicians nationwide.
Esophageal cancers are histologically classified as squamous cell carcinoma (SCC) and adenocarcinoma. SCC is the most common histological type in China (more than 90%), and adenocarcinoma is more common in North America and most European countries (about 70%).Smoking and alcohol abuse are major risk factors for SCC.Risk of SCC increases 3-8 times among the smokers and 7-50 times among the heavy drinkers. In the highprevalence areas in China, nitrosamine and certain mold/fungi are the major risk factors. For adenocarcinoma,gastroesophageal reflux disease (GERD) and Barrett’s esophagus are the two major risk factors.
High-risk group for esophageal cancer refers to people over 40 years old living in the high-prevalence areas with family history of esophageal cancer, malignancies of digestive system or other malignancies, or with precancerous diseases or lesions. The methods to decrease the incidence rate of esophageal cancer include restraining from high-risk factors (smoking, alcohol abuse, etc.),adopting mold resisting measures, avoiding intaking nitrosamine, life style modification, and nutrition/hygiene improvement. Screening for individuals with high-risk factors is essential to the prevention and treatment of esophageal cancer that helps early detection, diagnosis and treatment.
The treatment of esophageal cancer is based on the stage of the disease. For early esophageal cancer which is limited in mucosa, endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD) is the option for treatment. For early-mid esophageal cancers that invade into the submucosal layer, surgery is the main treatment.Postoperative adjuvant chemotherapy or radiotherapy may be offered if necessary. For mid-late esophageal cancer, the surgery-based comprehensive treatment is the choice.Either preoperative chemoradiotherapy, chemotherapy, or radiotherapy followed by surgery is usually utilized for the patients with lesions difficult to dissect or with more than 2 lymph nodes metastases. Postoperative chemotherapy or radiotherapy should be considered for these patients if necessary.
2. Diagnosis and treatment process for esophageal cancer
Procedure of diagnosis and treatment on esophageal cancer is shown in Figure 1.
3. Principles of diagnosis and treatment
3.1 Clinical diagnosis
3.1.1 Risk factors and high-risk group
Risk factors are as follows: over 40 years old, smoking,alcohol abuse, family history of esophageal cancer or malignancies, with precancerous diseases or lesions of above diseases. High-risk group refers to individuals with above risk factors, especially living in the high-prevalence areas of esophageal cancer.
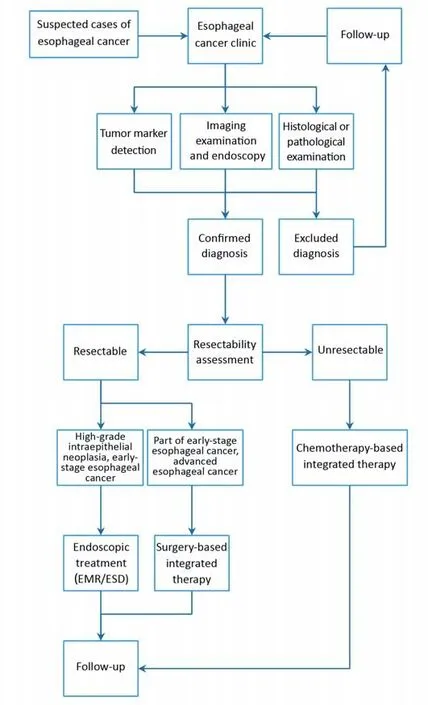
Figure 1 Standardized diagnosis and treatment process for esophageal cancer. EMR, endoscopic mucosal resection; ESD, endoscopic submucosal dissection.
3.1.2 Clinical manifestations
The symptoms of esophageal cancer include: dysphagia(difficulty in swallowing), foreign body sensation and retrosternal pain.
Symptoms of esophageal cancer are usually insidious.Patients may repeatedly complain block of food, foreign body sensation with swallowing, or retrosternal pain.Consistency or worsening of these symptoms indicates mid-late esophageal cancer.
If the patients present with chest pain, cough or fever,perforation needs to be considered. Hoarseness, dysphagia,cachexia, supraclavicular lymph node lesions or dyspnea is usually signs of late-stage esophageal cancer.
Although most patients with esophageal cancer do not have positive physical examination findings for the primary tumor, the distant metastasis may present with bone pain,hepatomegaly, pleural effusion, ascites, subcutaneous nodule, cervical lymph node lesions, headache, nausea or other neurological signs.
3.2 Examinations
3.2.1 Lab tests
Lab tests include CBC, hepatorenal function, coagulation function and screening of sexually transmitted diseases(STDs). These tests are employed to assess the general condition of the patients to determine the most suitable treatment for them. The increasing of ALP or calcium may refer to bone metastasis, while the increasing of GGT,ALP, GOT, LDH or bilirubin may refer to the possible liver metastasis. In addition, prealbumin and albumin are the indicators of nutrition status of the patients, especially those with dysphagia.
3.2.2 Tumor marker
Currently, tumor markers for the early diagnosis of esophageal cancer are still unmatured. However,cytokeratin fragment (CYFRA21-1), carcinoembryonic antigen (CEA), squamous cell carcinoma antigen (SCC)and tissue polypeptide specific antigen (TPS) can be utilized in combination with differentiation diagnosis,prognosis and radiation sensitivity forecasting, as well as follow-up monitoring.
3.2.3 Images
(1) Double-contrast barium enema (DCBE)
It is an economic, direct and easy method to detect esophageal cancer. DCBE can detect the relatively early mucosal lesions, and directly demonstrate the location and the size of the lesion. It is especially useful for the diagnosis of mid-late stage esophageal cancer. However, it is not accurate on the diagnosis of tumor evasion and cannot assess lymph nodes metastasis.
(2) Computed tomography (CT)
As a noninvasive test, CT is one of the best image tests for staging and prognosis forecasting of esophageal cancer. It is utilized to assess the tumor location, depth of invasion and the relationship between the esophageal lesion and the surrounding structures. In addition, CT is also utilized to detect the regional lymph nodes or distant metastasis, and the surrounding blood vessels invasion to provide evidence for clinical staging. Thoracic + upper abdominal CT with contrast is routinely recommended. If the lesion locates in cervical esophagus or less than 5 cm distal to cricopharyngeal muscle, cervical + thoracic + upper abdominal CT is recommended. If the contrast agent is contraindicated, (cervical) thoracic/upper abdominal CT without contrast, cervical and abdominal ultrasound are recommended. Besides preoperative assessment, CT can also be utilized postoperatively on the outcome of chemo/radiotherapy. Regarding clinical staging, the accuracy of CT on T staging is about 58%, on lymph nodes metastasis is about 54%, and on distant metastasis(e.g. liver, lung, etc.) is around 37%-66%.
(3) Magnetic resonance imaging (MRI)
MRI is a nonradiative test with high resolution. The development of high-field strength MRI equipments greatly fastens the speed of MRI scan, enables the completion of thin-slice scan and multi-phase dynamic contrast-enhanced scan as CT, improves the understanding of tumor invasion and its relationship between surrounding structures, and increases detection rate of lymph nodes. In addition, techniques of functional MRI (e.g. diffusion weighted imaging, perfusion weighted imaging, and spectrum analysis) also provide valuable complementary information for cancer detection and determination.Comparing with CT, MRI provides more details on location, invasion and surrounding structures of the esophageal lesion, and helps more accurate clinical staging.However, the time needed for MRI scan is longer than CT,movement of the heart may cause more image artifacts.Therefore, MRI is not routinely recommended for the assessment of treatment response.
(4) Ultrasound
Instead of detecting esophageal lesions, ultrasound is mainly utilized on discovery of cervical lymph nodes and distant metastasis (e.g. liver, kidney, etc.) to help clinical staging. It can also detect and locate pleural or pericardial effusion. Ultrasound guided percutaneous biopsy on suspected cervical lymph node or parenchyma organ metastasis can further help pathological diagnosis.
(5) Positron emission tomography/computed tomography(PET/CT)
Comparing with CT, PET-CT has higher sensitivity and specificity on detection of esophageal cancer, thus providing more accurate TNM staging data by confirming location of the lesion, discovering regional lymph nodes metastasis and finding more distant metastasis. Distant metastasis may be diagnosed by PET in 15%-20% of patients with negative results in regular exams. PET-CT is considered as a promising tool for therapeutic assessment and prognosis forecasting. PET-CT is recommended in patients with locally advanced esophageal cancer before surgery, preoperative therapy and radical radiochemotherapy. It is also recommended as a complimentary test to assess the response of preoperative therapy and radical radiochemotherapy. However, the threshold of SUV and timing of posttreatment PET-CT are controversial.Considering some inflammatory conditions (e.g. radiationinduced esophagitis or biopsy-induced inflammation) may influence the explanation of PET-CT result, we recommend the interval of PET-CT and above treatment should be no less than 2 weeks, and no biopsies should be done before PET-CT test. For patients without distant metastasis, PET-CT needs to cover areas from the brain to the groin. General check-up is indicated for patients with suspected distant metastasis.
The above described image tests have their own advantages and disadvantages. The combination of different tests helps provide more information of the patients.
3.2.4 Endoscopy
(1) Regular fiberoptic endoscopy
Under the observation of regular endoscope, the early esophageal cancer may be presented as 1) red area, with clear margin and flatten base; 2) local erosion, with clear margin and red pitting base; 3) plaque, usually is white bump, with clear margin; 4) nodule, usually <1 cm, is bumped superficial coarse mucosa or eroded nodule; 5)coarse mucosa, with unclear margin; 6) regional thickened mucosa, usually covers the underlying texture of blood vessels, and presents with disorder, lack of or termination of the mucosal vascular network. Physicians should be sensitive to these characteristics, and carefully observe the subtle alterations of the mucosa. Multifocal biopsy on suspicious lesions is the key to detect early esophageal cancer. However, most early esophageal cancers do not have the above typical presentations, and regular endoscopy combined with staining helps improve the detection of suspicious lesions. Mid-late stage esophageal cancer is usually easy to be recognized during endoscopy. Most of them are presented with nodule or cauliflower neoplasm,with hyperemia, eroded or pale mucosa, sometimes with ulcers. These lesions intend to hemorrhage when touched.Stenosis in various degree can also be observed in some patients. Once the CT finds the lesion(s) locate in the cervical or upper/middle segment of thoracic esophagus and is closely related to the membranous trachea or left main bronchus, bronchoscope is essential to confirm or exclude invasion of primary esophageal cancer to the trachea or bronchus.
(2) Chromoendoscopy
Certain dyes can be sprayed on the surface of the esophageal mucosa to differentiate the cancer lesions and normal mucosa to guide biopsy. Most frequently utilized dyes are iodine, toluidine blue, etc. They can be used solely or in combination.
(3) Endoscopic ultrasound (EUS)
EUS can clearly identify changes of the esophageal wall structure, the depth of cancer invasion and the relationship between primary lesion and surrounding organs. The accuracy of EUS on T staging is about 74%-86%,although this accuracy may be disturbed by size and location of the tumor. The sensitivity of EUS on regional lymph node metastasis is 80% which is higher than CT(50%) and PET (57%), while the specificity (70%) is lower(83% in CT and 85% in PET). On abdominal lymph nodes metastasis, the sensitivity and specificity of EUS(85% and 96%, respectively) are both higher than CT(42% and 93%, respectively). The combination of EUS and fine-needle aspiration (FNA) can be utilized on improvement of pathological diagnosis of suspected lymph node involvement.
3.2.5 Other tests
(1) Electrocardiogram (EKG): preoperative screening for arrythmia or myocardial infarction (MI) history.
(2) Pulmonary function test (PFT): preoperative assessment of lung volume, ventilation and diffusion.
(3) Exercise cardiopulmonary function test: it is used when the above two tests cannot reach clear conclusion on tolerance of surgery.
(4) Ultrasonic cardiogram: it is recommended to patients with history of heart diseases, to assess the modifications of heart structure and function.
(5) Coronary angiogram: it is recommended to the aged and patients with history of coronary disease, to assess the heart blood supply and risk of surgery.
3.3 Diagnosis
3.3.1 Clinical diagnosis
If the patients belong to any one of the following two criteria, the clinical diagnosis of esophageal cancer should be considered:
(1) Food blocking, foreign body sensation with swallowing,retrosternal pain or obvious dysphagia, and local esophageal mucosa thickening, stiffness, filling defect or niche on esophageal imaging tests;
(2) Food blocking, foreign body sensation with swallowing,retrosternal pain or obvious dysphagia, and circular or irregular thickening of esophageal wall on CT.
The clinical diagnosis of esophageal cancer needs pathological confirmation. Chemo/radiotherapy or experimental chemo/radiotherapy should not be based on the clinical diagnosis without pathological support.
3.3.2 Pathological diagnosis
If the patients belong to any one of the following two criteria, the confirmative diagnosis of esophageal cancer is established:
(1) Endoscopic brush cytology or biopsy confirms carcinoma; or
(2) After the establishment of clinical diagnosis of esophageal carcinoma, the metastasized lesions out of esophagus (supraclavicular lymph nodes, subcutaneous nodule) was pathologically diagnosed as metastasis of esophageal cancer.
Endoscopy plus pathological test is the “golden standard” for the diagnosis of esophageal cancer. The other tests are utilized to understand the location, size, staging of the tumor, to provide information for the choice of treatment.
3.4 Differential diagnosis
Esophageal cancer needs to be differentiated from other esophageal diseases (benign or malignant), as well as diseases of adjacent organs that compressing the esophagus.
3.4.1 Other malignancies of esophagus
Although not common, malignancies other than carcinoma of esophagus include sarcoma, leiomyosarcoma, melanoma and invasion of mediastinal lymph nodes metastasis from primary malignancies of other organs.
(1) Esophageal sarcoma: sarcoma and carcinoma of esophagus share similar image characteristics. The most common type of sarcoma is pedicle neoplasm in the lumen of esophagus, causing stenosis of esophagus and irregular filling defect on image test.
(2) Esophageal leiomyosarcoma: includes two types: polyp and invasive leiomyosarcoma. The polyp leiomyosarcoma is usually pedicle soft tissue neoplasm covered by mucosa,growing in the lumen of esophagus. The invasive leiomyosarcoma grows simultaneously toward in and out the lumen of esophagus, cause thickening of the esophagus wall. Ulceration usually occurs in the center of the neoplasm surface. Esophageal leiomyosarcoma can be demonstrated as mediastinal lesion on chest X-ray. On esophagus image, it may be shown as huge in-lumen lesion,with stenosis or regional dilation of esophageal lumen, with various filling defects. The mucosa of esophagus is usually flat or damaged, sometimes with niches.
(3) Esophageal melanoma: primary esophageal melanoma is rare. It can be brown nodule or lobulated lesion intrudes into the lumen of esophagus. The lesion sometimes has pedicle. The image test is similar to intraluminal esophageal carcinoma.
(4) Metastasis to esophagus: the primary trachea, thyroid,lung, kidney or breast cancer involves esophagus by either direct invasion or lymph nodes metastasis. Endoscopy can find compression from external lesions out of esophagus.Hematogenous spread of other cancers to esophageal wall is rare. The image test is also similar to intraluminal esophageal carcinoma.
3.4.2 Benign tumors or tumor-like lesions of esophagus
The most common benign tumor of esophagus is leiomyoma (50%-70%). Others include adenoma, lipoma,papilloma, hemangioma, etc. The tumor-like lesions of esophagus include polyp, cyst, diffused leiomyomatosis and endometriosis.
(1) Esophageal leiomyoma: observed through endoscopy, it is single or multiple mobile nodules covered by normal esophageal mucosa. The most common type is a single round, oval, dumbbell shape, or irregular lesion. Double air-barium contrast examination may find sharply marginated round or oval lesion on the esophageal wall.The mucosal folds on the surface of the lesion disappear while the opposite side is normal. There is no barium on the surface of the lesion, presented as uniform filling defect that is called “daubing-trace” or “falls sign”. Biopsy should not be implemented if esophageal leiomyoma is suspected,because the inflammation caused by biopsy may lead to the perforation of mucosa during surgery.
(2) Other benign tumors in the esophageal wall:
hemangioma, lipoma and polyp share similar image presentations with leiomyoma. Fibrovascular polyp is usually found in the cervical segment of esophagus.Sometimes it is mobile in esophagus and can even be observed in the oral cavity. Lipoma is a soft lesion with limited mobility, demonstrated as low density or lipid signal on CT or MRI.
3.4.3 Benign esophageal diseases
(1) Benign esophageal stricture: patients have history of mistaken strong acid or alkali intake. The stricture usually occurs proximal to the physical stricture of the esophagus,most commonly seen in the lower segment of esophagus.
(2) Achalasia: usually first reported in young age. The patient complaints long-term repeated dysphagia and needs of water to help food to pass through the esophagus.Symmetrical stricture above esophagogastric junction(funnel-shaped or “bird’s beak” stenosis) with proximal dilation of esophagus is demonstrated in esophageal image test. Food retention without damaged mucosa can be found by endoscopy. The endoscope can move forward into the stomach by passing through the stricture. It is important to notice that the stricture caused by achalasia should be differentiated from that caused by some rare invasive cancer of lower segment of esophagus.
(3) Peptic esophagitis: patient complaints long-term odynophagia, regurgitation and heart-burning, and further dysphagia due to cicatricial stricture caused by repeated inflammation. In double air-barium contrast test, one can see spastic constriction of the lower segment of esophagus,with rough or unclear mucosa. Barium retention or niche can also be seen if the mucosal erosion or ulceration exists.Long-term disease may cause symmetrical stricture due to fibrosis while the esophagus is still plastic. The endoscopy may find mild stricture of esophagus and formation of mucosal erosion and small ulcerations, usually accompanied by hiatus hernia or gastroesophageal reflux. The symptoms and involved mucous will be improved by anti-acid treatment (e.g. Omeprazole).
(4) Esophageal varices: the patient usually complains history of liver cirrhosis but no obvious dysphagia. The image test presents with polypoid filling defect. In serious conditions,the thickened mucosa is worm-like or bean-like. The involved esophagus is still soft without stricture and conserves some degree of constriction/dilation function.The soft thicken tortuous veins can be seen under endoscopy. Biopsy is contraindicated for esophageal varices to avoid bleeding.
(5) Compressive stricture: the compressive stricture of esophagus may be caused by benign tumors neighboring to esophagus, cervical or mediastinal lymph nodes metastasis,disease/deformation of great vessels or other mediastinal diseases (e.g. lymph nodes tuberculosis). It is demonstrated as compressive change with sharp edge under endoscopy,without mucosal damage. However, if the malignant lymph node or tuberculosis invades into the esophageal wall, the involved mucosa may be eroded or ulcerated. Biopsy confirms the diagnosis.
(6) Esophageal tuberculosis: Rare. Usually occurs in young patients who complain history of food blockage with swallowing. The routes of infection include: 1) extension of pharynx or larynx tuberculosis; 2) invasion of swallowed sputum for patients with pulmonary tuberculosis; 3)invasion of spinal tuberculosis; 4) hematogenous spread of TB infection to the esophageal wall; 5) invasion of the caseous mediastinal lymph nodes next to the esophagus (the most common type in clinical practice). On image test,slightly narrowed and stiff lesion, usually with formation of relatively big ulceration can be seen. The filling defect and mucosal damage are not as obvious as esophageal cancer. In endoscopy, big and deep ulceration can be observed, but the mucosal erosion, stricture or multiple nodules is not as obvious as esophageal cancer. Biopsy is utilized for differentiation.
3.5 Pathological classification and staging
3.5.1 Segment of esophagus
(1) Cervical esophagus: extending from the pharynx to the thoracic inlet (level of suprasternal notch). Adjacent to trachea, carotid sheath and spine. The distance is 15-20 cm from the incisors in endoscopy.
(2) Upper segment of thoracic esophagus: continuing from thoracic inlet to inferior margin of the azygos vein arch(above the level of hilum). Anteriorly adjacent to trachea,the three branches of aorta and brachiocephalic veins.Posteriorly adjacent to spine. The distance is 20-25 cm from the incisors in endoscopy.
(3) Middle segment of thoracic esophagus: continuing from inferior margin of the azygos vein arch to inferior margin of the lower pulmonary vein. It travels between left and right hilum anteriorly, adjacent to descending aorta on its left and to spine posteriorly. The distance is 25-30 cm from the incisors in endoscopy.
(4) Lower segment of thoracic esophagus: continuing from inferior margin of lower pulmonary vein to esophagogastric junction. The distance is 30-40 cm from the incisors in endoscopy.
3.5.2 Macroscopic type of esophageal carcinoma (Appendix A)(1) Early esophageal carcinoma: includes concealed, eroded,plaque and papillary types.
(2) Mid-late stage esophageal cancer: includes medullary,mushroom, ulceration, constrictive and intraluminal types(Appendix B).
3.5.3 Pathological subtype and classification
(1) Terminology
1) Esophageal carcinoma Derived from the epithelial cells of esophageal mucosa, including squamous cell carcinoma and adenocarcinoma. The squamous cell carcinoma located in the esophagogastric junction is considered esophageal cancer.
2) Intraepithelial neoplasia/dysplasia Precancerous lesions of esophageal carcinoma, including intraepithelial neoplasia/dysplasia of either squamous epithelium or glandular epithelium. The term intraepithelial neoplasia and dysplasia are interchangeable.
Intraepithelial neoplasia/dysplasia of squamous epithelium is a precancerous lesion characterized by various degrees of heteromorphic squamous cells in the squamous epithelium of esophageal mucosa. According to the depth of invasion, it is classified as low-grade intraepithelial neoplasia/dysplasia (limited in the superficial 1/2 of the squamous epithelium) and high-grade intraepithelial neoplasia/dysplasia (involved exceed 1/2 of the squamous epithelium).
Intraepithelial neoplasia/dysplasia of glandular epithelium is a precancerous lesion characterized by various degrees of heteromorphic glandular cells in the glandular epithelium of esophageal mucosa, usually seen in Barratt’s esophagus. It is also classified as low-grade and high-grade intraepithelial neoplasia/dysplasia. The standard of classification is same as that of squamous epithelium.
3) Early esophageal carcinoma Limited in the mucosal layer regardless of regional lymph node metastasis.
4) Superficial esophageal carcinoma Limited in the mucosal or submucosal layer regardless of regional lymph node metastasis.
5) Advanced esophageal carcinoma Invaded into or over muscular layer.
6) Adenocarcinoma of esophagogastric junction
Anatomically, the esophagogastric junction is the place where the tubular esophagus continued with cystic stomach. It is the end of the esophagus and the beginning of the stomach. It locates on the level of peritoneal refection, or angle of His, or inferior margin of esophageal sphincter. The location of the esophagogastric junction is not necessarily at the histological junction of squamous cells and columnar cells.
7) GERD and reflux esophagitis GERD is the reflux of gastric or duodenal contents into the esophagus, causing uncomfortable symptoms and/or complications. The characteristic symptoms include heart-burning and regurgitation. Reflux esophagitis is one of the main pathological presentations of GERD, presented as inflammation, erosion and ulceration of the esophageal mucosa. In late stage, even fibrosis and esophageal stricture can also be seen.
8) Barrett’s esophagus According to the consensus of diagnosis and treatment on Barratt’s esophagus by Digestive Division, Chinese Medical Association, once the columnar metaplasia exists in the distal mucosa of esophagus, the diagnosis of Barratt’s esophagus is established. The single columnar metaplasia may be gastric type, or intestinal type with goblet cells. Importantly, the intestinal type of columnar metaplasia predisposed to the development of adenocarcinoma. Therefore, the diagnostic report must note the histological type of the columnar metaplasia, and the existence of the intestinal epithelial metaplasia or intraepithelial neoplasia/dysplasia.
(2) Pathological classification, grade and staging 1) Histological types (Appendix B)
We recommend the 2010 WHO classification of digestive neoplasms.
2) Histological grade
According to the degree of differentiation, the squamous cell carcinoma and adenocarcinoma of esophagus are classified into well differentiated, moderately differentiated and poorly differentiated groups.
3) Staging (Appendix C)
We recommend the American Joint Committee on Cancer(AJCC) TNM staging system (8th edition).
(3) Pathological assessment of specimen from radical esophagectomy after neoadjuvant therapy (Appendix D)
The main pathological characteristics of the tissue after neoadjuvant therapy include: degeneration, reduction and massive necrosis of tumor cells, proliferation of fibrous tissue, interstitial inflammatory cells infiltration and deposition of calcium. After neoadjuvant chemotherapy,the squamous cell carcinoma may present with keratins without remnant cancer cells, while adenocarcinoma may present with massive lakes of mucus without remnant cancer cells. Both conditions should not be considered as residual tumors.
College of American Pathologists (CAP)/The National Comprehensive Cancer Network (NCCN) guidelines should be employed to the assessment of therapeutic outcome of esophageal cancer treatment.
3.5.4 Types of specimen and principle of specimen fixation(1) Types of specimen
The types of specimen are usually classified by their sources: specimen from endoscopic biopsy, EMR/ESD and radical surgery resection.
(2) Fixation of specimen
1) Neutral buffer formalin fixative solution (10%) should be utilized as soon as possible (best immediately after the specimen is acquired; the surgical dissected specimen should be fixated in 30 min). The volume of the fixative solution should be more than 10 times than that of specimen. The fixation time is 6-72 h.
2) Specimen from endoscopic biopsy: after the separation of the specimen, endoscopy physician or his/her assistant should obtain the tissue from the biopsy forceps and unbend the specimen on his/her finger. Place the unbend mucosa on the surface of a piece of small filter paper, and then place it into the fixative solution immediately.
3) Specimen from EMR/ESD: the endoscopy physician should unbend the specimen with the surface of the mucosa faced up, and then fix the specimen on cork board (or foam board) by fine steel needles. The specimen should be fixed without folds or excessive traction (which may cause the deformation of the specimen) should be avoided. The specimen should be immersed completely into the fixative solution after the distal and proximal margins have been marked respectively.
4) Specimen from radical dissection: disclosing the esophageal wall alongside the opposite side of the tumor.With the mucosa faced up, fixing the specimen on cork board (or foam board) covered with gauge by pins. Then prone the mucosa and then immerse the specimen into the fixative solution.3.5.5 Principle of tissue dissection and description(1) Verification
Before tissue dissection, basic information should be verified. (i.e. name, submitting department, bed ID,admission number and type of the specimen, etc.)
(2) Specimen from biopsy
1) Gross inspection and recording: describing the size and number of the submitted tissue.
2) Tissue dissection All the submitted mucosa should be dissected. The mucosa should be kept in folded filter paper to secure no loss. Eosin should be added before tissue dissection to help embedding and be recognized by technicians. Specimen with clearly different size should be placed in different dehydration box to avoid missing or over-cut of the small tissue. The unfolded flat mucosa should be embedded vertically (the mucosa is perpendicular to the bottom of the embedding box). One paraffin block should include no more than 3 (since most of the precancerous lesion and early esophageal cancer are flat,and the diagnosis of these lesions needs to understand the ratio of the dysplastic cells that invaded to the squamous epithelial layers, and whether the propria lamina mucosa is involved. The requirement on the clarity of the tissue layers is strict. The above described methods of unfolding and embedding are essential for the accurate diagnosis of early esophageal cancer and precancerous lesions). The margin of the paraffin block that does not contain tissue should be removed by knife. We recommend 6-8 consecutive tissue slices on every glass sheet.
(3) Specimen from EMR/ESD
1) Gross inspection and record The size of the specimen(length × width × thickness) needs to be measured and recorded. The length and width of the esophagus and stomach should be measured respectively for esophagogastric junction specimen. Other features that need to be recorded include color of the mucosa, size of the lesion(length × width × thickness), gross type (Appendix A),existed visible lesion, obvious bumps or depressions,erosion or ulcers, and distance between the lesion and margin of the specimen (at least record the distance between the lesion and the nearest margin of the mucosa).The dissected multi-block specimen should be reconstructed before fixation by the surgeon on basis of endoscopic outline of the lesion/outline of iodine unstained area (esophageal squamous epithelial lesion). Before the treatment of complex specimen, communication between surgeon and pathologist, or sketch map on extension and reconstruction of the specimen provided by the surgeon is recommended.
2) Tissue dissection All the specimen should be selected.Iodine solution is preferred (taking the specimen out of fixative solution and washing it at least 30 min before iodine staining) for identification of the lesion (unstained area) and the nearest margin. The specimen should be cut vertically to the nearest margin. To locate the margin and assess its condition, the margin of the mucosa and the base need to be marked by prepared Chinese ink or carbon ink(different color can be utilized to mark distal and proximal margin for differentiation, if possible). The esophagogastric junction specimen should be cut from distal to proximal end to demonstrate the relationship between the tumor and the esophagogastric junction. All the specimen should be cut parallelly every 2-3 mm. If the specimen is too large,the method of cutting should be modified by dividing one slice to mutiple slices, marked with a, b, c, etc. Embedding on same direction (embedding the first and the last cut slices. If there are any endoscopic lesions are detected, reembedding after 180° turning, to secure all the margins surround the specimen can be observed), and recording the order/location of the embedded specimen. Recording the corresponding location of the tissue block (well-marked picture or sketch map is recommended). We recommend number and cut the multiple dissected specimen, regardless of the condition of the margin.
(4) Specimen from radical resection
1) Gross inspection and record Record the length of the dissected esophagus, stomach (if included), and whether the esophagogastric junction is visible. The location of the tumor (based on the description of surgery and endoscopy):cervical esophagus, upper segment of thoracic esophagus,middle segment of thoracic esophagus, lower segment of thoracic esophagus, and esophagogastric junction.Recording the distance between the lesion and the distal or proximal end, the gross type (including description of the appearance) (Appendix A), size, quality, depth of invasion of the tumor, color of the slice, and whether the esophagogastric junction is involved (relationship between tumor and esophagogastric junction: tumor completely located in esophagus without involvement of esophagogastric junction; the center of the tumor locates in the distal esophagus involving the esophagogastric junction; the center of the tumor locates in esophagogastric junction; the center of the tumor locates in the proximal stomach,involving esophagogastric junction). If the esophagogastric junction is involved, distance between center of the tumor and esophagogastric junction should be recorded.
We recommend Siewert classification (Appendix E) for esophagogastric junction adenocarcinoma.
2) Tissue dissection If necessary, iodine solution should be utilized (taking the specimen out of fixative solution and washing it at least 30 min before iodine staining) for identification of the lesion (unstained area) and the nearest margin. One strip of the esophagus cutting from distal to proximal ends (passing center of the tumor) should be embedded (including tumor, paraneoplastic mucosa and both distal and proximal margins). The corresponding location of the tissue block should be recorded (wellmarked picture or sketch map is preferred). Longitudinal cutting is recommended while transverse cutting is acceptable if the tumor locates far away from both margins.The submitted margin in the stapler should be all cut for observation. The place where the tumor invaded most, and the suspected involved circumferential resection margin deserves more attention. Prepared Chinese ink or carbon black ink is recommended to mark the circumferential resection margin.
For early esophageal cancer, or specimen of radical surgery after neoadjuvant therapy with unconspicuous tumor, all the suspected lesions and tumor beds should be cut.
Areas with eroded, rough mucosa or unstained by iodine,nodules in esophageal or gastric wall, and tissue of esophagogastric junction should be dissected respectively.Submitted grouped lymph nodes should be all embedded and dissected. The submitted adjacent organs, such as mediastinal pleura, lung or diaphragm, should be checked and dissected.
We recommend the size of dissected tissues to be no more than 2.0 cm × 1.5 cm × 0.3 cm.
More than 12 lymph nodes should be harvested from the patients without neoadjuvant therapy undergoing standard two or three fields lymph node dissection.
3.5.6 Content and principle of pathological report
The pathological report of esophageal cancer should include all the contents associated to patient’s treatment and prognosis, i.e. source, size and number of the specimen, location of the tumor, gross classification,histological type, subtype and grade, depth of invasion,vessels and nerve invasion, spread or metastasis in the wall,status of the surrounding mucosa, lymph nodes,circumference and both margins. Finally, pTNM staging should be noted.
(1) Gross description Including source of the specimen,location, gross classification, size ( size of the tumor should be measured by three dimensions) and number of the tumors.
(2) The tumor Histological type, subtype and grade(Appendix B), depth of invasion (including propria lamina mucosa, mucosal muscularis, submucosal, superficial muscularis, deep muscularis, fibrous membranes and surrounding tissues or organs). For cancer invades into submucosa, if the specimen is acquired from endoscopic resection, the depth (µm) of the submucosal invasion should be measured and differentiation between SM1(depth of submucosal invasion ≤200 µm) and SM2 (depth of submucosal invasion >200 µm) is recommended; if the specimen is acquired from radical surgery, differentiation among SM1 (upper 1/3 of submucosa), SM2 (middle 1/3 of submucosa) and SM3 (lower 1/3 of submucosa) is recommended, margin (side margin and basement margin of endoscopic specimen; distal, proximal and circumferential margins of radical surgical specimen) (The status of the margins needs to be reported, such as invasive carcinoma, intraepithelial neoplasia/dysplasia, Barrett esophagus, or Barrett esophagus accompanied with intraepithelial neoplasia/dysplasia; distance from the tumor to the margins should be noted; 0, 0-0.1 cm and ≥0.1 cm system are recommended to mark the distance from tumor to the circumferential margin), vessels invasion (especially for endoscopic resected specimen, if vessels invasion is suspected, immunohistochemical CD31, D2-40 are recommended for confirmation of vessels invasion, while EVG staining is recommended for diagnosis of venous invasion), nerve invasion and metastasis in the wall.
(3) Paracancerous tissue Intraepithelial neoplasm/dysplasia and its grade, Barrett esophagus, existence of esophagitis, gastritis, and their types.
(4) Lymph node metastasis “Number of lymph nodes metastasis/total submitted lymph nodes number”. The number of the lymph nodes invaded exceed to capsule should be reported.
(5) Response of treatment (for neoadjuvant therapy cases).(6) Other accompanied diseases should also be reported.
(7) Immunohistochemistry on HER2 and mismatch repair protein (MLH1, PMS2, MSH2, MSH6) and/or MSI tests should be done for esophagogastric junction adenocarcinoma.
(8) Important history should also be addressed (i.e. history of tumor and neoadjuvant therapy).
(9) pTNM staging (Appendix C)
4. Standard Treatment of esophageal carcinoma
4.1 Principle of treatment
We recommend personalized treatment based on the decision of multiple discipline team (MDT). The treatment regimen should consider status of the patient, pathological type, invasion of the tumor and the possible prognosis. For patients planning to accept chemotherapy or radiotherapy,Karnofsky or Eastern Cooperative Oncology Group(ECOG) assessment is essential before treatment(Appendix F).
4.2 Surgery
Surgery is one of the most important radical treatment for patients with esophageal carcinoma. The early esophageal cancer is curable by surgery. The combination of surgery and adjuvant therapies can cure some of the mid-late esophageal cancer patients while improve the survival of the rest. Left transthoracic esophagogastrostomy and right transthoracic esophagogastrostomy are the two most commonly utilized surgical approaches in China. Before 2000, left transthoracic esophagogastrostomy is the predominant approach for the surgical treatment of esophageal cancer in China. However, because the aortic arch locates in the left thoracic cavity and the space superior to the aortic arch is limited, the dissection of upper mediastinal lymph nodes may not complete.Therefore, the rate of cervical or upper mediastinal lymph nodes metastasis is as high as 30%-40% and the 5-year survival rate is about 30%-40% in the recent 30 years for the patients underwent left transthoracic esophagogastrostomy. With the implement of national lesson on standardized treatment of esophageal cancer and the widely application of minimal invasive technique, more and more patients accepted right transthoracic esophagogastrostomy,with about 50% of cases for left and right approaches each in recent years. Without the blockage of aortic arch in right thoracic cavity, the thoracic lymph nodes are more likely thoroughly dissected. The supine position during abdominal operation also helps the dissection of abdominal lymph nodes. In China, most hospitals employ cervical lymph node dissection for selected patients, while some hospitals consider it as routine procedure for patients with esophageal cancer. Comparing with left approach,thorough two-field (thoracic-abdominal) or three-field(cervical-thoracic-abdominal) lymph node dissection through right approach may help to decrease the recurrence and metastasis of cervical and thoracic lymph nodes after surgery, thus to improve 5-year survival about 10% to 50% postoperatively. The adjuvant therapies include preoperative neoadjuvant therapy (chemotherapy,radiotherapy or radiochemotherapy) and postoperative adjuvant therapy. It is reported that preoperative radiochemotherapy is superior to chemotherapy or radiotherapy. For patients with thoracic esophageal cancer difficult to dissect or with two or more lymph nodes metastasis, most of Chinese hospitals consider neoadjuvant radiochemotherapy. Surgery is usually followed in 6-8 weeks if the stage of the tumor is down-graded after neoadjuvant therapy. Otherwise, continuation of radiochemotherapy is another choice. For patients with tumor residuals or with risk factors (positive lymph nodes,poorly differentiated, vascular invasion), postoperative chemotherapy/radiotherapy should be considered.
4.2.1 Principle of surgery
(1) Complete all the examinations to assess the status of patients and cTNM stage of esophageal cancer. The following tests must be done to make reasonable personized treatment regimen: endoscopy, EUS(recommend), histological or cytological exam, CT scan with contrast on the neck, chest and abdomen, cervical ultrasound, upper gastrointestinal series, pulmonary function test, EKG, PET-CT (for selected patients),nutritional status assessment and risk screening(recommend), CBC, UA, hepatorenal function, screening of STDs, bleeding time and coagulation test. Clinical T and N staging should be achieved by CT or PET-CT and EUS, M staging should be achieved by brain MRI/CT and bone scan or PET-CT.
(2) Risk assessment
1) Cardiovascular function Patients with grade I-II heart function can tolerate the esophagectomy. Otherwise,further tests or treatment will be needed. For patients with history of MI or cerebral infarction, the interval between the treatment for MI (or cerebral infarction) and esophageal surgery should be at least 3-6 months. The anti-coagulate agents, such as aspirin or clopidogrel should be stopped at least 1 week before surgery. For patients with cardiothoracic ratio (CTR) >0.55 and left ventricle ejection fraction (EF) <0.4, treatment and re-assessment are needed.Mild to moderate hypertension can be effectively controlled by medication. The antihypertensive drugs can be used until the morning of the surgery day. For patients with history of organic heart disease or myocardium infarction, ultrasonic cardiogram is recommended. For patients with serious arrythmia, e.g. tachycardia,atrioventricular block or sinus syndrome, 24-h Holter monitor is recommended and surgery should be planned after the treatment of the arrythmia.
2) Pulmonary function test Patients with normal or mildmoderate abnormal PFT (VC% >60%, FEV1 >1.2 L,FEV1% >40%, DLco >40%) can tolerate the esophagectomy. However, the risk of postoperative pulmonary complications increases in patients with moderate abnormal PFT results. If necessary, exercise cardiopulmonary exam or stair climbing are the choices of further evaluation. The patients are required to continuously to climb more than 3 stairs or with result of VO2max/(kg.min) >15 mL in exercise cardiopulmonary exam to be eligible for open surgery for esophageal cancer.
3) Hepatorenal function Child-Puhg score is utilized to assess the hepatic function. Low-risk group refers to patients with score 5-6, moderate-risk group for score 8-9 and high-risk for score >10. The assessment of renal function is mainly based on the preoperative UA, creatine and BUN. Patients with mild renal function abnormality can tolerate the surgery. Counseling of specialists is recommended for patients with moderate to serious abnormality of renal function. The surgery of esophageal cancer usually does not directly impact the function of hepatorenal function. However, it is not the case for perioperative medication, blood loss or hypotension. These factors should be monitored postoperatively.
4) Nutritional status The patients with mid-late stage esophageal cancer are usually accompanied by dysphagia,even symptoms of malnutrition, emaciation or dehydration.The change of weight and serum albumin should be noted before surgery. More than 5 kg weight loss is the indicator of poor prognosis, while albumin <30 g/L increase the risk of postoperative anastomosis leakage. If the planned surgery is not emergent, enteral or parenteral support should be given to improve the nutritional status of the patients, finally to minimize the surgery associated complications.
(3) It is the responsibility of thoracic surgeons to decide the possibility and regimen of surgery, to choose suitable approach of surgery based on the location and stage of the tumor, status and accompanying diseases of patients, and surgical skills of the surgeons themselves. The thoracic surgeons should try every effort to achieve complete dissection of the tumor and regional lymph nodes.
(4) Surgical approaches
Left transthoracic esophagogastrectomy: It is reported that for middle to lower segment of esophageal cancer, right transthoracic esophagogastrectomy is superior on lymph node dissection and prognosis. So Ivor Lewis esophagogastrectomy (right thoracotomy and laparotomy) is recommended. However, other retrospective researches found there is no difference on survival and recurrence rate between left and right approaches for patients without upper mediastinal lymph nodes metastasis. As a result, left transthoracic esophagogastrectomy (Sweet esophagogastrectomy) is still a choice for early-mid esophageal cancer located in mid-lower segment of thoracic esophagus without upper mediastinal lymph nodes metastasis.
Right transthoracic esophagogastrectomy: For thoracic esophageal cancer patients with upper mediastinal lymph node metastasis, Ivor Lewis esophagogastrectomy (right thoracotomy laparotomy followed by right thoracotomy)and McKeown esophagogastrectomy (right thoracotomy followed by laparotomy and cervical anastomosis) are the two standard options. Two-field (thoracic-abdominal) or three-field (cervical-thoracic-abdominal) lymph node dissection is recommended.
Transhiatal esophagectomy, THE: This approach is used for esophageal cancer without obvious invasion or mediastinal lymph node metastasis, especially for the aged or patients with impaired cardiac or pulmonary function.The inversion esophagectomy is utilized in the past.Comparing with transthoracic esophagectomy, it is associated with smaller trauma and less cardiopulmonary function loss. However, the operation in the thoracic cavity is not directly visivble that increases the risk of trachea injury or posterior mediastinal bleeding. Moreover, the thoracic lymph node dissection cannot be achieved through this approach. It is now not a routine approach for the majority of the patients with esophageal cancer in China.With the development of minimal invasive technique, some thoracic surgeons are now exploring esophagectomy and mediastinal lymph node dissection by laparoscopy or videoassisted thoracic surgery. This approach can replace the inversion esophagectomy and dissect the mediastinal lymph nodes. Although the surgery procedure is complex and time consuming, the postoperative recovery is faster. It is a choice of the aged or patients with impaired cardiac or pulmonary function who are not candidates of open esophagectomy. The indications of this approach are still exploring.
(5) Choice of surgical approach
Regular transthoracic esophagectomy and minimal invasive esophagectomy (MIE) with lymph node dissection are both standard options. MIE strategies may be associated with less complications, especially respiratory complications.Retrospective researches found the MIE may also associated with somehow better prognosis. For patients with T1-3N0-1M0 esophageal carcinoma, right transthoracic MIE is recommended, especially for those have underwent neoadjuvant radio/chemotherapy.Nevertheless, thoracic surgeons should carefully choose the type of surgery based on the level of centers and their own surgical skills.
(6) Lymph node dissection
Based on the grouping and terminology of International Society for Diseases of Esophagus, Japanese Society of Esophageal Diseases, the regional lymph node of esophagus includes (Figure 2):
Cervical lymph nodes: cervical paraesophageal (right, left)lymph nodes, deep cervical (right, left) lymph nodes,supraclavicular (right, left) lymph nodes.
Thoracic lymph nodes: based on the Chinese version by the society of esophageal tumor, China Anti-Cancer Association:
C201: right recurrence laryngeal nerve nodes (lymph nodes and fat tissues surrounding the right recurrent exercise laryngeal nerve, located between the recurrence of the right vagus nerve and the end of the right subclavicular artery).
C202: left recurrence laryngeal nerve nodes (lymph nodes and fat tissues left to the upper 1/3 of the trachea and superior to the aorta arch).
C203: upper thoracic paraesophageal lymph nodes(lymph nodes surrounding the trachea between the top of the lung and inferior margins of azygus vein arch).
C204: paratracheal lymph nodes (lymph nodes on the right of the trachea, between right recurrence laryngeal nerve nodes and paraesophageal lymph nodes).
C205: subcarinal lymph nodes (lymph nodes located between trachea and bifurcate of left and right main brochus).
C206: middle thoracic paraesophageal lymph nodes(lymph nodes between tracheal bifurcate and inferior margin of the lower pulmonary vein).
C207: lower thoracic paraesophageal lymph nodes(paraesophageal lymph nodes between inferior margin of the lower pulmonary vein and gastroesophageal junction).
C208: inferior pulmonary ligament lymph nodes (lymph nodes in the inferior pulmonary ligament, closely beside the lower pulmonary vein).
C209: paradiaphragmatic lymph nodes (lymph nodes located in the cardiophrenic angle).
Abdominal lymph nodes: paracardial lymph nodes, left gastric artery and lesser curve lymph nodes, celiac axis lymph nodes, splenic artery lymph nodes, and common hepatic lymph nodes.
It is reported that the prognosis of three-field lymph node dissection is superior to two-field lymph node dissection. However, three-field dissection is associated with more complications, especially recurrent laryngeal nerve paralysis, anastomosis leakage, and aspiration pneumonia. Therefore, the en bloc surgery of esophageal cancer should include routine thoracic and abdominal lymph node (two-field) dissection. All the dissected lymph nodes should be sent to pathological analysis with locations marked. It is recommended that at least 15 lymph nodes should be dissected for accurate pN staging and radical resection of the disease. The following lymph node drainage regions should be included in thoracic and abdominal lymph node dissection: thoracic: left and right recurrent laryngeal nerve, upper, middle and lower thoracic paraesophageal, subcarinal, left bronchus, inferior pulmonary ligament, and paradiaphragmatic lymph nodes;abdominal: paracardial left gastric artery and lesser curve,celiac axis, splenic artery, and common hepatic lymph nodes.
To minimize the postoperative complications and fasten recovery, cervical lymph nodes dissection is recommended only for selected patients currently. For example: upper segment of thoracic esophageal cancer, or when cervical lymph nodes metastasis is suspected before surgery, or fast biopsy confirmed metastasis of any recurrent laryngeal nerve lymph nodes (left or right). The area of dissection is shown in Figure 3.
(7) Substitutions
Stomach is the most frequently selected substitution for dissected esophagus. Colon or jejunum are also options for some patients.

Figure 2 Diagram of the Chinese version of naming and grouping of mediastinal lymph nodes in esophageal cancer: “C” represents Chinese nomenclature, and “2” represents thoracic lymph nodes. Station C201, right recurrent laryngeal nerve nodes; Station C202, left recurrent laryngeal nerve nodes; Station C203, upper thoracic paraesophageal lymph nodes; Station C204, right thoracic paratracheal lymph nodes; Station C205, subcarinal lymph nodes; Station C206, middle thoracic paraesophageal lymph nodes; Station C207, lower thoracic paraesophageal lymph nodes; Station C208, inferior pulmonary ligament lymph nodes; Station C209, paradiaphragmatic lymph nodes.
(8) Pathway of substitution
Prevertebral reconstruction is the most common choice.Retrosternal or subcutaneous reconstruction are also selected to leave the esophageal bed for further radiotherapy.
It is reported that the volume of esophageal cancer surgeries is an important factor to impact the morbidity and mortality. We recommend patients to receive surgical treatment in specialized thoracic department or centers with high volume of surgery.
4.2.2 Indication of surgery
(1) Based on International Union Against Cancer(UICC)/AJCC staging system (8th edition): T1aN0M0:mainly recommend EMR or ESD; T1b-3N0-1M0:surgery; T3-4aN1-2M0: recommend neoadjuvant radiochemotherapy or radiotherapy or chemotherapy, then reassess the possibility of surgery; Any T4b or N3 or M1:radical radiotherapy (surgery is NOT recommended)(Figure 4).
(2) Resectable recurrent esophageal cancer after radiotherapy without distal metastasis.
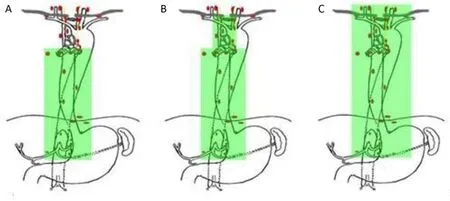
Figure 3 Areas of lymphadenectomy. (A) Incomplete two-field lymphadenectomy (light thoracic approach); (B) Total two-field lymphadenectomy (right thoracic approach); (C) Total three-field lymphadenectomy.
4.2.3 Contraindication of surgery(1) Cachexy;
(2) Any T4b or N3 or M1;(3)Serious malfunctions of heart, lung, liver, brain or kidney, e.g. low PFT, heart failure, MI no more than 6 months, serious cirrhosis or renal malfunction.
4.2.4 Follow-up
For patients with esophageal cancer who have already received surgery, we recommend follow-up every 3 months in the first 1-2 years and every 6 months in the 3rd-5th year, then annually in the following years. The follow-up examinations include history and physical examination, and additional tests based on the clinical conditions, e.g. CBC,biochemistry (hepatorenal function, albumin or tumor markers), endoscopy, upper gastrointestinal contrast and CT. If metastasis is suspected, PET-CT, MRI and bone scan are the further choices for confirmation, and promptly radio/chemotherapy counseling is recommended.
4.3 Radiotherapy
Radiotherapy is a very important component of treatment on esophageal cancer. In China, about 70% of the patients with esophageal cancer are categorized into mid-late stage at the time of diagnosis. These patients are not considered as candidates of immediate radical surgery. However, more than 95% of the esophageal cancers in China are squamous carcinoma that is sensitive to radiotherapy. Therefore,neoadjuvant radiotherapy followed by surgery, or radical chemoradiotherapy, is essential to improve the prognosis of these patients. For resectable esophageal carcinoma,preoperative radiotherapy improves the 5-year survival rate from 33% to 47%. Comparing with radiotherapy alone, the intensity modulated radiation therapy (IMRT) and concurrent radiochemotherapy also increases the 5-year survival from 5% to 15%-20%, for unresectable esophageal carcinoma. As a result, for the mid-late stage esophageal carcinoma, preoperative concurrent radiochemotherapy combined with surgery, or radical concurrent radiochemotherapy is an important treatment option.
4.3.1 Indications for radiotherapy
Radiotherapy is the choice when patients are intolerant to concurrent radiochemotherapy.
(1) Neoadjuvant radiotherapy/concurrent radiochemotherapy: T3-4aN+M0 patients who can tolerate surgery.
Note: If the esophageal cancer is resectable after neoadjuvant radiotherapy, the following surgery is recommended. If not, the following radical radiotherapy is recommended.
(2) Adjuvant radiotherapy/concurrent radiochemotherapy
1) R1 (include circular edge) or R2 resection;
2) For squamous carcinoma, R0 resection, pathologically N+, or T4N0, lymph node capsule involved.
For adenocarcinoma, pathologically N+, or N3-4aN0,or T2N0 lower segment of thoracic esophageal/esophagogastric junction adenocarcinoma with risk factor(s) (e.g.poor differentiation, tumor embolism in vessels, nerve invasion, under 50 years old).

Figure 4 Flowchart of standardized diagnosis for esophagus cancer. CT, computed tomography; EMR, endoscopic mucosal resection;VATS, video-assisted thoracoscopic surgery; LN, lymph node.
Currently, there are no evidence-based reports on the order of postoperative chemotherapy and radiotherapy. In general, after R1 or R2 resection, we recommend postoperative radiotherapy or concurrent radiochemotherapy, then followed by chemotherapy. For squamous carcinoma after R0 resection, we recommend postoperative radiotherapy or concurrent radiochemotherapy, then followed by chemotherapy. For adenocarcinoma after R0 resection, we recommend chemotherapy first, then followed by radiotherapy or concurrent radiochemotherapy.
(3) Radical radiotherapy/concurrent radiochemotherapy
1) T4bN0-3.
2) Cervical esophageal cancer, or cancer located in the junction of cervical and thoracic esophagus but less than 5 cm to cricopharyngeal muscle.
3) Unresectable esophageal cancer even after neoadjuvant radiotherapy.
4) Contraindicated to surgery.
5) Patients with unacceptable risks of surgery, e.g. aged,serious diseases of heart or lungs.
6) Patients refuse surgical treatment.
(4) Palliative radiotherapy
1) Limited regional recurrence after surgery (without preoperative radiotherapy).
2) Widespread multi-station lymph node metastasis.
3) To alleviate symptoms of distant metastasis, e.g. bone or brain metastasis.
4) Primary lesion radiotherapy for late-stage patients with decreased or stable distant metastasis lesions after chemotherapy.
5) To alleviate obstruction and improve the nutritional status for patients with late esophageal carcinoma.
6) To alleviate symptoms caused by compression of lymph nodes metastasis.
4.3.2 Assessment of radiotherapy
We recommend completing all the assessment tests in 2 weeks before radiotherapy.
1) Endoscopy, EUS (pharyngoscope for cervical esophageal cancer).
2) Histological or cytological exam.
3) CT on neck, thorax and abdomen, ultrasound on neck.4) Upper gastrointestinal contrast.
5) PFT.6) EKG.
7) PET-CT (selective).
8) MRI on esophagus (selective).
9) Nutritional status assessment and risk screening.
10) Lab tests: CBC, UA, stool routine test, hepatorenal function, etc.
4.3.3 Principle of radiotherapy
(1) Techniques
We recommend three-dimensional conformal radiation therapy or IMRT. Many researches have confirmed that these two types of radiotherapy are superior to twodimensional radiotherapy on targeted area dose distribution, normal organ protection (especially heart and lung protection), and minimization of radiotherapy associated complications.
(2) CT simulation orientation
The patient is supine, with arms on the side of the trunk, or arms crossed and placed on the forehead. For cervical or upper segment of thoracic esophageal cancer, head-neckshoulders immobilization is recommended. For mid-lower segment of thoracic esophageal cancer or esophagogastric junction cancer, trunk immobilization is recommended.Four-dimensional CT simulation orientation can also be utilized to increase the accuracy of radiotherapy, because the location of the lesion in the esophagus is usually influenced by respiration and movement of the heart. If not contraindicated, CT with contrast is recommended, with 0.5 cm slice thicknesses.
If the length of the esophageal lesion can not be confirmed by CT, reorientation after endoscopic sliver clip mark is needed. The interval between sliver clip placement and reorientation has to be as short as possible, because the silver clips are easy to drop.
For lower segment of thoracic esophageal cancer, or esophagogastric junction cancer, or the targeted area involves left gastric artery/abdominal lymph nodes, in order to minimize the discrepancy of the irritation volume caused by gastric filling, the patients need to fast 3-4 h before CT simulation orientation, and then orally take 200-300 mL semiliquid diets right before the CT scan, or 15 min before each radiotherapy. If the residuals of the stomach located in the mediastinum, the best way to orientation and radiotherapy is keeping the stomach empty.
If the residuals of the stomach locate in the mediastinal,the best way to orientation and radiotherapy is keeping the stomach empty.
(3) Definition of targeted area
A. Neoadjuvant radiotherapy/concurrent radiochemotherapy or radical radiotherapy/concurrent radiochemotherapy
1) Gross tumor volume (GTV) and GTVnd
The visible primary esophageal lesion (on exams) is GTV.Confirmed or suspected metastased lymph nodes are GTVnd. If silver clips are placed, the margins of the GTV depend on the location of the silver clips.
2) Clinical target volume (CTV)
Cervical/upper thoracic segment: the primary tumor (GTV)plus 3-5 cm, or GTVnd plus 0.5-1 cm expansion superiorly and inferiorly along the length of the esophagus.CTV should also cover elective nodal regions such as 1(lower cervical, supraclavicular), 2, 4, 7 lymph node drainage regions.
Middle thoracic segment: GTV plus 3 cm, or GTVnd plus 0.5-1 cm expansion superiorly and inferiorly along the length of the esophagus. It usually needs to cover 1, 2, 4, 7 and part of 8 lymph node drainage regions.
Lower thoracic segment/Siewert I/Siewert II: GTV plus 3-5 cm, or GTVnd plus 0.5-1 cm expansion superiorly and inferiorly along the length of the esophagus and cardia. It usually needs to cover 7, part of 8, cardia, left gastric artery and celiac trunk lymph node drainage regions.
Note: We recommend GTV plus 3 cm expansion superiorly and inferiorly for patients without enlarged lymph nodes are found 3 cm away. If suspected lymph nodes are found in 3-5 cm, 5 cm expansion is recommended. If more distant lymph nodes are suspected,radiation to involved fields should be considered.
3) PTV
CTV plus 0.5 cm expansion. For cervical or upper thoracic segment of esophageal cancer with head-neck-shoulder immobilization, CTV plus 0.3 cm expansion is acceptable.4) PGTV (when sequential or concurrent enhanced radiotherapy) GTV + GTVnd plus 0.5 cm expansion.
B. Adjuvant radiotherapy/concurrent radiochemotherapy
The residual stomach is hard to tolerate radiation. If it is located in the mediastinum (targeted postoperative radiotherapy field), active prophylactic radiotherapy is NOT recommended, unless the resection is R1 or R2. If the residual stomach is located in left or right thoracic cavity, and the patient is indicated to posteoperative radiotherapy, the prophylactic radiotherapy on mediastinal lymph node drainage areas is acceptable.
1) GTV and GTVnd: .
Residual primary tumor after R1 or R2 resection,anastomosis with positive margin, residual metastased or suspected lymph nodes is GTV or GTVnd.
2) CTV
Cervical/upper thoracic segment: including GTV + GTVnd(if exists), anastomosis, 1 (lower cervical, supraclavicular), 2,4, 7 lymph node drainage regions. For cervical esophageal carcinoma, No. 7 drainage area is selective.
Middle thoracic segment: including GTV + GTVnd (if exists), 1, 2, 4, 7 and part of 8 lymph node drainage regions.If the tumor is T4b, the tumor bed should be involved.
Lower thoracic segment/Siewert I/Siewert II: including GTV + GTVnd (if exists), 1, 2, 4, 7, 8, cardia, left gastric artery and celiac trunk lymph node drainage regions. If the tumor is T4b, the tumor bed should be involved.
3) PTV
CTV plus 0.5 cm expansion. For cervical or upper thoracic segment of esophageal cancer with head-neck-shoulder immobilization, CTV plus 0.3 cm expansion is acceptable.4) PGTV (when sequential or concurrent enhanced radiotherapy): GTV + GTVnd plus 0.5 cm expansion.
4.3.4 Dose
(1) Neoadjuvant radiotherapy/concurrent radiochemotherapy or radical radiotherapy/concurrent radiochemotherapy
95% PTV 41.4-50 Gy/1.8-2.0 Gy, once a day, 5 times per week. If possible, concurrent enhanced radiotherapy can also be used.
(2) Adjuvant radiotherapy/concurrent radiochemotherapy 1) After R0 resection: 95% PTV 50-56 Gy/1.8-2.0 Gy,once a day, 5 times per week.
2) After R1/R2 resection: 95% PTV 50 Gy/1.8-2.0 Gy,sequential radiotherapy 95% PGTV 10-14 Gy/1.8-2.0 Gy,once a day, 5 times per week. If possible, concurrent enhanced radiotherapy can also be used.
(3) Radical radiotherapy/concurrent radiochemotherapy
1) 95% PTV 60-64 Gy/1.8-2.0 Gy, once a day, 5 times per week.
2) 95% PTV 50 Gy/1.8-2.0 Gy, sequential radiotherapy 95% PGTV 10-14 Gy/1.8-2.0 Gy, once a day, 5 times per week. If possible, concurrent enhanced radiotherapy can also be used.
4.3.5 Normal tissue tolerance dose-limits
(1) Lung: average dose 14-16 Gy, V5≤60%, V20=30%,V30≤20%. Concurrent radiochemotherapy V20≤28%;
(2) Heart: V30≤40%, V40≤30%;
(3) Cord PRV: Dmax≤45 Gy;
(4) Stomach: V40≤40%, Dmax≤55-60 Gy;
(5) Intestine: V40≤40%, Dmax≤55 Gy;
(6) Kidneys: V20≤30%;
(7) Liver: V30≤30%.
4.3.6 Concurrent chemotherapy regimens and dosing
(1) Paclitaxel and platinum
Paclitaxel 45-60 mg/m2on d 1;
Carboplatin AUC 2 on d 1 (or Nedaplatin 20-25 mg/m2on d 1; or cisplatin 20-25 mg/m2on d 1). Weekly for 5-6 weeks.
Weekly for 5-6 weeks.
(2) Cisplatin and 5-fluoropyrimidine or capecitabine or Tegafur
5-fluoropyrimidine can be replaced by capecitabine or Tegafur because of their better therapeutic effect, less side effects and availability for oral intake.
Cisplatin 30 mg/m2on d 1;
Capecitabine 800 mg/m2, P.O., Bid on d 1-5 (or Tegafur 40-60 mg/m2, P.O. Bid on d 1-5).
Weekly for 5-6 weeks.
(3) Paclitaxel and 5- fluoropyrimidine or capecitabine or Tegafur
Paclitaxel 45-60 mg/m2IV on d 1;
Capecitabine 625-825 mg/m2, P.O., Bid on d 1-5 (or Tegafur 40-60 mg/m2, P.O. Bid on d 1-5).
Weekly for 5-6 weeks.
(4) Oxaliplatin and 5- fluoropyrimidine or capecitabine or Tegafur
Oxaliplatin 85 mg/m2IV on d 1,15,29
Capecitabine 625 mg/m2, P.O., Bid on d 1-5 (or Tegafur 40-60 mg/m2, P.O. Bid on d 1-5).
Weekly for 5-6 weeks.
Pleas also see Appendix G on Assessment of Therapeutic Effect.
4.3.7 Radiotherapy associated complications(1) Malnutrition
Esophageal cancer patients suffer the highest occurrence rate of malnutrition (60%-85%) among all the malignant tumors. The obstruction of food and increasing of basic metabolism are main reasons. Side effects of the radiochemotherapy, such as radioactive esophagitis, acid regurgitation and loss of appetite may also worsen the malnutrition. Nutrition support can improve the nutrition status of patients to tolerate the radiochemotherapy better.It can also help shorten the duration of complications and intervals between different treatments.
1) Assessment of nutrition status
All patients should be assessed for nutrition risk and status,then personalized nutrition support regimen should be decided. The methods of nutritional support include education and enteral or parenteral support. We recommend enteral nutrition support as the first choice.The indications of enteral nutrition support include: more than 5% weight loss in 1 month, body mass index (BMI)<18.5 kg/m2, PG-SGA≥4, intake less than 60% normal daily requirement for consecutively 3-5 days.
2) Enteral nutritional support
We recommend all indicated patients or those with anticipated high nutrition risk during radiotherapy to accept enteral nutrition support [through gastric tube or gastrostomy (gastrostomy not for patients with resectable lesion)]until at least 1-2 week after the end of radiochemotherapy. If the patient can not tolerate radiotherapy because of malnutrition, 1-2 weeks preradiotherapy nutrition support is recommended. Total energy of 25-30 kcal/kg·d is recommended.
(2) Perforation of esophagus
Perforation of esophagus is one of the most common serious complications of esophageal carcinoma. It can occur before, during or after radiotherapy. Xiao et al. reported modality rate of 62% in 3 months and 82% in 6 months once perforation happens in patients with esophageal cancer. Invasion of the tumor and its response to radiotherapy are the two main reasons of perforation. If the tumor shrinks too fast and combined with infection, the impaired tissue repair may cause “retreat” perforation,which includes cancerous and non-cancerous subtypes.About 20%-30% of these perforations are non-cancerous,with much better prognosis.
1) Symptoms
The clinical manifestation of pre-perforation condition usually includes fever, chest pain or back pain, and increased inflammatory indicators. Once the perforation occurs, the chest or back pain may disappear, and drinking may cause choking.
2) Treatment
If any signs of perforation are found before radiotherapy,e.g. specul sign or niche in image tests, antibiotics and strengthened nutrition support are recommended. Patients should drink water after each meal to minimize the residuals of food in esophagus. Oral gentamicin may also be used. For patients already suffering perforation of esophagus, the treatment needs to include stopping radiochemotherapy, nothing by mouth, intravenous antibiotics, anti-acid, placement of gastric tube or gastrostomy, and supplement of protein. Esophageal stent can be considered based on the location of perforation.
Perforation is not an absolute contraindication for radiotherapy. Non-cancerous perforation or small esophagomediatinal fistula may close after intravenous antibiotics and improvement of nutrition status. As a result,the radiotherapy may be continued in these patients.
(3) Radioactive esophagitis
Most of the patients may suffer radioactive esophagitis during radiotherapy (usually begin with 20 Gy). The main manifestations are dysphagia and worsen sensation of food obstruction (Appendix H). If the daily intake is not affected,the patient may take soft or semiliquid food, and drink water. Mid-serious pain impacts oral intake, therefore,intravenous hydration, antibiotics and corticosteroids can be given. Analgesics can be considered to patients without obvious ulcerations.
(4) Response of airway to radiation
The response of airway to radiation is usually manifested by irritating dry cough, worsen during night. However,other reasons that cause cough need to be differentiated,such as upper airway infection or esophageal reflux. Aerosol inhalation is the treatment for most of these patients. It can be given several times per day, 15-20 min each. Ambroxol,impratropium bromide, chymotrypsin, or small dose of corticosteroids can also be given through aerosol inhalation.
(5) Obstruction of esophagus
Edema of regional esophagus during radiotherapy may worsen the existed obstruction, manifested by increased saliva and difficulty in feeding. For patients with placed gastric tube or gastrostomy, no other special treatments are needed. If gastric tube or gastrostomy is not placed, the patients need parental nutrition support, oral intake of liquid nutritious food, or temporary gastric tube placement to secure daily energy intake. Antibiotics and corticosteroids may help relieve edema that may usually automatically improves after 40 Gy dose of radiation.
If the obstruction occurs after radiotherapy, the recurrence of tumor needs to be differentiated first. If the endoscopy excludes the recurrence of tumor, radiation induced fibrosis is considered the reason of the regional stenosis of esophagus. Then endoscopic esophageal dilation can be implemented to improve oral diet intake.
4.3.8 Follow-up after radiotherapy
(1) Follow-up after preoperative radiotherapy
We recommend reassessment of CT, gastrointestinal imaging, ultrasound, endoscopy and lab tests 1 month after the accomplishment of radiotherapy. We also recommend surgery 6-8 weeks after the accomplishment of radiotherapy.
(2) Follow-up after postoperative radiotherapy
After the postoperative radiotherapy, we recommend follow-up of every 3 months for 2 years, then 6 months for the following 3 years, and then annually. The routine tests include CT, gastrointestinal imaging, ultrasound,endoscopy and lab tests. The selective tests include PETCT, bone scan, brain MRI, etc.
(3) Follow-up after radical radiotherapy
We recommend reassessment by CT, gastrointestinal imaging, ultrasound, and lab tests 1-2 months after the accomplishment of radical radiotherapy. If there are any positive findings in image tests or the patient is symptomatic, endoscopy, PET-CT, bone scan or brain MRI should to be scheduled accordingly.
4.4 Medical management
Since most of the esophageal cancers are usually found in locally advanced or late stage, chemotherapy is essential for the treatment of esophageal cancer to control the spread of the tumor. With the emergence of new drugs of molecular targeting therapy and immunotherapy in recent years, the role of medical management is promising in the comprehensive treatment of esophageal cancer.
Currently, the medical management of esophageal cancer includes neoadjuvant chemotherapy and adjuvant chemotherapy for locally advanced patients, and chemotherapy, molecular targeting therapy and immunotherapy for late stage patients.
Some patients may even be benefitted from clinical researches after the failure of current routine treatments.Since no standard regimens can be provided for the medical management of esophageal cancer, we recommend physicians to encourage patients with informed consent to participate clinical researches. Considering the nutrition status of the patients may be influenced by the existence of primary tumor, and the possible complications of esophageal cancer, e.g. bleeding, obstruction or perforation etc., physicians need to pay close attention to the patients’nutrition stats, prophylaxis and treatment of complications through the entire anti-tumor treatment, to sustain or improve the life quality of the patients.
4.4.1 Indications of chemotherapy for esophageal cancer
(1) Neoadjuvant chemotherapy
The benefit of neoadjuvant chemotherapy includes possible down-staging of tumor, elimination of micro metastasis and observation of tumor response to chemotherapy that may help guide the postoperative treatment. Based on current available large randomized controlled clinical studies on esophageal cancer, we recommend neoadjuvant chemotherapy for patients with clinically T3-4N0M0 or T1-3N1-3M0 resectable esophageal cancer to increase the en-bloc (R0) dissection rate and improve overall survival(OS), without increasing postoperative complications. We suggest preoperative radiochemotherapy for patients with esophageal squamous cell carcinoma, because the evidence of neoadjuvant chemotherapy is not persuasive. However,enough evidence has been established to support perioperative chemotherapy for esophageal adenocarcinoma.We recommend neoadjuvant chemotherapy for patients with resectable adenocarcinoma locating in lower segment of esophagus or esophagogastric junction to improve 5-year survival without increasing postoperative mortality and morbidity.
(2) Postoperative adjuvant chemotherapy
Without the support of large randomized controlled clinical researches, it is still controversial whether patients with esophageal squamous carcinoma should accept routine postoperative adjuvant chemotherapy. Based on the results of current prospective stage II and retrospective clinical researches, we recommend 2-3 cycles of postoperative adjuvant chemotherapy for patients with pathologically confirmed regional lymph nodes metastasis (N+).
Evidence on postoperative chemotherapy of esophageal adenocarcinoma originates from the researches on its perioperative chemotherapy. For patients who have accepted radical surgery after preoperative neoadjuvant chemotherapy, the same regimen of chemotherapy can be utilized after surgery.
Adjuvant chemotherapy usually starts 4 weeks after surgery. All recovered patients ready to accept postoperative chemotherapy need to accomplish routine prechemotherapy tests. If the patient is still not recovered from the surgery, the chemotherapy should be delayed accordingly, but no more than 2 months after surgery.
(3) Palliative chemotherapy
If tolerated, we recommend chemotherapy for all patients with metastasized esophageal cancer. If the disease progresses after systemic treatment for these patients,different regimen can be chosen for further chemotherapy.We also recommend chemotherapy for all patients with local recurrence or distant metastasis after radical treatment, if they can tolerate.
4.4.2 Assessment before chemotherapy
(1) Assessment of tumor
Pathological subtype needs to be confirmed by histological or cytological tests. History and physical (H&P) and image tests are needed to understand the location and fields of the tumor involved, and to evaluate the tendency of the disease.Thoracic-abdominal CT or cervical-thoracic-abdominal CT should be done before chemotherapy as baseline for outcome assessment after chemotherapy or long-term follow-up.
(2) Assessment of patient
The patient should be generally in good condition, with ECOG PS 0-1 (see details in Appendix F). CBC,hepatorenal function and EKG should be done no more than 1 week before chemotherapy. There are no obvious abnormalities in heart function, hepatorenal function or hematopoietic function. Chemotherapy can be considered if neutrophil ≥1.5×109/L, platelet ≥80×109/L, and HGB ≥80 g/L.
(3) Assessment of concomitant diseases
The patient should accompany with no serious diseases,such as active digestive tract bleeding, gastrointestinal obstruction, perforation, embolism or shock. If nonneoplastic fever occurs, the temperature needs to be no more than 38 °C. Other concomitant heart, lung or chronic diseases should be checked accordingly, e.g. myocardial enzymes, dynamic electrocardiogram (Holter monitor),ultrasonic cardiogram, brain natriuretic peptide or PFTs.
4.4.3 Regimens and dosing
(1) Cisplatin and 5-fluoropyrimidine
Cisplatin 75-100 mg/m2IV in 4 h on d 1 5-fluoropyrimidine 750-1,000 mg/m2IV drip on d 1-4 Cycled every 21-28 weeks
(2) Paclitaxel and cisplatin
Paclitaxel 135-175 mg/m2IV in 3 h d 1
Cisplatin 75 mg/m2IV d 1
Cycled every 21 d
(3) Paclitaxel and cisplatin
Paclitaxel 90-150 mg/m2IV in 3 h d 1
Cisplatin 50 mg/m2IV d 1
Cycled every 14 d
(4) Epirubicin, cisplatin and 5-fluorouracil (ECF)
Epirubicin 50 mg/m2IV push on d 1
Cisplatin 60 mg/m2IV push on d 1
5-fluorouracil 200 mg/m2IV continuous infusion over 24 h daily on d 1-21
Cycled every 21 d
(5) Epirubicin, oxaliplatin and capecitabine (EOX)
Epirubicin 50 mg/m2IVP d 1 Oxaliplatin 130 mg/m2IV in 2hon d1 capecitabine 625 mg/m2PO BID on d 1-21 Cycled every 21 d
(6) Oxaliplatin, leucovorin and 5-fluorouracil (FLO)
Oxaliplatin 85 mg/m2IV in 2 h on d 1
Leucovorin 200 mg/m2IV in 2h on day 1, then followed by 5-fluorouracil
5-fluorouracil 2,600 mg/m2IV continuous infusion over 24 h daily on d 1
Cycled every 14 d
(7) Docetaxel, cisplatin and 5-fluorouracil (DCF modification
Docetaxel 60 mg/m2IV in 1 h on d 1
Cisplatin 60 mg/m2IV in 1-3 h on d 1
5-fluorouracil 750 mg/m2IV continuous infusion over 24 h daily on d 1-4
Cycled every 21 d
(8) Irinotecan and 5-fluorouracil/leucovorin
Irinotecan 80 mg/m2IV for 30 min on d 1 Leucovorin 500 mg/m2IV in 2 h on d 1
5-fluorouracil 2,000 mg/m2IV continuous infusion for 22 h on d 1
Weekly for 6 weeks followed by 2 weeks off treatment
(9) Irinotecan and 5-fluorouracil/leucovorin
Irinotecan 180 mg/m2IV for 30 min on d 1
Leucovorin 125 mg/m2IV in 15 min on d 1
5-fluorouracil 400 mg/m2IV push for 22 h on d 1
5-fluorouracil 1,200 mg/m2IV continuous infusion over 24 h daily on d 1 and 2
Cycled every 14
4.4.4 Assessment of therapeutic effect
Appendix G.
4.4.5 Prophylaxis and treatment of chemotherapy associated complications
Regular lab tests should be scheduled during chemotherapy based on the characteristics of different regimens.Supportive care should also be given if necessary. Bone marrow suppression, gastrointestinal reactions and hepatorenal malfunction are the most common side effect of chemotherapy.
(1) Bone marrow suppression
We recommend CBC test once or twice per week after chemotherapy. The interval between two CBC tests can be different according to the regimen of chemotherapy, and the results of patients’ CBC test. Chemotherapy should be paused if grade 3-4 leukopenia or neutropenia occurs.Granulocyte colony-stimulating factor (G-CSF) or granulocyte-macrophage colony-stimulating factor (GMCSF) should be given to these patients. The next cycle of chemotherapy may need to be delayed, or the dose of future chemotherapy may need to be adjusted. IL-11 or recombinant human plateletin (TPO) should be given when platelet <50×109/L. If necessary, hemostatic agents should be considered. Prophylactic G-CSF or TPO may also be considered according to the results of patients’ CBC and regimen of the chemotherapy.
(2) Gastrointestinal reactions
Chemotherapy associated nausea and vomiting: usually occur several hours or days after chemotherapy. Single or combined use of 5-HT3 receptor inhibitor, glucocorticoid,and neurokinin-1 receptor inhibitor, etc. are options of treatment. Combination of metoclopramide and diphenhydramine improves the effect of antiemetic, and helps control of extrapyramidal symptoms. Physicians need to closely monitor and correct the disorder of water and electrolytes caused by serious vomiting.
Loss of appetite: especially postoperatively. Since the digestive system has been changed by surgery, nutrition support deserves more attention during chemotherapy.Oral nutrition supplements or appetite increasing agents,such as megestrol acetate, can be given. Enteral nutrition support can be given through placed gastric or jejunal nutrition tube. If necessary, parental nutrition support should be considered.
Diarrhea: the patients should avoid intaking cold water/food, and diet rich in coarse fiber. Timely antidiarrheic agents are needed. The chemotherapy should be paused if the diarrhea lasts more than 5 consecutive days or bloody diarrhea is observed, and dehydration and disorder of electrolytes need to be corrected.
(3) Malfunction of liver and kidneys
The history of hepatitis before chemotherapy is essential.We recommend hepatorenal function test once per cycle of chemotherapy. Once liver damage occurs, the overall hepatic function assessment and liver-protecting agents should be given. For patients with renal insufficiency, the nephrotoxic agents are contraindicated. If the chemotherapy regimen includes cisplatin, enough hydration should be given, and physicians should be attentive to the interactions of different agents.
(4) Neurotoxicity
Before the infusion of Oxaliplatin, the patients should be instructed not to touch any cold objects. Neurotrophic drugs should also be given. The chemotherapy should be stopped if serious neurotoxicity occurs.
(5) Anaphylaxis
Prophylactic glucocorticoids, H2 receptor inhibitor or diphenhydramine may decrease the possibility of anaphylaxis. If the regimen of chemotherapy includes agents with high possibility of anaphylaxis, the patients should be closely monitored in the first 2 h since the infusion of the agent. The infusion should be stopped immediately once anaphylaxis occurs. The emergenct treatment includes epinephrine, glucocorticoids, oxygen inhalation and hyperensorts.
4.4.6 Follow-up after chemotherapy
(1) For patients with resectable esophageal cancer who accept neoadjuvant chemotherapy: we recommend H&P before every cycle of chemotherapy, and image tests after 2-3 cycles of chemotherapy. If the results of above tests indicate progress of the disease, the chemotherapy should be stopped and the resectability of the tumor should be reassessed. For resectable lesions, patients should accept surgery in time.
(2) For patients underwent radical surgery who accept adjuvant chemotherapy: we recommend image tests after the accomplishment of scheduled chemotherapy. If the patient is asystematic or the disease is stable, we recommend follow-up of every 3-6 months for 2 years,then 6-12 months for the following 3 years, then annually.The routine tests include H&P, image tests, and CBC,blood biochemistry or endoscopy, if necessary.
(3) For patients with metastasized esophageal cancer who accept palliative chemotherapy: we recommend image tests after the accomplishment of scheduled chemotherapy because of short median duration of remission. If the patient is asystematic and the disease is stable, we recommend follow-up of every 2 months. The routine tests include H&P, image tests, and CBC, blood biochemistry or endoscopy, if necessary.
4.4.7 Progress in molecular targeted therapy and immunotherapy
Based on results of current clinical research, molecular targeted therapy and immunotherapy are options of second-line or further treatment of metastasized esophageal cancer. They are not currently recommended as part of the routine.
(1) Molecular targeted therapy
Stage III randomized clinical researches have revealed the effect of second-line epithelial growth factor receptortyrosine kinase inhibitor (EGFR-TKI) on metastased esophageal cancer. Comparing with placebo group, the progression-free survival (PFS) of EGFR-TKI group is a little longer, while there is no difference on OS. Further research indicates the patients with EGFR mutation may be potentially benefit from EGFR-TKIs.
In research on EGFR monoclonal antibody combined with chemotherapy versus chemotherapy alone for metastasized esophageal squamous carcinoma and esophagogastric junction adenocarcinoma, no differences were found on median PFS or median OS. Treatment effect of EGFR monoclonal antibody on late-stage esophageal cancer needs further investigation.
(2) Immune check point inhibitors
In recent years, some domestic and international clinical researches have observed inspiring effect of second-line immune check point inhibitors on treatment of metastasized esophageal cancer. KEYNOTE-028 research in Japan recruited 23 cases of PD-1 positive patients to accepted single agent Pembrolizumab treatment. The objective release rate (ORR) is 30%, including 7 cases of partial response (PR), 2 cases of stable disease (SD) and 13 cases of progressive disease (PD) [no complete response(CR)]. One single-arm stage II research from Japan reported the effect and safety of Nivolumab on late-stage esophageal cancer patients who failed to respond to, or were intolerant of fluorouracil/platinum/taxane. In 64 cases of assessable patients, ORR is 17%, with 1 CR case. The median PFS and OS are 1.5 months and 2.3 months,respectively. More researches on treatment of late-stage esophageal cancer by immune check point inhibitors are still ongoing. This treatment may be a second-line choice for the metastasized esophageal cancer in the future.
4.4.8 Supportive and palliative treatment
(1) Nutritional support
Patients with esophageal carcinoma usually also suffer from malnutrition because of obstruction of the esophagus and energy exhaustion from the tumor itself. The malnutrition can further lead to intolerance of anti-tumor therapy,reducing the effect of treatment, or increasing possibility of complications. For esophageal cancer patients with malnutrition, active nutrition support should be given.
Oral formula is the choice of nutrition support for patients who can still eat. For patients with esophageal obstruction, enteral nutrition support can be given after endoscopic stent placement or through nasojejunal tube.For selective patients, gastrostomy can also be considered.
(2) Palliative therapy
The concept of palliative therapy should be kept in mind through entire treatment of all patients with esophageal cancer. To improve the life quality of the patients with esophageal cancer, physicians should provide suitable treatment and/or support on the physical, psychological and social problems of these patients.
The palliative therapy of esophageal cancer includes:pain relieving, guidance of sleep, psychological support,and guidance/education on end-stage patients and their families.
4.5 Principle of early-stage cancer/precancerous disease of esophagus and endoscopic treatment
Early diagnosis and treatment are essential to improve the prognosis of esophageal cancer and patients’ life quality, as well as to reduce personal and national medical burden.Routine screening can help detect precancerous lesions or early esophageal cancer, and prevent the lesion progress to mid-late stage.
4.5.1 High-risk group of esophageal cancer
The incidence rate of esophageal cancer increases with age.99% of patients with esophageal cancer are over 40 years old. Accordingly, the screening of esophageal cancer in China usually begins at 40 years old. Whether screening is necessary for people over 70 years old still need investigation.
Based on the characteristics of China and the risk factors and epidemiology of esophageal cancer, we recommend to consider people matching the following first criteria plus any one of 2nd-6th criteria as high-risk group and should be screened for esophageal cancer:
(1) Over 40 years old;
(2) Live in the area with high incidence rate of esophageal cancer;
(3) With upper digestive tract symptoms;
(4) With family history of esophageal cancer;
(5) With preesophageal cancer diseases or precancerous lesions;
(6) With other high-risk factors of esophageal cancer(smoking, heavy use of alcohol, head and neck or respiratory tract squamous carcinoma).
4.5.2 Methods of screening
(1) Preparation
A. Patients should be abstained from food ≥6 h, NPO ≥2 h.The time NPO should be prolonged for patients with symptoms of partial or full obstruction.
B. The informed content is required. The physicians should explain the procedure to the patients to eliminate the sense of fear. The patients should be told to breath peacefully, without swallowing to avoid unnecessary nausea.
C. The mucous removing agents (e.g. pronase) and defoaming agents (e.g. simethicone) can be orally given to the patients 10-20 min before examination to minimize the mucous and foam in the upper digestive tract, to improve the endoscopic fields and increase the detection of minimal lesions.
D. Orally 5-10 mL 1% dacromine hydrochloride gel, or 1% lidocaine gel, or pharyngeal sprayed anesthesia should be given. IV sedation or anesthesia by anesthesiologists can also be used in selected centers to improve patients’tolerance to endoscopy.
(2) Endoscopy
A. White light endoscopy
The mucous lesion of esophagus includes: 1) red area: with clear edge and flatten base; 2) erosion:is usually red,slightly sunken erosion with clear edge; 3) plaques: usually off-white color, slightly elevated plaques with clear edge; 4)nodules: the nodular lesion with rough or eroded mucous,diameter <1 cm; 5) rough mucous: focal irregular rough mucous, without clear edge; 6) focal mucosal epithelia thickness: usually covers the underlying texture of vessels,presented with derangement, loss or disconnect of mucosal rete vasculosum. Endoscopic physicians should be sensitive to these characteristics of mucous and carefully observe the slight changes of mucous. Multi-focal biopsy is essential to improve the chance of early detection. However, most of early esophageal cancers do not have typical characteristics and may be missed. The combination of chromoendoscopy or electronic staining can help to improve the detection rate.
B. Chromoendoscopy
After dyes are spread on the surface of esophageal mucous,the difference in color between the lesion and normal mucous clarifies the field of lesion, and therefore to help biopsy and improve the detection rate of early esophageal cancer. Iodine solution and toluidine blue are most frequently used alone or in combination during chromoendoscopy.
(a) Iodine solution: the rich glycogen in normal squamous epithelia cells becomes deep brown when iodine is added.However, early-stage cancer and dysplastic hyperplasia tissue contain less or even no glycogen, these areas may be lightly stained or even can not be stained. The color of the stainless areas may variant from light yellow to deep yellow,depends on the grade of dysplasia. In combination with indicated biopsy, the detection rate of early squamous cell cancer or dysplastic hyperplasia may be improved in highrisk group based on color, field and shape of the lesion.Please note iodine solution is contraindicated in patients with hyperthyroidism or who are allergic to iodine.
(b) Toluidine blue: it is an alkaline dye. When binding with acid materials in cells, it turns blue. The cancer cells are easy to be stained to blue due to that these active proliferating cells contain rich nucleic acid, while the stain of normal cells is not obvious because of less genetic material contained. Comparing with iodine solution,toluidine blue stain is not usually used in China because it requires better operation technique, longer time and yields higher false positive rate.
(c) Combined staining: the detection effect of a single agent staining on early esophageal cancer and precancerous lesion is influenced by mechanism and concentration of the dye.Combination staining may overcome the shortcoming of single agent staining. It is reported that the accuracy on detection of early esophageal squamous cell cancer and precancerous lesion by iodine solution - toluidine blue or iodine solution — methylene blue staining is better than single iodine solution staining, and these combined staining methods are valuable in assessment of tumor invasion.
C. Digital chromoendoscopy
By special optical methods, digital chromoendoscopy can display clearer structure of esophageal mucous, shape of microvessels and range of the lesion than white light endoscopy, as well as overcome the defects of chromoendoscopy, such as side effects of dyes and longer time consumption. In addition, digital chromoendoscopy can also switch to white light endoscopy during the operation if necessary.
Narrow band imaging (NBI) has been widely utilized in clinical practice, and its value on diagnosis of early-stage esophageal cancer has been recognized. Researches have reported the advantage of NBI over white light endoscopy on screening of esophageal squamous cell cancer. However,whether the accuracy and specificity of NBI on diagnosis of esophageal squamous cell carcinoma is better than iodine staining still need to be further investiged. The combination of NBI and magnifying endoscopy may help differentiation of lesion from normal mucous, and assessment of tumor invasion by observation of esophageal intrapapillary capillary loops (IPCL) and microstructure of the mucous. It has become an important endoscopic method on early detection of esophageal cancer.
Flexible spectral imaging color enhancement (FICE)resolve white light to different wave bands. By combining as many as 50 kinds of spectrum, the best image of different mucosal lesions can be obtained and IPCL can be clearly demonstrated. It can be an important compensation of iodine staining. I-Scan enhances the color contrast of esophageal mucous, and greatly improves the surface enhancement, contrast and tonal processing.
D. Magnifying endoscopy
When placed on the top of normal endoscope, the amplifying system can magnify the esophageal mucous for tens to hundreds of times, to observe microstructure of the surface of mucous, and the tiny alteration of the mucosal microvascular network. The characteristics of the mucous can be shown more clearly when magnifying endoscopy is combined with digital chromoendoscopy. This combination can not only help the differentiation of the lesion to improve the accuracy of early esophageal cancer detection, but also clearly present the edge of the lesion to guide the choose of treatment.
E. Confocal laser endomicroscopy (CLE)
CLE can magnify the tissue for 1,000 times and permit physicians to observe the cellular and sub-cellular structures microcosmically. The “optical biopsy” can even be achieved by CLE because the lesions can be differentiated histologically without real biopsy. CLE can also provide real-time histological image of early stage esophageal cancer with high accuracy. The time needed for diagnosis is greatly shortened because the biopsy may be omitted. Assessment on the maturity of surface of esophageal squamous epithelia by CLE 3D reconstruction can effectively differentiate squamous intraepithelial neoplasia from non-neoplasia epithelial. The sensitivity is 81% while specificity is over 90%.
F. Autofluorescence imaging
The autofluorescence imaging can transfer the different autofluorescence spectrum from normal tissues and neoplasia into the discrepancy of colors to help the differentiation of the lesion. However, it is not widely used in clinical practice because this examination requires certain equipments and is expensive. The sensitivity and positive predictive value on dysplasia of esophageal squamous epithelium is relatively low.
The meticulous examination of early esophageal cancer should be on basis of the white light endoscopy. Physicians should carefully observe every part of the esophagus. Based on the conditions of medical centers and experience of endoscopy physicians, special techniques such as chromoendoscopy, magnifying endoscopy or confocal endomicroscopy can be also combined to show the endoscopic presentation of early-stage esophageal cancer,therefore to help understand the field, invasion and pathological type of the lesion, and to guide the choice of treatment. Meticulous examination process of early-stage esophageal cancer is shown as Figure 5.
(3) Endoscopic classification and invasion of early-stage esophageal cancer and precancerous lesion
A. Endoscopic classification: according to the Paris classification in 2002 and renewed Paris classification in 2005, superficial esophageal cancer and precancerous lesion(Type 0) are divided into protruded (0-I), flat (0-II) and excavated (0-III) subtypes. The 0-I type includes pedunculated (0-Ip) and sessile (0-Is) subtypes. 0-II type includes elevated (0-IIa), flat (0-IIb) and depressed (0-IIc)subtypes. The threshold of 0-I subtype and 0-IIa subtype is 1.0 mm of elevation (the single cup of openned biopsy forceps is approximately 1.2 mm), while the thresholds of 0-III and 0-IIc is 0.5 mm of depression (half of one cup of biopsy forceps is approximately 0.6 mm). According to the elevation/depression proportion, the lesion simultaneously includes slightly elevation and slightly depression is classified 0-III + IIc and 0-IIc + III subtypes, as shown in Figure 6.
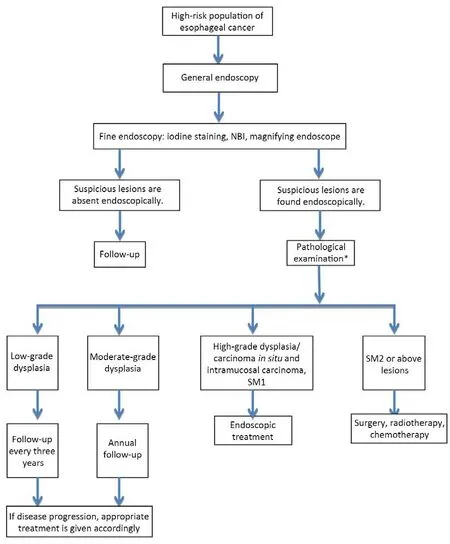
Figure 5 Flowchart of esophageal cancer screening. NBI, narrow band imaging; *, Biopsy pathological examination mainly aims at a certain point, but it has some limitations to reflect the whole picture of lesion. Endoscopic resection for diagnosis can be performed if necessary after careful assessment.
B. Classification of invasion
The lesion limited in epithelium and not involve basement membrane is described as M1 (cancer in situ/severe dysplasia, Tis). The early esophageal cancer includes intramucosal carcinoma and submucosal carcinoma. The intramucosal carcinoma includes M2 (the lesion breaks through basement membrane and invades lamina propria mucosa, LPM) and M3 (the lesion invades into muscularis mucosa, MM) subtypes. According to the depth of invasion,the submucosal carcinoma is classified into SM1 (invades upper 1/3 of submucosa), SM2 (invades middle 1/3 of submucosa) and SM3 (invades lower 1/3 of submucosa).For the endoscopic dissected specimen of esophageal squamous carcinoma, 200 µm thickness is the threshold between superficial and deep layer of submucosa. The risk of lymph node metastasis of these two layers is obviously different.
C. Relationship between shape and invasion of the lesion
The intramucosal carcinoma usually presented as 0-IIb,0-IIa or 0-IIc type, with smooth or regular granular surface. The submucosal carcinoma often presented as 0-I or 0-III type, with irregular rough granular or rugged nodular surface. Physicians can initially predict the depth of invasion by the above described criteria. Chinese researchers classified the pathological features of early esophageal carcinoma into concealed (congestive), eroded,plaque and papillary subtypes. The concealed subtype is usually related to carcinoma in situ. The eroded subtype is mostly related to carcinoma in situ, sometimes to early invasive carcinoma with poor differentiation. Plaque subtype is the most common type. Most carcinomas of this type are well differentiated early invasive carcinoma. The papillary subtype is often related to well-differentiated early-stage invasive carcinoma.
(4) Biopsy
Biopsy should be done if any suspected lesions are found endoscopically. The sample number of biopsy should be determined by the field and size of the lesion. Indicative biopsy by chromoendoscopy or other new endoscopic techniques are recommended. The specimen of the mucosa should be big enough and made to reach muscularis mucosa.
4.5.3 Preoperative assessment of endoscopic treatment on early esophageal cancer
(1) Assessment of extension and invasion of the lesion and lymph node metastasis
Today, endoscopic minimal invasive treatment is recommended for early esophageal cancer without lymph node metastasis. For those with lymph node metastasis, or with distant metastasis, or limited SM2/SM3 esophageal cancer with high-risk of lymph node metastasis, surgery is still the preferred treatment. Therefore, the preoperative assessment of invasion and extent of tumor, and involvement of lymph nodes are essential for selection of reasonable treatment and prognosis prediction. The extent of the lesion mainly is assessed by chromoendoscopy and digital chromoendoscopy, while the depth of the lesion is mainly assessed by endoscopic ultrasound, squamous epithelial IPCL classification and endoscopic feature of the lesion. However, the uniform standard is still not established, and the technique and experience of the operator can easily influence the outcome. The golden standard is still postoperative pathology.

Figure 6 Endoscopic classification of early esophageal cancer (Paris classification, 2005).
EUS: the typical presentation of early esophageal carcinoma in EUS is hypoecho lesion limited in mucosa (do not exceed submucosa). EUS can clearly show the changes of esophageal wall structures, depth of tumor invasion, and the relationship between the lesion and the adjacent organs.Although the accuracy of T staging is as high as 74%-86%,the assessment on the depth of invasion by EUS can be influenced by the size and location of the lesion. On diagnosis of regional lymph node metastasis, the sensitivity of EUS is 80%, obviously higher than CT (50%) and PET(57%). However, the specificity (70%) is a little lower(83% for CT and 85% for PET). The sensitivity and specificity of EUS on diagnosis of abdominal lymph node metastasis in esophageal cancer patients are 85% and 96%,respectively, both higher than CT (42% and 93%). The combination of EUS and FNA can further improve the capability of diagnosis on suspected lymph node metastasis.The distant metastasis can hardly be assessed by EUS.Therefore, CT, MRI or PET-CT should be employed.
This guidelines recommend combination of EUS and CT with contrast to obtain the information of lymph node and distant metastasis for preoperative staging of esophageal carcinoma.
(2) Criteria of pathological classification and principle of clinical treatment
Clinical treatment should be determined based on the endoscopic and pathological diagnosis (refers to the Vienna classification of gastrointestinal epithelial neoplasia) (Table 1).
4.5.4 Endoscopic therapy for early-stage esophageal carcinoma(1) Principle of therapy
Comparing with traditional surgery, endoscopic dissection for early esophageal cancer/precancerous lesion leads to similar prognosis (5-year survival rate reaches as high as 95%), less surgical trauma and complications, faster recovery and less expenses. In principle, patients without lymph node metastasis, or those with extremely low risk of lymph node metastasis, or with low risk of residual/recurrence are candidates for endoscopic dissection. It can be considered as preferred treatment for these patients.
A. Indications for esophageal squamous carcinoma
(a) Absolute indications:
1) T1a esophageal squamous carcinoma limited in epithelium (M1) or lamina propria mucosa (M2), without evidence of lymph node metastasis;2) Precancerous lesions.b) Relative indications:
1) the lesion invades into muscularis mucosa (M3) or superficial submucosa (T1b-SM1, submucosal invasion<200 µm), without evidence of lymph node metastasis;
2) the lesion extends more than 3/4 cycle of the esophagus,high-risk of stenosis after dissection, and havingcontraindications to surgery. The patient should be well informed the risks of endoscopic treatment like esophageal stenosis.

Table 1 Revised Vienna classification of gastrointestinal epithelial neoplasia
B. Indications for esophageal adenocarcinoma
The currently well-admitted indications of endoscopic dissection are:
1) The lesion’s diameter ≤2 cm, can be dissected completely, well or moderately differentiated(pathologically confirmed), the invasion does not exceed superficial submucosa, without evidence of lymph node metastasis.
2) Precancerous lesions.
All the endoscopic dissected specimen should be pathologically tested to decide if further treatment is needed.
C. Contraindications
Absolute contraindications:
1) Lymph node metastasis has been confirmed;
2) If the lesion invades into deep layer of submucosa, the endoscopic dissection would be not radical for a considerable proportion of these patients. Surgery should be preferred treatment for these patients;
3) Intolerant to endoscopic dissection.
Relative contraindications:
1) Positive non-lift sign;
2) Patients with coagulation disorders that not corrected, or those who is taking anti-coagulate agents;
3) Lesion invades to deep layer of submucosa; patients refuse or are not suitable for surgery.
(2) Endoscopic dissection
The methods of endoscopic dissection for early esophageal carcinoma include EMR and ESD. In 1989, Saitoh et al..firstly employed EMR technique on dissection of superficial esophageal squamous cell carcinoma. Hosokawa et al.. designed and utilized insulation-tipped knife on treatment of early digestive tract cancer, which represents the beginning of “ESD time” of endoscopic treatment.ESD makes en bloc dissection of relatively big digestive tract mucosal lesion possible, and extends the indications of endoscopic treatment for early digestive tract cancer and precancerous lesions. Today, esophageal ESD has been matured.
A. EMR
(a) Definition: EMR is endoscopic en bloc or part by part dissection of mucosal lesion. It is a method for diagnosis and treatment of gastrointestinal superficial tumors.
(b) Methods: EMR technique is still developing with innovation of endoscopic equipments and development of endoscopic techniques. New techniques like EMR with a cap (EMRC), EMR with ligation (EMRL), and endoscopy piecemeal mucosal resection (EPMR), etc. have been established on basis of traditional submucosal injectionlifting-dissection method. Different EMR techniques share similar mechanisms that are separating submucosa and muscularis propria by submucosal injection, and then dissecting the regional lifted mucosal lesion.
EMRC aspirates the lesion by a transparent cap on top of the endoscope, and then dissects the lesion. It is easy to operate with less complications, however the range of dissectable mucosa is limited by the size of the cap. The procedure of EMRC is shown in Figure 7. EMRL ligates the lesion to block the blood flow first, and then dissects the lesion after the formation of sub-pedicle. EMRL results in clear field and less bleeding. EPMR is employed for big lesions that cannot be completely dissected by single traditional EMR. This technique dissects the lesion part by part and is suitable for lesion >2 cm. However, the part by part dissected specimen can hardly be jointed together to evaluate the outcome of dissection. Residual lesions or recurrence can occur.
(c) Outcome: The international researches report EMR eliminates 57.9%-78.3% of T1a esophageal carcinomas and precancerous lesions. The rate of en bloc dissection is 46%-78.6% and the 5-year survival rate reaches 95%.Chinese researchers also reported en bloc dissection rate of EMR for early esophageal cancer and precancerous lesions is 44.1%-84.5%, and complete resection rate is 44.8%-100%.
B. Multi-band mucosectomy (MBM)
MBM is a modified multi-mucosa dissection technique on basis of esophageal varices ligator. The main process includes marking, capped dissection and treatment of the wound (Figure 8).
Comparing with EMR, MBM obviously shortens the operation time because submucosal injection is not needed.In addition, MBM is effective, safe, easy to operate and inexpensive. The endoscopic physician should follow the standard operation process to avoid residual of the lesion.
C. ESD
(a) Definition: ESD is a method to completely dissect the diseased mucosa and submucosa for lesions of various location, size and depth of invasion. In ESD, the tissue between mucosa and muscularis propria is separated by a special electric scalpel after submucosa injection.
(b) Procedure: 1) marking the lesion; 2) submucosal injection to lift the lesion; 3) partial or circled dissection of the mucosa; 4) submucosal decollement to completely separate the mucosa and propria muscularis, then completely remove the lesion once for all; 5) treatment of the wound: includes treating the vessels of the wound and check-up of the lesion margin (Figure 9). The Chinese researchers modified the classical ESD and invented channeled mucosal decollement (mark-injection-distal opening-proximal dissection-establishment of the channeldissect from both sides). This technique is an effective method to treat esophageal lesions with large areas. It simplifies the procedure, shortens operation time and makes endoscopic operation safer and faster.
Figure 7 Endoscopic mucosal resection with a cap (EMRC) procedure. (A) Rough and erosive esophageal mucosa is showed endoscopically.Biopsy result reveals high-grade dysplasia; (B) The lesion is dark brown under narrow band imaging (NBI) mode; (C) Positive iodine staining; (D) After marking; (E) After submucosa injection; (F) Endoscopic mucosal resection with transparent cap method; (G) Wound surface after resection; (H) Re-iodine staining after resection. Positive lesions are absent around artificial ulceration; (I) Resected specimen.
(c) Therapeutic effect: ESD is widely utilized for treatment of early esophageal carcinoma in Japan, but it is not the case in the United States. In Europe, the case of ESD is increasing in recent years. The en bloc dissection rate of ESD for treatment of esophageal squamous cell carcinoma is 93%-100%, and the R0 dissection rate is over 88%. In China, the en bloc dissection rate is 80%-100%, and the R0 dissection rate is 74%-100%. The average operation time is 40-95 min.
(3) Indications and contraindications
The endoscopic dissection mainly treats the esophageal lesions with low risk of lymph node metastasis and those can be completely dissected. In China, uniform indications for endoscopic dissection has not been established yet.Since the incidence rate of esophageal cancer, and proportion of esophageal squamous carcinoma is low in Europe and North America, and their utilization of endoscopic dissection is different from China, the endoscopic dissection for treatment of early esophageal cancer in China usually consults the Japanese guideline.

Figure 8 Multi-band mucosectomy (MBM) procedure. (A) Esophageal mucosal erosion is shown on white-light endoscopy; (B) Narrow band imaging (NBI) image of the lesion; (C) Positive iodine staining of the lesion; (D) The range of the lesion is marked; (E) The lesion is removed with a snare; (F) Wound surface after resection; (G) All of the resected specimens are pieced together into the probable shape.
The esophageal cancer practice guidelines 2012 edited by the Japan Esophageal Society: absolute indications of endoscopic dissection for early esophageal cancer: T1a lesion limited in epithelium or mucosa lamina propria, with extremely low risk of lymph node metastasis, can be radically treated by endoscopic dissection. Relative indications of endoscopic dissection: the lesion invades into muscularis mucosa (M3) or superficial submucosa(T1b-SM1, depth of invasion <200 µm). If the depth of invasion is more than 200 µm submucosally, the possibility of lymph node metastasis is high, and radical treatment can hardly be achieved by endoscopic dissection.
Most of the data regarding indications of endoscopic treatment are from the researches outside China. It is reported that some non-indicated patients still present with good prognosis. The indications of endoscopic treatment still need to be confirmed by multicenter researches in China.
(4) Complications
Although it is a minimal invasive treatment, endoscopic dissection can still cause complications because of the influence of equipment, technique, experience of the operator and patients’ status. The most common complications are bleeding, perforation, postoperative esophageal stenosis and infection.

Figure 9 Endoscopic submucosal dissection (ESD) procedure. (A) The white light endoscopy shows that the esophageal mucosa of the lesion was rough, and the dendrite vascular network disappeared; (B) White light magnifying endoscopy for intrapapillary capillary loops(IPCL) classification of lesion; (C) Narrow band imaging (NBI) was used to observe the IPCL classification of the lesion, which was IV type(Inoue Haruyo classification); (D) Positive iodine staining; (E) Endoscopic ultrasonography reveals that the lesions are mainly located in the mucosal layer; (F) Observe the boundaries of lesion carefully; (G) after marking; (H) A mucosal defect is made after submucosal injection; (I)Remove the lesion completely and examine the wound surface carefully; (J) The resected specimens.
A. Bleeding: Intraoperative bleeding refers to the local wound bleeding that need hemostatic therapy. Delayed postoperative bleeding presents with hematemesis and melena in 30 d after the operation, and Hb drops more than 20 g/L.
Incidence rate and risk factors: Researches from China reported the incidence of bleeding in EMR is 1.52%-11.7% intraoperatively and 0-7.04% postoperatively, while 22.9%-59.6% and 0-4.88%, respectively in ESD. Researches from other countries also reported incidence of EMR associated bleeding is 2%, and bleeding is more common during ESD than EMR, with less than 1% delayed postoperative bleeding. Bleeding in EMR is associated with the size of the lesion. Lesions >2 cm increase the risk of bleeding. Hybrid electric dissection associates with more intraoperative bleeding, while coagulation current dissection associates with more postoperative bleeding. The possibility of ESD-associated bleeding may be influenced by the location, size, type and adhesion of the lesion, layer of the dissection, distribution of vessels, and experience of the operator.
Principle of treatment on bleeding: intraoperative bleeding is the most common type. For small amount of bleeding, endoscopic spray of adrenalin saline is effective.For massive bleeding, endoscopic submucosal adrenalin saline injection, clamps by hot biopsy forceps, APC, and clipping of the bleeding site are the choices. Postoperative bleeding is relatively rare. Conservative treatment is usually enough for patients with stable hemodynamics. If the hemodynamics is still unstable after supportive care,emergent endoscopic electrocoagulation or clipping is needed. Surgery is rarely needed. Prophylaxis is essential for prevention of intraoperative bleeding, because most of these bleeding is caused by damage of submucosal vessels during the operation. The methods of intraoperative bleeding include submucosal injection of adrenalin saline to constrict the vessels, and electrocoagulation by hot biopsy forceps for risky vessels. Careful treatment of the wound and pre-coagulation to visible vessels can help minimize the possibility of postoperative bleeding. Postoperative hemostatics and antacids can also be used for prophylaxis.
B. Perforation: Perforation seldom occurs in EMR;however, it is not rare in ESD. Intraoperative perforation can usually be detected in time. Postoperative perforation should be considered if physical examinations find subcutaneous emphysema around chest and neck, or chest X-ray/CT reveals mediastinal air.
Incidence rate and risk factors: as reported by foreign researches, the incidence of perforation in EMR is less than 2%, while 2%-10% in ESD. The Chinese researches reported less than 6.3% incidence of perforation in EMR and 0-11.5% in ESD. The perforation in ESD is associated with experience of operator, location and extent of the lesion, and the existence of ulcer besides the lesion.Utilization of CO2gas during operation and prophylactic clipping of damaged muscular layer help decrease the incidence of perforation while exposure of wounded muscular layer may increase the possibility of perforation.Massive gas accumulation in the digestive tract may cause break of the small muscular layer wound to form perforation. Therefore the gas in the digestive tract should be exhausted promptly during the operation. Strict adherence to indications of endoscopic dissection, sufficient submucosal injection, and suitable equipments may also help prevent perforation.
Principle of treatment on perforation: If the perforation is detected during the operation, injection of gas and water should be minimized during the rest of the operation.Clipping should be employed after dissection. Perforations usually are healed by postoperative NPO, gastrointestinal decompression, intravenous broad-spectrum antibiotics,and supportive care. Surgery may be needed when endoscopic clipping is failed, or the perforation is too big to be treated endoscopically. If pneumothorax occurs,negative pressure drainage should be placed timely. Latent perforation usually healed after supportive care.
C. Esophageal stenosis: It refers to the stenosis of the esophageal lumen after endoscopic dissection that needs endoscopic treatment. This kind of stenosis usually occurs in 1 month after operation and presents with various degree of dysphagia.
Incidence rate and risk factors: the incidence of esophageal stenosis is mainly influenced by the extent,depth of invasion of the lesion, and circumferential ratio and longitudinal length of the wound. Dissection exceeding 3/4 circle of esophagus and tumor invasion exceeding M2 are independent risk factors of postoperative stenosis. More than 3/4 circle of esophageal lesion dissection increases the incidence of stenosis after endoscopic dissection to 88%-100%.
Principle of treatment on esophageal stenosis:endoscopic esophageal dilation is the most common treatment. Most of the stenosis can be alleviated by several times of dilation treatment. Prophylactic dilation can also decrease the chance of stenosis for patients with risk factors. Stent placement is choice for refractory cases,however complications such as pain, granulation growth and esophageal ulceration are still exist. Some of the stents even cannot be taken out. Recent researches report prophylactic placement of covered stents can effectively decrease the incidence of postoperative stenosis in patients underwent nearly full circle ESD dissection. The longterm outcome of biodegradable stents is not as expected because of the decreasing braced force and dislocation during degradation. Oral administration or submucosal injection of glucocorticoids is essential for prophylaxis of stenosis, to decrease the degree of stenosis and needs of dilation. Nevertheless, the best regimen has not been widely admitted. Current regimens include: glucocorticoid injection: injecting triamcinolone acetonide (dilute to 5 mg/mL) into the residual submucosa after ESD. The injection usually begins at the margin of the ulcer, linear,from distal to proximal end. 20-40 points need to be injected, with 0.5-1 mL each. The total dose is 100 mg.Multiple glucocorticoids injection is also reported for the prophylaxis of stenosis, that is, injection of betamethasone to the residual submucosa after ESD. 8-20 points were needed, and the total dose is 4-8 mg. The injection should be given once or twice a week until the complete epithelization of the wound. The local injection of glucocorticoid should not reach muscular layer to avoid delayed perforation. Oral intake of glucocorticoid includes long-term (high-dose) and short-term (low-dose) regimens.Long-term (high dose) oral prednisolone regimen begins on the 3rd day postoperatively at starting dose of 30 mg/d for 2 weeks, and then followed by tapering the dose as: 25 mg/d for 2 weeks, 20 mg/d for 1 week, 15 mg/d for 1 week,10 mg/d for 1 week, 5 mg/d for 1 week; totally 1,120 mg/8 weeks. Short-term (low dose) oral prednisolone begins on the 2nd day postoperatively at starting dose of 30 mg/d for 1 week, and then followed by tapering the dose as: 20 mg/d for 1 week, 10 mg/d for 1 week; totally 420 mg/3 weeks.Regenerative medical techniques like cell patch are still under investigation.
(5) Endoscopic treatment other than resection
Radiofrequency ablation (RFA) utilizes the heat effect of electromagnetic wave to lead dehydration and coagulation necrosis of the tumor. It is special advantageous for multiple, long lesions, or early esophageal cancer or precancerous lesion that involves full circumference of esophagus. The depth of the therapy can be controlled around 1,000 µm to decrease incidence of peroration and postoperative stenosis. RFA is reported to be utilized for type IIb lesion, pathologically confirmed esophageal squamous epithelial moderate and/or severe dysplastic hyperplasia, and moderate-well differentiated squamous cell carcinoma limited in M2 layer. The 12-month postoperative CR rate of the suitable candidates of RFA is as high as 97%. However, researches with large sample size are lack on the therapeutic effect of RFA on early flatten esophageal squamous cell carcinoma. Further evidence is still needed for long-term outcome of RFA. Circumferential ablation system is usually used to treat multiple,long or circled lesions. The process of the treatment includes recording location of the ablation, measuring inner diameter of esophagus and ablation with placed ablation catheter. According to the conditions of the lesion and the first ablation, second ablation can be considered after the clearance of the ablated mucosa (Figure 10,11).Local ablation system is usually used on the treatment of local or postoperative remnant lesions, without measurement of the diameter of esophagus.
Other endoscopic non-resection treatments include photodynamic therapy (PDT), APC, laser therapy, thermal probe therapy, and cryotherapy. These techniques can be utilized solely or in combination with endoscopic resection.PDT utilizes special laser to provoke the selectively gathered photosensitizer around the tumor to produce singlet oxygen that leads to tumor necrosis through complex physics, chemical and immune mechanism. PDT can be used on the treatment of large area multiple early lesions. The physicians should take care of the complications such as photosensitive reaction and postoperative perforation. APC is a non-contact thermocoagulation method. It can effectively treat precancerous lesion of esophagus while the indication on treatment of early esophageal cancer should be strictly controlled. The non-resection treatment causes the destruction of the lesion which leads to non-available tissue specimen for precise pathological assessment. In addition,whether the tumor is completely removed cannot be assessed in APC. Close follow-up is needed, and long-term therapeutic outcome still needs further confirmation.
4.5.5 Follow-up for high-risk group and patients underwent endoscopic therapy
The follow-up requirement for patients with mild dysplastic hyperplasia is every 3 years, and for patients with moderate dysplastic hyperplasia is once a year. The followup after endoscopic resection should be at postoperative 3,6 and 12 months, and then annual endoscopy if no recurrence found. Combination of staining and/or magnifying endoscopy during follow-up is needed. Biopsy and pathological assessment should be done if positive or suspected lesions are found. In addition, tumor markers and related image tests should not be ignored, and physicians should be alert about multiple primary esophageal squamous cell carcinoma and second primary cancer (e.g. head and neck squamous cell carcinoma, gastric cancer, etc.)

Figure 10 Flowchart of endoscopic therapy process for early stage esophageal cancer. EP, epithelium; LPM, lamina propria mucosa; ESD,endoscopic submucosal dissection; EMR, endoscopic mucosal resection; MBM, multiband mucosectomy; RFA, radiofrequency ablation.
Prophylaxis and treatment of recurrence: physicians should carefully observe the wound after resection of the lesion. Staining or NBI may be utilized if necessary.Endoscopic re-treatment helps decrease recurrence rate if remnant lesion is found. The remnant or recurrent lesions can usually be removed endoscopically. Further surgery or radiochemotherapy can be added to patients with failed endoscopic treatment.

Figure 11 Radiofrequency ablation (RFA) procedure. (A) Esophageal squamous epithelium with high-grade dysplasia is about 4 cm long and nearly circumferential; (B) Narrow band imaging (NBI) image of the lesion; (C) Positive iodine staining; (D, E) The proximal and distal end of treatment range are marked; (E) RFA balloon is positioned at the position of proximal mark (6 o’clock position); (F) Appearance of mucosa after first RFA; (G) Appearance of mucosa after removal of the ablated lesion; (H, I) Appearance of proximal (H) and distal (I)mucosa after the second RFA.
4.6 Treatment model for esophageal cancer
Surgery-centered comprehensive treatment is the mainstay of therapy for esophageal cancer, and personalized comprehensive treatment regimen should be determined by MDT (including specialists on surgery, radiation therapy,chemotherapy and endoscopic treatment) after staging of esophageal cancer. The below described is UICC/AJCC staging (8th edition).
4.6.1 Stage I (T1N0M0)
If the stage Ia lesion is suitable for endoscopic therapy,ESD or EMR is the preferred treatment. If the length of the lesion >3 cm, or extent >3/4 circumference, or invasion into submucosa, or lymph node metastasis is suspected,surgery is recommended. Surgery is the preferred treatment for stage Ib lesions. If the patient is not willing to accept surgery, or with cardiopulmonary malfunction,endoscopic ESD plus postoperative radiotherapy is the choice. Postoperative adjuvant therapy usually does not need for completely resected (R0 resection) stage I esophageal cancer.
4.6.2 Stage Ib, stage II and part of stage IIIa (T1b-3N0M0,T1-2N1M0)
Surgery is preferred. Radical radiochemotherapy is the choice for patients with cardiopulmonary malfunction or who do not willing to accept surgery. We do not recommend postoperative adjuvant radiotherapy or chemotherapy for completely resected (R0 resection)T2-3N0M0 esophageal squamous carcinoma or T2N0M0 esophageal adenocarcinoma. For pathological T4N0 or T1-4N1-3M0 esophageal squamous carcinoma underwent R0 resection, postoperative adjuvant radiotherapy or chemotherapy can be considered. Fluorouracil-based postoperative radiochemotherapy is the choice for R0 resected T3N0M0 and T1-2N1M0, or R1/R2 resected esophageal adenocarcinoma.
4.6.3 Stage III (T3N1M0, T4N0-1M0)
For T1-3N1-2M0 and some resectable T4aN0-1M0 esophageal cancer, preoperative neoadjuvant radiochemotherapy/radiotherapy/chemotherapy and posttherapy reassessment are recommended.
Preoperative radiotherapy increases the resection rate of out-invaded tumor which is hard to be removed completely by surgery, while preoperative radiochemotherapy improves the OS of esophageal squamous cell carcinoma.Therefore, for resectable tumors over T3 or with lymph node metastasis, preoperative adjuvant radiochemotherapy should be considered. Routine postoperative chemotherapy is not recommended for esophageal squamous cell carcinoma without lymph node metastasis after R0 resection. Fluorouracil-based postoperative radiochemotherapy is the choice for patients with esophageal adenocarcinoma who underwent R0 resection and those who underwent R1, R2 resection.
For stage III patients unsuitable for surgery, current routine treatment is concurrent radiochemotherapy.
4.6.4 Stage IV (any T, any N, M1, N3 or T4b)
The main treatment is radical radiochemotherapy/radiotherapy/chemotherapy. If the patients cannot tolerate the above treatments, palliative and supportive care should be provided. The destination of the treatment is to extend life and improve life quality.
Palliative treatment includes endoscopic treatment(esophageal dilation, stent, etc.), pain relieving,symptomatic treatment and nutrition support.
4.7 Traditional Chinese Medicine
As an important adjuvant method in treatment of esophageal cancer, traditional Chinese medicine therapy may help decease postoperative complications and alleviate the side effects of radiotherapy and chemotherapy. It can be an adjuvant therapy for elderly patients or those who cannot tolerate the western medicine treatment.
For early detected precancerous lesions of esophagus (i.e.esophageal ulcer and esophagitis, esophageal leukoplasia,epithelial dysplastic hyperplasia, cicatricial stricture of esophagus, etc.), traditional Chinese medicine therapy combined with diet and life model adjustment may delay the incidence of tumors.
 Chinese Journal of Cancer Research2019年2期
Chinese Journal of Cancer Research2019年2期
- Chinese Journal of Cancer Research的其它文章
- Nomograms based on HPV load for predicting survival in cervical squamous cell carcinoma: An observational study with a longterm follow-up
- Efficacy of endoscopic treatment on patients with severe dysplasia/carcinoma in situ of esophageal squamous cell carcinoma: A prospective cohort study
- Histogram analysis of apparent diffusion coefficient predicts response to radiofrequency ablation in hepatocellular carcinoma
- Alisol B 23-acetate-induced HepG2 hepatoma cell death through mTOR signaling-initiated G1 cell cycle arrest and apoptosis: A quantitative proteomic study
- Sequential therapy according to distinct disease progression patterns in advanced ALK-positive non-small-cell lung cancer after crizotinib treatment
- Synthesis and evaluation of 64Cu-radiolabeled NOTA-cetuximab(64Cu-NOTA-C225) for immuno-PET imaging of EGFR expression
