Hepatotoxicity associated with Garcinia cambogia:A case report
Muhammad Nadeem Yousaf,Fizah S Chaudhary,Seyed Mohammad Hodanazari,Charmian D Sittambalam
Muhammad Nadeem Yousaf,Seyed Mohammad Hodanazari,Charmian D Sittambalam,Department of Medicine,Medstar Union Memorial Hospital,Baltimore,MD 21218,United States
Muhammad Nadeem Yousaf,Seyed Mohammad Hodanazari,Charmian D Sittambalam,Department of Medicine,MedStar Good Samaritan Hospital,Baltimore,MD 21239,United States
Muhammad Nadeem Yousaf,Charmian D Sittambalam,Department of Medicine,Medstar Franklin Square Medical Center,Baltimore,MD 21137,United States
Fizah S Chaudhary,Department of Internal Medicine,American University of Barbados,Wildey 11100,Barbados
Abstract
Key words:Hepatotoxicity;Drug induced liver injury;Acute liver failure;Herbal supplements;Garcinia cambogia;Obesity;Case report
INTRODUCTION
In the United States,the prevalence of obesity is 39.8%,which is even higher among individuals aged 40 to 59 years old(42.8%)[1].Individuals are using various modalities for weight loss including lifestyle modifications,pharmacologic,and surgical approaches.Herbal supplements(HS)have become a common method for weight loss due to accessibility without prescriptions,relatively low cost,and false perception of safety as widely advertised in the ads.Currently,there is lack of tight regulation of HS by the United States Food and Drug Administration(FDA)which raises a concern for safety.Every year millions of American use over-the-counter herbal products and most of them are unaware of the potential harmful effects of these products.Among these individuals,58% failed to report use of HS to their primary care providers[2].Since they are often not viewed as medications with potential side effects,usage of these HS may be missed because patients may not report their use unless specifically asked about these products.The United States Drug Induced Liver Injury Network(DILIN)noted increasing rates of hepatotoxicity due to HS in the past 10 years,ranging from 2%-16% of all reported liver injuries[3,4].
Garcinia cambogia(GC),a widely available “natural” HS,is found within a tropical fruit,commonly found in South Asia.Its extract is frequently used for weight loss and has been extensively marketed as such for the past decade.Herein we report a case of hepatotoxicity associated with use of the extract of GC.
CASE PRESENTATION
Chief complaints
A 21-year-old African American female with noted obesity(basic metabolic index 40.34 kg/m2),without significant past medical history,presented with abdominal pain for 1 wk.
History of present illness
Her abdominal pain was described as 7 out of 10 on a pain scale,diffuse,and nonradiating.It was associated with nausea,multiple episodes of non-biliary and nonbloody vomiting,anorexia,and myalgias.She denied any jaundice,pruritis,change in bowel habits,urinary symptoms,or extremity swelling.There was no history of fever,sick contacts,or recent blood transfusions.
History of past illness
There was no significant past medical illness.
Personal and family history
She denied smoking tobacco,drinking alcohol,usage of illicit drugs,hormonal contraceptives,or energy drinks.She mentioned that she was taking a HS,GC(1400 mg daily),for weight loss since 4 wks.Family history was unremarkable.
Physical examination upon admission
Vital signs were notable for tachycardia(133 bpm).On examination,she had epigastric and right upper quadrant tenderness,without jaundice or hepatosplenomegaly.
Laboratory workup
Laboratory workup(Table 1)revealed elevated alanine aminotransferase(ALT)981 U/L,aspartate aminotransferase(AST)1062 U/L,alkaline phosphate 248 U/L,international normalized ratio(INR)1.6,prothrombin time 19 s,and ammonia level 44 μmol/L.Acetaminophen and alcohol levels were negative,as was her urine toxicology.Testing for hepatitis A,hepatitis B,hepatitis C,human immunodeficiency virus,herpes simplex virus,cytomegalovirus,Epstein Barr virus,parvovirus,and rapid plasma regain were negative.Autoimmune work-up including antinuclear antibody,antimitochondrial antibody,and anti-smooth muscle antibody were also negative.Serologies for alpha-1 antitrypsin,ceruloplasmin,iron studies,alpha fetoprotein,and carcinoembryonic antigen were unremarkable.
Imaging examination
Abdominal ultrasound showed hepatosplenomegaly with heterogenous increased echogenicity compatible with fatty liver.Abdominal computer tomography(CT)scan showed hepatosplenomegaly with heterogeneous-appearing liver.
FINAL DIAGNOSIS
The final diagnosis of presented case is acute liver failure associated with GC.
TREATMENT
GC was stopped,and she was provided supportive care at the liver transplant center.
OUTCOME AND FOLLOW-UP
Patient’s symptoms resolved,and liver enzymes improved gradually(Figure 1)by day 7(ALT 125 U/L,AST 46 U/L,alkaline phosphate 248 U/L).Her liver function test returned to her baseline at 42 days follow-up from discharge.
DISCUSSION
Herbal and dietary supplements are the second most common cause of drug-induced liver injury(DILI),after antibiotic therapy,in the United States[5].Americans spend an estimated $66 billion annually on weight loss products[6].Approximately 10% of obese population are using over-the-counter weight loss products in the Unites States[7].HS are increasingly used for weight loss in the past decade,as these products are easily available over the counter and considered natural supplements without potential side effects.GC is one of the HS which is increasingly being used in the United States for weight loss.It contains hydroxycitric acid which is considered to be a “magical ingredient” responsible for weight loss.It affects the metabolism of citric acid cycle and inhibits thede novosynthesis of fatty acid[8].
“Hydroxycut” is a weight loss supplement which was commonly used for weight loss about a decade ago.GC was one of the active ingredients in Hydroxycut supplement.In April 2009,the FDA reported 23 cases of severe hepatotoxicity attributed to Hydroxycut[9]and issued a public warning in May 2009 causing Hydroxycut product to be recalled by its manufacturer.A reformulated form of Hydroxycut without GC extract was manufactured and reissued within the market for weight loss.Since May 2009,multiple case reports have identified the causal relationship of GC with severe hepatotoxicity(Table 2)[7,10-16].These case reports reinforce the potential toxic effects of GC contributing to hepatotoxicity.
Due to multitude of ingredients in the supplement formulations,it is difficult to establish correlation of hepatotoxicity with GC.The exact mechanism by which it causes liver failure is unclear.A rodent study revealed that GC may exacerbate steatohepatitis by increasing hepatic collagen accumulation,lipid peroxidation,oxygen free radical injury,and levels of proinflammatory cytokines like tumor necrosis factor-alpha and monocyte chemoattractant protein-1[17].The pattern of liver injury caused by GC was noted to be hepatocellular and cholestatic in most of the case reports(Table 2).The most common symptoms of presentation are nausea,vomiting,abdominal pain,anorexia,jaundice,fatigue and generalized myalgias.The duration of GC use before onset of symptoms was ranged from 7 to 28 days however,it was found to be 2 days and 150 days in two case reports,respectively.In most patients,there was an improvement of symptoms and liver function with stopping GC and providing supportive care.Liver transplantation was required in 3 patients.In our case,the patient developed acute liver failure within 4 wks after starting GC.DILI is diagnosis of exclusion of other possible etiologies of acute liver failure,as was investigated in this patient.

Table 1 Laboratory testing done to investigate acute liver failure etiology
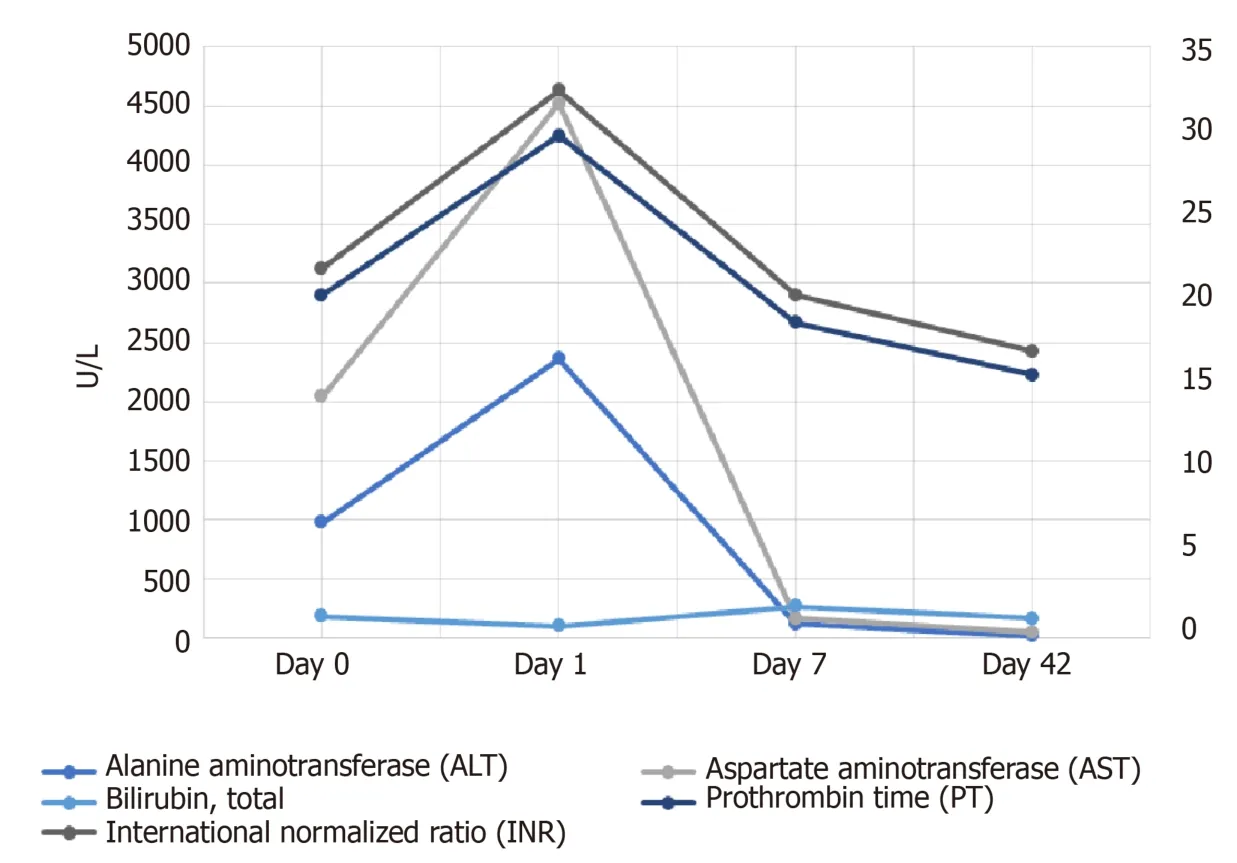
Figure 1 Trends of liver function test,prothrombin and international normalized ratio from day 0 to day 42.
To reduce the chances of overdiagnosis or misdiagnosis related to GC,The Council of International Organizations of Medical Sciences(CIOMS)and Roussel Uclaf Causality Assessment Method(RUCAM)scale is the “most commonly used scoring system to establish the etiology of DILI”(Table 3)[18].The “CIOMS/RUCAM scale”grades DILI into definitive(score > 8),probable(score 6-8),possible(score 3-5),unlikely(score 1-2),or excluded(scores<0).In this patient,a score of 9 was found and indicated acute liver failure secondary to use of herbal supplements.We excluded other possible etiologies of acute liver failure.Improvement in the patient’s symptoms and liver function with discontinuation of GC also indicated correlation of hepatotoxicity with GC.
CONCLUSION
Early recognition and discontinuation of GC can prevent progression of drug-induced liver failure to fulminant hepatic failure and the potential need for liver transplantation if not investigated and stopped rather quickly.Therefore,a medication reconciliation of both prescribed and over-the-counter supplements are prudent on an ongoing basis.Ingredients of herbal and dietary supplements should be regulated by FDA for adverse health consequences and safety profile;however,this may prove to be a daunting task given the number of HS that are on the market and continue to be developed.Further clinical trials are needed to recognize the association between GC and hepatotoxicity and whether this ingredient needs to be closely regulated,given its high propensity for detrimental and potentially fatal complications.

Table 2 Case reports of hepatotoxicity related to non-Hydroxycut formulation of Garcinia cambogia since 2009
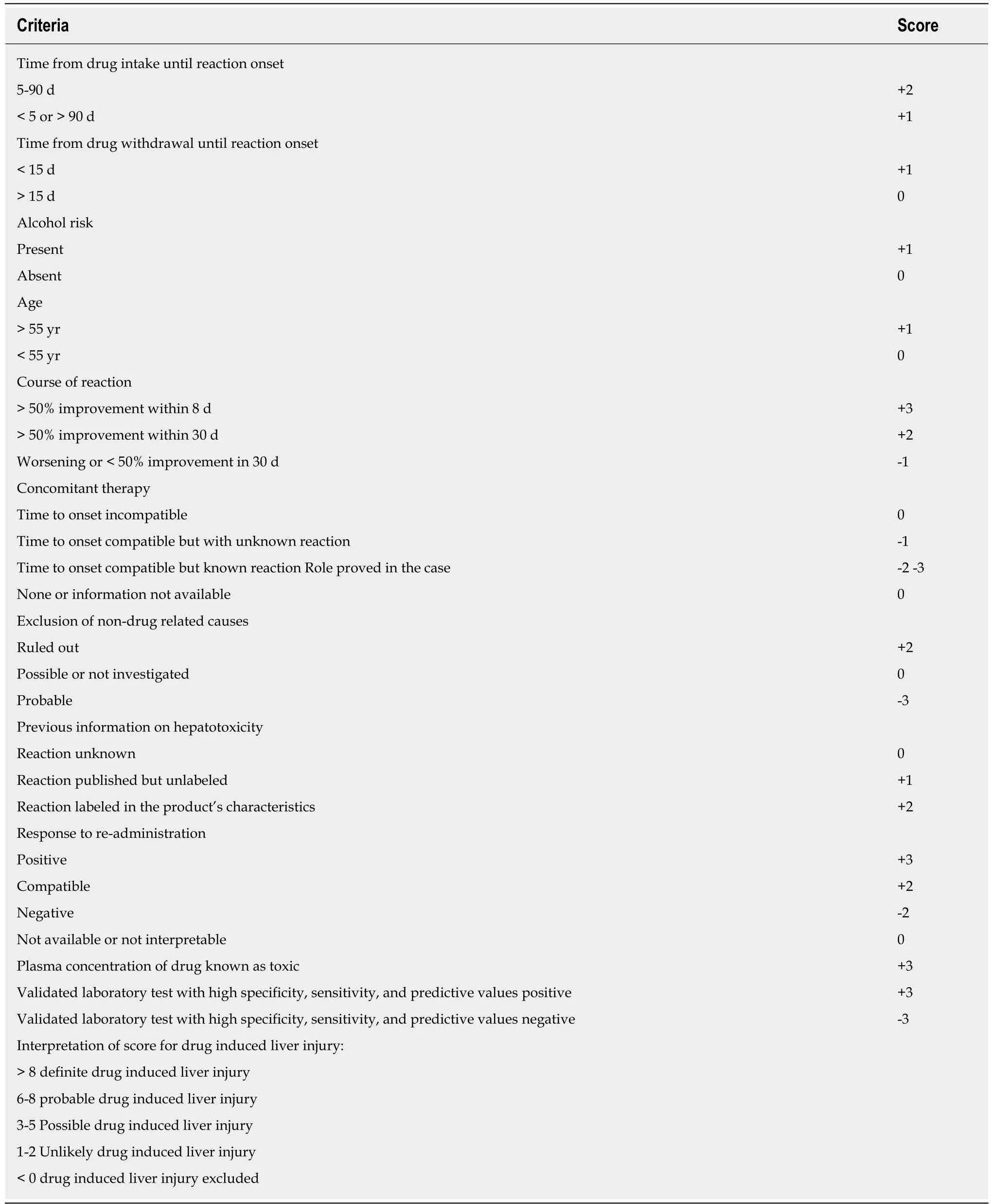
Table 3 The Council of International Organizations of Medical Sciences and Roussel Uclaf Causality Assessment Method Scale

