Superior intestinal integrity and limited microbial translocation are associated with lower immune activation in SIVmac239-infected northern pig-tailed macaques(Macaca leonina)
Ming-Xu Zhang,Tian-Zhang Song,Hong-Yi Zheng,Xue-Hui Wang,3,Ying Lu,2,Han-Dan Zhang,Ting Li,Wei Pang,Yong-Tang Zheng,4,*
1 Key Laboratory of Animal Models and Human Disease Mechanisms of the Chinese Academy of Sciences/Key Laboratory of Bioactive Peptides of Yunnan Province,National Kunming High Level Biosafety Research Center for Non-human Primates,KIZ-CUHK Joint Laboratory of Bioresources and Molecular Research in Common Diseases,Kunming Institute of Zoology,Chinese Academy of Sciences,Kunming Yunnan 650223,China
2 Kunming College of Life Science,University of Chinese Academy of Sciences,Kunming Yunnan 650204,China
3 School of Life Sciences,University of Science and Technology of China,Hefei Anhui 230026,China
4 KIZ-SU Joint Laboratory of Animal Models and Drug Development,College of Pharmaceutical Sciences,Soochow University,Suzhou Jiangsu 215123,China
ABSTRACT Microbial translocation is a cause of systemic immune activation in HIV/SIV infection. In the present study, we found a lower CD8+ T cell activation level in Macaca leonina (northern pigtailed macaques, NPMs) than in Macaca mulatta(Chinese rhesus macaques, ChRMs) during SIVmac239 infection. Furthermore, the levels of plasma LPS-binding protein and soluble CD14 in NPMs were lower than those in ChRMs.Compared with ChRMs, SIV-infected NPMs had lower Chiu scores, representing relatively normal intestinal mucosa.In addition,no obvious damage to the ileum or colon epithelial barrier was observed in either infected or uninfected NPMs,which differed to that found in ChRMs. Furthermore, no significant microbial translocation (Escherichia coli) was detected in the colon or ileum of infected or uninfected NPMs, which again differed to that observed in ChRMs.In conclusion,NPMs retained superior intestinal integrity and limited microbial translocation during SIV infection, which may contribute to their lower immune activation compared with ChRMs.
Keywords: Immune activation; Intestinal integrity;Microbial translocation; SIVmac239; Macaca leonina; Northern pig-tailed macaques; Macaca mulatta;Chinese rhesus macaques
INTRODUCTION
Immune activation is a hallmark of HIV/SIV infection and is closely correlated with disease progression (Boasso &Shearer,2008;Hazenberg et al.,2003).In addition,T cell activation is more predictive of AIDS disease progression than plasma viral load and CD4+T cell count(Giorgi et al.,1999;Liu et al., 1998). Immune activation may accelerate the depletion or impede the reconstitution of CD4+T cells by exacerbating lymphoid tissue fibrosis(Estes et al.,2008;Hunt et al.,2003;Zeng et al.,2011).Furthermore,a number of non-AIDS diseases are also correlated with immune activation,such as cardiovascular disease and cancer (Cadogan &Dalgleish, 2008; Kaplan et al., 2011; Klatt et al., 2013).However,immune activation is difficult to alleviate,even in patients with sustained viral suppression during antiretroviral therapy(French et al.,2009).Therefore,therapeutic strategies to relieve persistent immune activation are needed for patients to improve their quality of life.
Microbial translocation is a significant factor that contributes to persistent immune activation (Brenchley et al., 2006;Sandler&Douek,2012).HIV/SIV infection can cause severe mucosal CD4+T cell depletion and damage to intestinal integrity,followed by translocation of microbial products into circulation,including 16S rDNA and lipopolysaccharide(LPS)(Brenchley et al.,2004;Dillon et al.,2012;Marchetti et al.,2013;Veazey et al.,1998).As a result,soluble CD14(sCD14)released by activated monocytes and LPS-binding protein(LBP)levels increase(Brenchley et al.,2006).Plasma sCD14 levels can predict mortality in HIV infection(Sandler et al.,2011). In addition, elevated microbial translocation is associated with increased risk of AIDS-related lymphoma and HIV-associated dementia(Ancuta et al.,2008;Marks et al.,2013). Therefore, therapeutic strategies targeting microbial products and their downstream effects will be of great benefit.
Pig-tailed macaques are divided into three species based on morphological characteristics(Spartaco,2001).Thereinto,Macaca nemestrina (southern pig-tailed macaques, SPMs)are suitable animal models to study the relationship between microbial translocation and disease progression of AIDS due to their compromised intestinal integrity and increased microbial translocation prior to SIV infection (Klatt et al.,2010).Our recent studies indicate,however,that pre-infection peripheral CD4+T cell counts in Macaca leonina(northern pigtailed macaques, NPMs) differ from those in Macaca nemestrina (Klatt et al., 2012; Zheng et al., 2014b). In addition,CD4+T cells also exhibit superior homeostasis in SIVmac239-infected NPMs compared with Chinese rhesus macaques (ChRMs), which is not reported in SIV-infected SPMs(Zhang et al.,2017a).This raises the question as to whether compromised intestinal integrity and increased microbial translocation occur in pre-and post-infected NPMs similarly.
In the present study,we analyzed the levels of CD8+T cell activation and plasma biomarkers of microbial translocation in SIVmac239-infected NPMs and ChRMs and normal NPMs.To further analyze the status of microbial translocation,intestinal integrity and microbial translocation of NPMs were also studied.
MATERIALS AND METHODS
Animals and sample collection
Viral seroprevalence in NPMs and ChRMs enrolled in this study was investigated as described previously(Zhang et al.,2016b).All NPMs and ChRMs were healthy males and were seronegative for SIV,STLV,SRV,and SFV prior to the study.Six NPMs and three ChRMs(6-8 years old)were inoculated with a 3 000 50%tissue culture infectious dose(TCID50)of SIVmac239 intravenously. Blood was collected periodically,and tissues were obtained from the macaques at necropsy.Tissue and blood samples from four uninfected NPMs were also obtained as the control. In addition, blood from five uninfected ChRMs and tissues from one SIVmac239-infected ChRM were also obtained.Blood was partially used for flow cytometry analyses and isolation of plasma and peripheral blood mononuclear cells(PBMCs).All tissues were partially preserved in 4%paraformaldehyde and-80°C immediately after collection. Detailed information on animals used in histopathological assessment is shown in Table 1.
All animal experiments and procedures were approved by the Ethics Committee of the Kunming Institute of Zoology,Chinese Academy of Sciences(approval No.:SYDW-2015023;approval date:20 June 2015).

Table 1 Animals enrolled in this study
Flow cytometry
Multiparameter flow cytometry was performed using peripheral blood,as described previously(Ma et al.,2012;Xia et al.,2009;Zhang et al.,2017b;Zheng et al.,2014a).For the frequency of HLA-DR+CD8+T cells,blood was treated with lysing buffer(BD Biosciences,CA,USA)for 10 min and then incubated with a mixture of flow cytometry antibodies at 4°C for 30 min.For staining with ki67,surface-labeled cells were further treated with fixation and permeabilization solution(BD Biosciences,CA,USA),followed by perm/wash buffer(BD Biosciences,CA,USA).Cross-reactive flow cytometry human antibodies anti-CD3 APC-Cy7(clone SP34-2),anti-CD8 PECy7(clone RPA-T8),anti-HLA-DR APC(clone G46-6),and anti-ki67 PE (B56) were purchased from BD Pharmingen(Franklin Lakes, NJ, USA). Anti-CD4 PerCP-Cy5.5 (clone OKT4)was obtained from Biolegend(San Diego,CA,USA).Flow cytometry acquisition was performed on a BD FACSVerseTMflow cytometer(BD,Franklin Lakes,NJ,USA)and flow cytometric data analysis was performed using FlowJo vX.0.7(TreeStar,Ashland,OR,USA).
Enzyme-linked immuno sorbent assay(ELISA)
Plasma used for ELISA was isolated from blood by centrifugation(room temperature,500 g,10 min).Plasma was preserved at-80°C until detection.Plasma sCD14,LBP and IFABP levels were detected using commercially available ELISA kits obtained from R&D Systems(Minneapolis,MN,USA).
Histopathological assessment
Intestinal tissues fixed in 4% paraformaldehyde were dehydrated in a graded ethanol series and then embedded in paraffin.We collected 4 μm thick paraffin sections,which were stained with hematoxylin and eosin(H&E).Intestinal injury was evaluated using the Chiu score system following the grading principles described by Chiu et al.(1970).
Immunofluorescence
Immunofluorescence was performed as described previously(Zhang et al., 2016a). The 4 μm thick paraffin sections mentioned above were baked at 60 °C for 2 h before dewaxing in xylene and rehydrating through a graded ethanol series.The sections were then washed in flowing water for more than 15 min.Antigen retrieval was performed using the high-pressure method. The sections were then naturally cooled to room temperature and washed in flowing water for 5 min.For immunofluorescence,sections were treated with 1%sodium borohydride solution for 30 min and washed in phosphate buffered saline. After treatment with blocking buffer,sections were incubated with primary and secondary antibodies.The sections were then treated with Sudan Black for 20 min and washed with 50%ethanol.Mounting medium containing DAPI was used to adhere a coverglass to the microscope slide at last.Cross-reactive antibodies,including rabbit anti-claudin-3 antibody (polyclonal), mouse anticytokeratin antibody(clone MNF116),donkey anti-rabbit IgG(Alexa Fluor®488, polyclonal), and donkey anti-mouse IgG(Alexa Fluor®555,polyclonal),were purchased from Abcam(Cambridgeshire, UK). Rabbit anti-E. coli antibody was purchased from Dako (Glostrup, Denmark). Microscopic examinations were performed on a Leica DMI4000B Microsystem (Leica Microsystems, Wetzlar, Germany). All slides were inspected, with photographs taken using the system.
Statistical analysis
Two-way ANOVA was used to compare frequencies of HLADR+CD8+T cells,ki67+CD8+T cells and plasma I-FABP levels between NPMs and ChRMs.The Mann-Whitney nonparametric test was used to compare pre-infection plasma sCD14,LBP and I-FABP levels and Chiu scores.Spearman’s rank test was used to determine correlations between CD8+T cell activation and plasma sCD14, LBP and I-FABP levels in NPMs and ChRMs during SIVmac239 infection. All data analyses were performed using GraphPad Prism v6.01(GraphPad Software,San Diego,CA,USA).
RESULTS
NPMs have lower frequencies of activated and proliferating CD8+T cells than ChRMs during SIVmac239 infection
T cell activation, especially that of CD8+T cells, is more closely correlated with disease progression than plasma viral load or CD4+T cell count during HIV-1 infection(Giorgi et al.,1999;Liu et al.,1998).In our previous study,we also found a close relationship between T cell activation and disease progression in SIVmac239-infected NPMs (Zhang et al.,2017b). Therefore, here we compared the frequencies of activated and proliferating CD8+T cells in NPMs and ChRMs.The infection was divided into an acute and chronic phase at 12 weeks post infection(Figure 1).The frequencies of HLADR+CD8+T cells and ki67+CD8+T cells in NPMs were both lower than those in ChRMs,regardless of acute or chronic phase, which indicated that CD8+T cell activation and proliferation levels of NPMs were lower than those of ChRMs during SIVmac239 infection(Figure 1A,B).
NPMs have lower levels of plasma sCD14 and LBP than ChRMs during SIVmac239 infection
As microbial translocation is an important cause of immune activation, we further analyzed the plasma biomarkers of microbial translocation,including sCD14 and LBP(Figure 2).We found no significant differences in pre-infection plasma sCD14 and LBP levels between NPMs and ChRMs(Figure 2A). These results implied that there was no obvious difference in microbial translocation between NPMs and ChRMs in the absence of SIV infection.Plasma sCD14 levels in NPMs were lower than in those ChRMs during SIVmac239 infection, which indicated that SIVmac239 infection might cause more severe microbial translocation in ChRMs than in NPMs(Figure 2B).However,there was no obvious difference in LBP levels between SIVmac239-infected NPMs and ChRMs(Figure 2C).Furthermore,plasma sCD14 levels were directly correlated with frequencies of HLA-DR+CD8+T cells in both NPMs and ChRMs,which implied that higher sCD14 levels might contribute to higher CD8+T activation(Figure 2D). In contrast, plasma LBP levels were not directly correlated with the frequencies of HLA-DR+CD8+T cells in either NPMs or ChRMs(Figure 2E).Thus,not all microbial translocation-related products appear to be closely associated with immune activation.
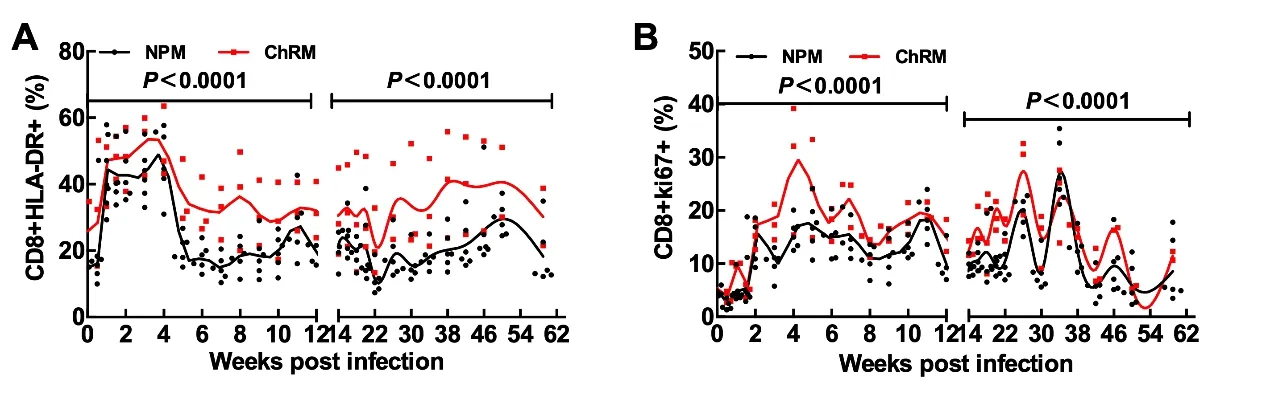
Figure 1 Comparisons of CD8+T cell activation and proliferation between NPMs and ChRMs
Intestinal mucosal injuries in ChRMs were more severe than in NPMs post SIVmac239 infection
Microbial translocation mainly occurs in the intestine;thus,we focused on mucosal injuries in the colon and ileum. The normal NPMs had relatively intact intestinal mucosa,whereas the SIVmac239-infected ChRMs and NPMs presented varying degrees of damage(Figure 3A).We evaluated the severity of mucosal injury using the Chiu score system (Chiu et al.,1970), which indicated that colon mucosal injuries in SIVmac239-infected ChRMs were more severe than those in SIVmac239-infected NPMs, though there was no obvious difference in the Chiu score of the ileum mucosa(Figure 3B,C). In addition, colon and ileum mucosal injuries in SIVmac239-infected NPMs were more severe than those in the normal group. These results indicate that SIVmac239 infection can damage intestinal mucosa and NPMs may be less affected than ChRMs.
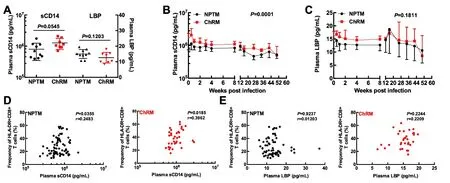
Figure 2 Plasma microbial translocation biomarker levels and their correlations with frequencies of CD8+HLA-DR+T cells
NPMs maintained superior intestinal epithelial integrity post SIVmac239 infection
Tight junctions between the intestinal epithelium play an important role in preventing translocation of gut microbe products from the lumen into the subepithelial region via the paracellular pathway (Milatz et al., 2010). Therefore, we detected the expression of tight junction protein claudin-3,which reflects the integrity of the intestinal epithelium.The intestinal epithelium in SIVmac239-infected ChRMs showed severe discontinuities in both the colon and ileum.In contrast,however, the intestinal epithelium of SIVmac239-infected NPMs showed superior continuities, as did normal NPMs(Figure 4A).These results indicate that SIVmac239 infection may not severely affect the expression of tight junction protein claudin-3 in NPMs.
Limited microbial translocation levels in SIVmac239-infected NPMs
Although SIVmac239-infected NPMs maintained superior intestinal epithelial integrity, whether the relatively intact intestinal epithelium was concomitant with lower microbial translocation was unclear.Therefore,we selected Escherichia coli as a representative microbial product and analyzed its infiltration into the intestinal epithelium (represented by cytokeratin).As expected,translocation of E.coli through the intestinal epithelium was obvious in SIVmac239-infected ChRMs, but rare in SIVmac239-infected or normal NPMs(Figure 4B).These results are consistent with the expression of the tight junction protein claudin-3.In summary,NPMs had limited microbial translocation due to superior intestinal epithelial integrity.
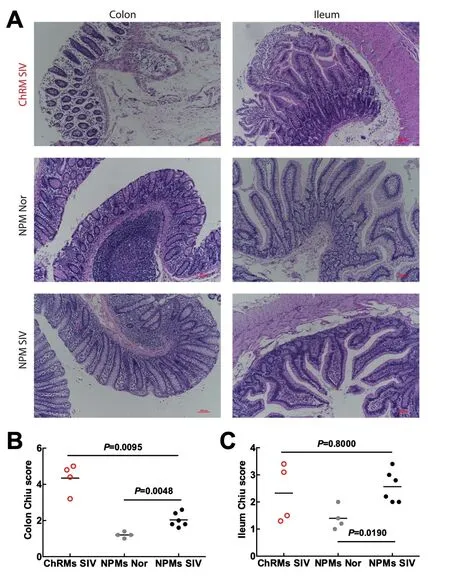
Figure 3 Intestinal mucosal injuries in the colon and ileum

Figure 4 Tight junctions and translocation of microbial products(E.coli)into intestinal lamina propria in the colon and ileum
DISCUSSION
Microbial translocation is associated with systemic immune activation during HIV/SIV infection,which further accelerates disease progression(Brenchley et al.,2006;Canary et al.,2013;Marchetti et al.,2011).
The pig-tailed macaque is an Old-World monkey known to be susceptible to HIV-1 infection(Agy et al.,1992;Liao et al.,2007). We previously identified a novel TRIM5-cyclophilin fusion gene(TRIMCyp)in the pig-tailed macaque that did not inhibit HIV-1 or SIV replication,thus explaining why the pigtailed macaque is prone to HIV-1 infection(Kuang et al.,2009;Liao et al.,2007).Recently,three NPM virus models,i.e.,HIV-1NL4.3,HIV-1R3A,and stHIV-1sv,were established in our lab and a long-term viral reservoir was detected in these NPMs,which indicated that NPMs might serve as a potential model for HIV-1 reservoir research(Pang et al.,2017,2018).In addition,we previously reported on various biological parameters of NPMs(Lian et al.,2016,2017,2018;Song et al.,2018;Zhang et al.,2014,2016b,2016c;Zheng et al.,2014b;Zhu et al.,2015),thus promoting the application of NPMs in AIDS and other biomedical research.SPMs are appropriate research models due to their compromised intestinal integrity and increased microbial translocation in the absence of SIV infection(Canary et al.,2013;Klatt et al.,2010).In the present study,however,we found lower levels of CD8+T cell activation in SIVmac239-infected NPMs than in ChRMs,which was partly attributed to superior intestinal integrity and limited microbial translocation.These results imply that the intestinal microenvironment of NPMs may differ from that of SPMs.Thus,further study on the differences and potential mechanisms may provide new insight into the therapeutic strategies of microbial translocation during HIV infection.
Several plasma biomarkers are commonly selected to evaluate the level of microbial translocation,including LPS,sCD14, LBP, and EndoCAb (endotoxin-core antibody)(Brenchley et al.,2006;Marchetti et al.,2013).In contrast to uninfected individuals, HIV-infected progressors have significantly higher levels of plasma sCD14 and LBP and significantly lower levels of EndoCAb(Brenchley et al.,2006).In the present study, we also observed direct correlations between plasma sCD14 levels and CD8+T cell activation in SIVmac239-infected NPMs and ChRMs,which implied that microbial translocation was closely associated with immune activation(Figure 2D).However,plasma biomarkers do not consistently reflect the real level of microbial translocation and limitations do exist(Marchetti et al.,2013).Here,we did not observe direct correlations between plasma LBP levels and CD8+T cell activation in either NPMs or ChRMs(Figure 2E).Previous research has demonstrated that gut commensal microbes are not the main contributors to circulating CD4+T cell activation(Zimmermann et al.,2015).Furthermore,CD8+T cells may be activated in an antigen-independent manner that is not associated with the level of microbial translocationrelated products (Bastidas et al., 2014). Therefore, the relationship between certain plasma microbial translocation biomarkers and immune activation needs further confirmation.
Histopathological assessment provides the most direct evidence of intestinal mucosal injuries.In the present study,superior intestinal integrity was only coincident with lower plasma sCD14 levels in NPMs.These results indicate that compromised intestinal integrity may cause severe microbial translocation,followed by systemic immune activation,which accelerates disease progression. Furthermore, microbial translocation resulting from breakdown of intestinal mucosa may result in microbial product translocation into draining or distant lymph nodes, followed by persistent local immune activation(Estes et al.,2010).Therefore,intestinal mucosal damage appears to be the fundamental cause of microbial translocation and subsequent immune activation.
To assess the level of intestinal injury,fatty acid-binding proteins are often used as plasma biomarkers of tissue injury(Pelsers et al.,2003,2005).In the present study,we selected intestinal-type fatty acid-binding protein (I-FABP) as the plasma biomarker of intestinal injury,as described elsewhere(Jenabian et al.,2015;Pelsers et al.,2003).As the plasma IFABP levels in most individuals were below the lower limit,we did not find any significant differences in plasma I-FABP levels between NPMs and ChRMs either prior to or post infection(Figure 5A,B).Further study showed that plasma I-FABP levels were directly associated with CD8+T cell activation in NPMs, but not in ChRMs (Figure 5C, D). In summary,although the plasma I-FABP level was correlated with immune activation to some extent,I-FABP may not fully reflect the level of intestinal injury or microbial translocation.

Figure 5 Plasma I-FABP levels and their corrections with CD8+T cell activation
There are some limitations in the present study.First,it is difficult to obtain absolutely accurate results using such a small number of animals.Given the differences between each macaque, more experimental animals would improve accuracy. In addition, infection time varies between individuals,which may also affect pathological consequences.
In the present study, we characterized the relationship between immune activation and microbial translocation in SIVmac239-infected NPMs for the first time. Intriguingly,NPMs maintained superior intestinal integrity and limited microbial translocation after SIV infection,which was different from that observed in ChRMs and SPMs.How NPM intestinal mucosa avoids damage from SIV infection is still an enigma that needs to be resolved.Research on intestinal mucosal immunity in NPMs is likely to provide guidance for therapeutic strategies of microbial translocation in HIV-1-infected patients.In addition,although pig-tailed macaques are susceptible to HIV-1 infection,how HIV-1 affects regional immunity in the intestinal tract of pig-tailed macaques is still unclear(Kuang et al.,2009;Liao et al.,2007;Pang et al.,2017).Studies on this problem may provide deeper insight into the mechanisms of microbial translocation during HIV-1 infection.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS CONTRIBUTIONS
Y.T.Z.and M.X.Z.conceived and designed the experiments.M.X.Z.,T.Z.S.,H.Y.Z.,X.H.W.,Y.L.,H.D.Z.,T.L.and W.P.performed the experiments.M.X.Z.analyzed the data.M.X.Z.and Y.T.Z.wrote the paper.All authors read and approved the final version of the manuscript.
ACKNOWLEDGEMENTS
We thank Jin Jiang,Yu Xiao,Xiao-Dong Lian,Ren-Rong Tian,Jia-Hao Song, and Min Chen from the Kunming Institute of Zoology, Chinese Academy of Sciences,for their assistance with the experiments.
- Zoological Research的其它文章
- From our roots,we grow Celebrating the 60th anniversary of the Kunming Institute of Zoology,Chinese Academy of Sciences
- Genomic insights into ruminant evolution:from past to future prospects
- Antimicrobial peptides:new hope in the war against multidrug resistance
- Chromosomal level assembly and population sequencing of the Chinese tree shrew genome
- Conserved sequences identify the closest living relatives of primates
- Systematic relationships of Chinese freshwater semisulcospirids(Gastropoda,Cerithioidea)revealed by mitochondrial sequences

