The f irst mitogenome of the Nile puff erf ish Tetraodon lineatus from Lake Turkana in East Africa: new insights into the genus*
CAO Liang SONG Xuelin ZHANG E
1 Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan 430072, China
2 University of Chinese Academy of Sciences, Beijing 100049, China
3 Sino-Africa Joint Research Center, Chinese Academy of Sciences, Wuhan 430074, China
Abstract A complete mitogenome of T. lineatus from Lake Turkana in the Kenyan part was determined.It had a length of 16 470 bp, including 37 genes as found in teleosts with the typical gene order in these f ishes. Mitogenomic comparison and phylogenetic analysis supported not only the morphology-based recognition of the puff erf ish specimen from Lake Turkana as the Nile puff erf ish, but also the identif ication of the Chinese specimen recently recorded as T. lineatus and its African source. The mitochondrial genome here amplif ied for the sample of T. lineatus from Lake Turkana also provides conclusive molecular evidence for the monophyletic nature of Tetraodon s.s., as it serves as a reference genome of this species used to clarify ambiguity in its identif ication in previous molecular studies.
Keyword: Tetraodon lineatus; Kenya; mtDNA; phylogenetic analysis
1 INTRODUCTION
The family Tetraodontidae, also popularly known as puff erf ish, is the most speciose group of the order Tetraodontiformes and is widespread across many tropical and subtropical regions of the world. It includes species that are typically marine, with occasional entering and occurring in brackish and freshwaters (Nelson, 2006). Puff erf ishes are notable for their conspicuous defensive behavior: when threatened, they inf late the body to a volume nearly four times larger than normal size (Brainerd, 1994).These f ishes are also remarkable for being the smallest genomes among vertebrates, approximately 400 MB or 1/8 the size of the human genome (Hinegardner and Rosen, 1972). Thus, two puff erf ish species,namelyDichotomycterenigroviridisandTakifugu rubripes, have been selected as model organisms to study the evolution of vertebrate genomes (Brenner et al., 1993; Crnogorac-Jurcevic et al., 1997).
The genusTetraodonLinnaeus, 1758, as traditionally conceived, is a group of puff erf ish widely occurring in tropical African and Asian regions where they are dwellers of marine and freshwater systems,particularly in estuaries and inshore waters (Kottelat,2013). A total of about 24 puff erf ish species have been designated to this genus. From Kottelat’s (2013) point of view,Tetraodons.s. is an endemic African genus including six valid species:T.duboisi,T.lineatus,T.mbu,T.miurus,T.pustulatus, andT.schoutede.Among them,T.lineatusis the most widespread,ranging from the Senegal and Volta River basins to the Niger and Nile River basins as far as to Lake Turkana, whileT.pustulatusis conf ined to the Cross River basin in southeastern Nigeria. All other four species are so far known from the Congo River basin(Ebert, 2001).

Fig.1 Lateral view of the Nile puff erf ish Tetraodon lineatus, IHB 2016077390, 199.5 mm SL, collected from Lake Turkana in Kenya
Recently, the Nile puff erf ishT.lineatuswas reported from Shanghai City, East China, and its complete mitogenome was determined and its phylogenetic relationship with other two African puff erf ishes was inferred based on mitogenome (Gong et al., 2016). Undoubtedly, this specimen found in a Chinese coastal location is completely outsideT.lineatus’ known distribution in Africa. The report on the occurrence of this African puff erf ish in China is not free from the question. The clarif ication of the source and species recognition of Chinese sample is thus warranted. Molecular technology can help to f ind solutions to these issues when fresh samples ofT.lineatusfrom the African continent are available.
The non-monophyletic nature ofTetraodons.l. was unveiled in many molecular phylogenetic analyses of this genus and the family Tetraodontidae (Yamanoue et al., 2011; Igarashi et al., 2013; Santini et al., 2013).In these analyses, all species ofTetraodonclustered into three distantly allied groups: Asian freshwater group, Asian brackish water group, and African freshwater group. This is one of the factors that led Kottelat (2013) to restrictTetraodons.s. to African freshwater group. Nonetheless, molecular evidence for the monophyletic nature of the genus is still not totally conclusive. The crucial reason for this is thatT.lineatus, the type species of the genus, was either not included or included but ambiguously identif ied in previous molecular studies.
Lake Turkana, the world’s largest permanent alkaline desert lake located in eastern Rift Valley in East Africa, is an endorheic lake situated in northern Kenya with its far northern end crossing into southern Ethiopia. It is home to around 50 f ish species (Nyingi,2013), most of which are present in the Nile River basin (Wakjira and Getahun, 2017). There is a common consensus that the Nilotic-Sudanic f ish fauna historically extended into the eastern Rift Valley lakes inclusive of Lake Turkana (Roberts, 1975). This lake, however, has its own unique f ish species, with a total of 11 endemics (Hardman, 2008; Nyingi, 2013).
Lake Turkana falls within the known distribution ofT.lineatus, and this species is the only puff erf ish presently reported from this Lake. During a f ish survey conducted by us in August, 2016 in Lake Turkana, Kenya, a single puff erf ish specimen was collected. We amplif ied its complete mitochondrial genome and inferred its phylogenetic relationship with other puff erf ishes. The aims of the present study are to conf irm the morphology-based recognition of the puff erf ish sample from Lake Turkana asT.lineatusutilizing molecular evidence, verify the source and species identif ication of the Nile puff erf ish sample recently reported from China and address issues regarding the monophyletic nature ofTetraodons.s.
2 MATERIAL AND METHOD
2.1 Taxon sampling and DNA extraction
One specimen of the Nile puff erf ish from Lake Turkana in Kenya (Fig.1) was collected in August,2016. Diagnostic characters provided by Lévêque(1992) forT.lineatusincludes: no scales present on body, but head and body covered with small spines except on snout and caudal peduncle; lateral line absent, a pair of fused teeth at front of each jaw; two pairs of non-perforated nasal tentacles; nostrils consisting of two f leshy lobes located in front of folded collar surrounding the opening; dorsal and anal f ins short, placed far back on body; pectoral f ins well-developed; no pelvic f ins; caudal f in truncate to rounded; yellow longitudinal stripes along sides of body in adults, but black-rimmed red ocelli (eyespots)present in juveniles. All these characters are shared with the puff erf ish specimen collected from Lake Turkana in Kenya, so the specimen was identif ied asT.lineatus. See Fig.1 for the picture of this species captured by us from Lake Turkana; its coloration is similar to Nyingi’s (2013) pictures.
The tissue (right pectoral-f in tip) employed for DNA extraction was sampled from the fresh specimen immediately after capture and stored in 95% ethanol.Genomic DNA was extracted from a small piece of the pectoral-f in clip (30 mg) using a TIANampGenomic DNA Kit (Tiangen Biotech, Beijing),following the manufacturer’s instructions. The voucher specimen (IHB 2016077390) is kept in the Freshwater Organism Museum at the Institute of Hydrobiology,Chinese Academy of Sciences, Wuhan, Hubei Province, China.

Table 1 Primers utilized in this study for amplifying and sequencing the mitogenome of the Nile puff erf ish Tetraodon lineatus from Lake Turkana
2.2 PCR and sequencing
The complete mitogenome was amplif ied in three overlapping segments by the long PCR technique(Lo, 1998). Software Primer Premier 5.0 was used for primer design (Singh et al., 1998; Lalitha, 2000).Long-range PCR primers were designed based on three versatile primers, which were used to amplify 16SrRNA (16Sar-L/16Sbr-H), COI (FishF1/FishR1)and Cytb(Cytball-Fl/ Cytball-Rl) segments. These three short sequences were utilized as templates to design three long-primers: Lincy16H/Lincy16L(amplif ication product was the fragment from Cytbto 16SrRNA), Lin16COH/Lin16COL (amplif ication product was the fragment from 16SrRNA to COI),and LinCOCYH/LinCOCYL (amplif ication product was the fragment from COI to Cytb) (Table 1). For the three short sequences, the PCR was performed in a total reaction volume of 25 μL containing 2.5 μL of 10×PCR buff er, 1.5 μL dNTPs (2.5 mmol/L), 0.5 μL of each primer (10 mmol/L), 0.5 μL rTaq(5 U/μL)and 1 μL genomic DNA template, and sterilized water was added to the PCR mixture to bring the f inal volume to 25 μL. For the three long-range segments,the PCR were conducted in 50 μL volume including 5 μL 10×PCR buff er, 8 μL dNTPs (2.5 mmol/L),0.5 μL of each primer (10 mmol/LM), 0.5 μL LATaq(5 U/μL), 1 μL genomic DNA template, and sterilized water was added to the PCR mixture to bring the f inal volume to 50 μL. The thermocycling conditions for these primers were as follows: initial denaturation for 5 min at 94°C, denaturation for 50 s at 94°C, annealing for 50 s appropriate temperatures (Table 1), extension for 1 min/kb amplif ication according to the product length at 72°C. After 35 cycles, f inal extension was done at 72°C for 10 min, and then the product was stored at 4°C. The PCR fragments were sequenced using the primer-walking strategy.
2.3 Annotation
DNA sequences were assembled by SeqMan program (DNAstar), checked, and edited manually.Annotation of 13 protein-coding genes was completed by comparing with closely associated species withinTetraodons.l. in ORF f inder tool of NCBI (https://www.ncbi.nlm. nih. gov/orffi nder/). The position and secondary structures of 22 tRNAs were determined by tRNAscan 2.0 (http://lowelab.ucsc.edu/tRNAscan-SE/). For the small and large subunit ribosomal RNA genes (12SrRNA and 16SrRNA), the start and stop base pair were assumed adjacent to the ends of their neighboring genes. The same method was utilized for two non-coding regions, D-loop and the replication origin of L strand (OL).The former was def ined as the sequence bounded by the genes for tRNA-Pro and tRNA-Phe, and the latter is a small fragment more than 30 bp in length and located between tRNA-Asn and tRNA-Cys (Bibb et al., 1981; Zhou et al., 1993).
2.4 Phylogenetic analysis
Four datasets were used for phylogenetic analysis:the complete mitogenomes, COI, Cytband 16SrRNAsequences (Table 2). For the complete mitogenome dataset, there were 52 mitogenomes of Tetraodontiformes available in GenBank (Table 2).Three partial segments of COI, Cytband 16SrRNA gene were separated from 52 available complete mitogenomes in order to derive phylogenetic relationship based on these genes. Single gene’s sequences of the three partial segments available in GenBank were added, reference to Table 2. The generic classif ication of the family Tetraodontidae given by Kottelat (2013) was followed.

Table 2 Taxa used in this study with GenBank accession numbers for genes, and sequence sources

Table 2 Continued
DNA sequences were translated into protein sequences to check if nonsense mutation occurred (Li et al., 2008). Sequences coding start- and stop-codons were excluded to eliminate homogeneity. Transition and transversion on the three codon positions were plotted against F84 genetic distance by DAMBE (Xia and Xie, 2001) to assess the saturation of the 13 genes.Sequences were aligned using MAFFT v7.407 (Katoh et al., 2002) and then aggregated into a sequence matrix. Phylogenetic trees were inferred by raxmlGUI for maximum-likelihood tree (Silvestro and Michalak,2012) by the ML+rapid bootstrap searching method and GTRGAMMA model for 1000 repetitions.Evolutionary models were chosen through PartitionFinder V1.1.1 (Lanfear et al., 2012) on the basis of AIC criterion, setting partition by codon for each gene and gene for the combined dataset. Final rooting was done utilizingTriodonmacropterus(Triodontidae),Molamola(Molidae) andDiodon holocanthus(Diodontidae), based on Yamanoue et al.’s (2011) results.
3 RESULT
3.1 Genome organization
The complete mitochondrial genome ofT.lineatusfrom Lake Turkana was 16 470 bp in length (GenBank No. MG913990) (Fig.2, Table 3). The mitogenome of this species, just like other four puff erf ishes of the genusTetraodons.s., consisted of 37 genes (for genes codes, please refer to legend in Fig.2), 13 of which areprotein-coding genes (PCGs), 22 tRNA genes, two rRNA genes and two non-coding regions, as found in other vertebrate animals (Wang et al., 2011a, b). Out of these, 28 encoded on the H-strand, and the remaining nine genes encoded on the L-strand,including one protein coding gene (ND6) and eight tRNA genes (tRNA-Gln, tRNA-Ala, tRNA-Asn,tRNA-Cys, tRNA-Tyr, tRNA-Ser, tRNA-Glu, and tRNA-Pro).

Table 3 Mitochondrial genome annotation of the Tetraodon lineatus

Fig.2 Mitogenomic organization of the Nile puff erf ish Tetraodon lineatus from Lake Turkana in Kenya
Ten intergenic spacers were recognized, ranging from 1-6 bp in size. Gene overlaps varying from 1-10 bp were observed in 13 regions, including four notable overlapping positions between genes: ATP6 and ATP8, ND4 and ND4L, ND5 and ND6, COI and tRNA-Ser. The f irst three overlaps have been also reported from other f ish species (Yu and Kwak, 2015).ATG is the most frequently used start codon for the 13 protein-coding genes, with a unique exception, GTG utilized by COI gene. GTG start codon for the COI gene is also present in other f ish species (Wang et al.,2011a, b; Yu and Kwak, 2015). Eight genes’ (ND1,ND2, ATP8, ATP6, COIII, ND4L, ND5, Cytb) stop codon is TAA, while two genes’ (COI and ND6) stop codons are AGG, and ND3’s is TAG. Both ND4 and COII exhibit incomplete stop codons with a single“T” residue. The presence of incomplete stop codons is common in f ish species mitochondrial genes (Peng et al., 2006; Kartavtsev et al., 2007; Yu and Kwak,2015).
The complete mitochondrial genome ofT.lineatusfrom Lake Turkana was 11 bp longer than the previously published one for a sample (KT715694,16 459 bp in length) recognized asT.lineatusfrom China by Gong et al. (2016). The overall nucleotide sequence similarity between these two mitogenomes was 98%. The 13 protein-coding genes and two rRNA genes exhibited 95%-100% similarities in nucleotide sequence; major length distinction came from 16SrRNA gene and the control region.
3.2 Sequence variations
The pairwise transition and transversion diff erences for each codon increased with F84 genetic distance,except TS diff erence of the third codon which asymptotically approached to saturation at about 0.4 distance and increases were hardly observed(Fig.3). Nucleotide frequencies at all codon positions diff ered between the two strands. On the H-strand, the nucleotide frequencies were C>A>G>T at the f irst codon position, T>C>A>G at the second codon position and C>A>T>G at the third codon position.Meanwhile, on the L-strand, the nucleotide frequencies were G>T>A>C at the f irst codon position, A>G>T>C at the second codon position and G>T>C>A at the third codon position.
3.3 Phylogenetic relationships
Phylogenetic trees generated from the complete mitogenome, COI, Cytb, and 16SrRNA gene for the family Tetraodontidae were shown in Fig.4. SinceTetraodons.s. was the target group of this study, each sample of this genus used in this study was retained as the terminal taxon in these resultant phylogenetic trees where they were marked as red lines.
In all trees constructed in this study, all analyzed samples ofTetraodongrouped together into a wellsupported monophyletic lineage (Fig.4). The sample of the Nile puff erf ish from Lake Turkana (MG913990)formed a well-supported monophyletic lineage, which representsT.lineatus(see the discussion below), with the sample (KT715694) from Shanghai, China in trees inferred from the complete mitogenome (Fig.4a).In COI, Cytband 16SrRNA gene-based trees(Fig.4b-d), samples ofT.lineatusformed a monophyletic clade, which then clustered withT.pustulatus. These samples together formed a wellsupported clade with other three species ofTetraodon(T.mbu,T.miurus, andT.duboisi).
4 DISCUSSION
Lake Turkana falls within the distribution of the Nile puff erf ishT.lineatusbased on known literatures.Deraniyagala (1948) was the f irst to describe the puff erf ish of the lake asTetraodonfahakarudolf ianus.The name was subsequently renamed toTetraodon fahakaby Hamblyn (1962), Mann (1964), and Hopson and Hopson (1982). Seegers et al. (2003)synonymizedT.fahakawithT.lineatus, a species initially described from the Nile River basin. This taxonomic treatment has thereafter been followed consistently by other authors (Habteselassie, 2012;Wakjira and Getahun, 2017). The puff erf ish specimen collected from Lake Turkana in the Kenyan section shared with all the morphologic characters provided by Lévêque (1992). The coloration of this specimen is the same as Nyingi’s (2013) and Wakjira and Getahun’s (2017) pictures. Therefore, this puff erf ish was identif ied asT.lineatus.
The complete mitogenome of the Nile puff erf ishT.lineatusfrom Lake Turkana in Kenya was determined for the f irst time. This complete genome sequence has strongly supported the morphologybased recognition of the puff erf ish specimen from this lake asT.lineatus. Its type locality is in the Nile River basin. Mohammed-Geba et al. (2016) analyzed COI gene genetic diversity of this species from the upper Nile River basin in Egypt. In our phylogenetic analysis based on a mitochondrial perspective, the Lake Turkana puff erf ish sample clustered with those of the upper Nile River basin and one sample(JQ681835) without precise collecting location into a strongly-supported monophyletic lineage (Fig.4c).Such a f inding further supports the hypothesis that the specimen from Lake Turkana, whose mitochondrial genome was sequenced in this study, belongs to the Nile puff erf ish speciesT.lineatus. There were three COI haplotypes ofT.lineatusfound by Mohammed-Geba et al. (2016) in 45 samples from three localities of the upper Nile River basin in Egypt. The most widespread haplotype among them was also shared with the puff erf ish sample collected from Lake Turkana. The shared presence of the common haplotype in Lake Turkana and the upper Nile River basin provides molecular evidence in favor of the existing geological hypothesis of existed historical connections of both water systems (Feibel, 2011).

Fig.3 Plot for transition and transversion on the three codon positions of the concatenated 13 protein coding genes (PCGs)versus pairwise F84 genetic distance of the 47 Tetraodontidae species and three outgroups
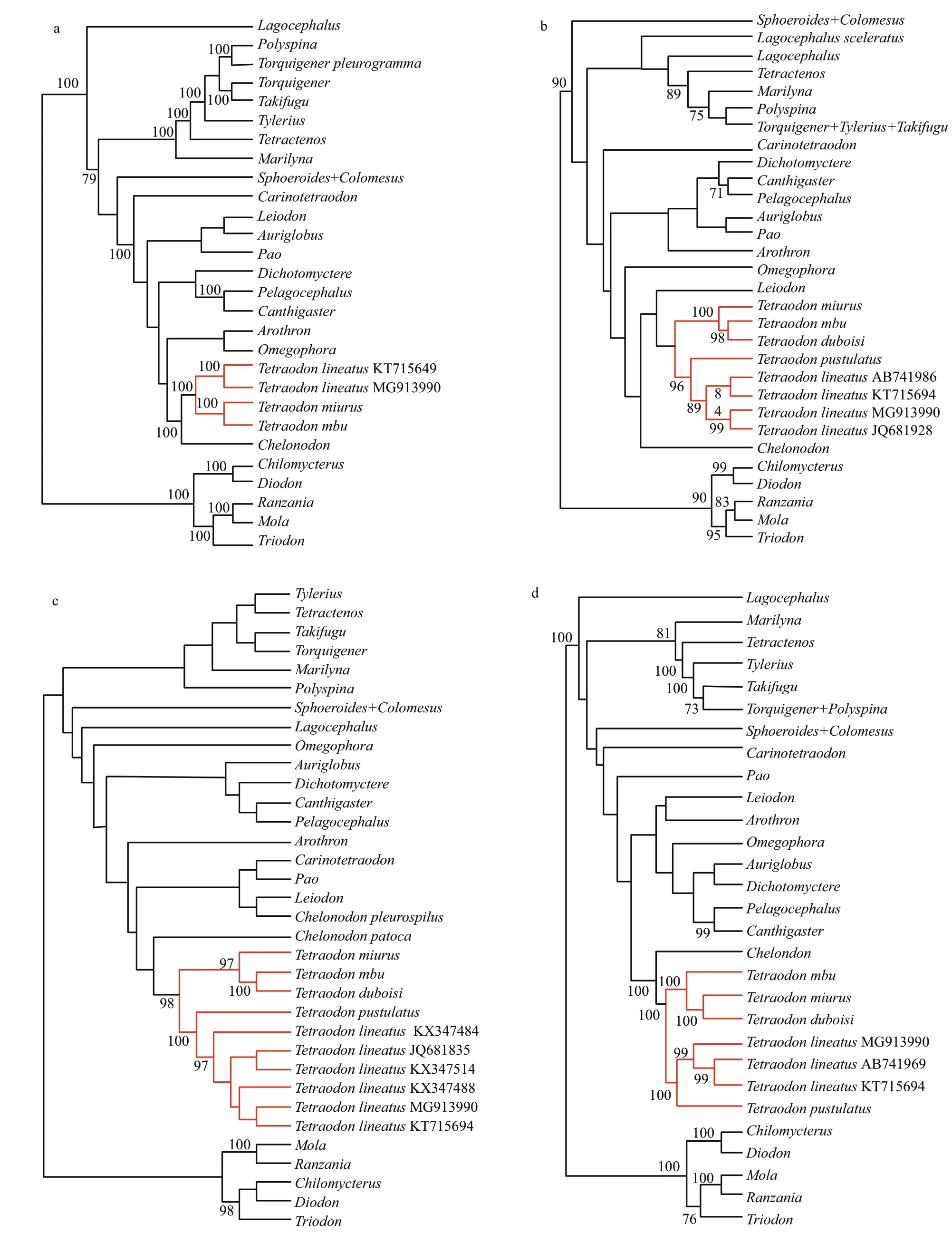
Fig.4 The maximum-likelihood (ML) trees of Tetraodontidae inferred from
The mitogenome ofT.lineatusfrom Lake Turkana from this study can be a reference genome to evaluate the recognition of the puff erf ish specimen of this species by Gong et al. (2016) collected in Shanghai City, East China. In their phylogenetic trees inferred from the complete mitogenomes for the family Tetraodontidae, the Chinese puff erf ish specimen was grouped with two African puff erf ish species ofTetraodonto constitute a monophyletic lineage.Nevertheless, the identif ication of this sample asT.lineatusneeds to be conf irmed based on comparisons with African sample of this species,particularly from its type locality (in the Nile River basin). Our comparison found that there was a high similarity (98%) in complete mitogenomes between Gong et al.’s (2016) puff erf ish sample and theT.lineatusspecimen from Lake Turkana in Kenya. In complete mitogenome-based trees (Fig.4a), these two samples were grouped with each other to form a lineage with four African species ofTetraodon. Based on a reanalysis of Yamanoue et al. (2011) dataset of puff erf ishes, the genetic distance in complete mitogenome between both was 1.7%, less than the minimum one (2.6%) found betweenSphoeroides annulatusandS.testudineus. These f indings affi rm that they are conspecif ic and belong to the same speciesT.lineatus.
Gong et al. (2016) reported that the Chinese specimen ofT.lineatuswas caught from Luchaogang,Shanghai, East China, without detailed information about its possible source. The Nile puff erf ish has not previously been recorded in f ish species list of China(Zhang et al., 2016) or Southeast Asia (Kottelat,2013). However, Liang et al. (2006) listedT.lineatusas introduced f ish species in Taiwan, China, pointing out that pet trade has served as the source of many exotic f ish. Accordingly, Xiong et al. (2015) includedT.lineatuson the species list of non-native freshwater f ish in China. Our COI gene sequence comparison unveiled that the most common haplotype found by Mohammed-Geba et al. (2016) for samples of this species from the upper Nile River basin was also shared with Gong et al.’s (2016) sample. It is highly likely that their sample from Shanghai, China was of African origin. The introduction of the Nile puff erf ish as pet f ish from Africa is, therefore, a reasonable explanation for its presence in China.
The genus nameTetraodonwas initially erected by Linnaeus in 1758, withT.lineatusas the type species.It has been widely considered as valid since the work by Tyler (1980). In previous molecular phylogenetic analyses of the family Tetraodontidae or the genusTetraodons.l. (Yamanoue et al., 2011; Igarashi et al.,2013; Santini et al., 2013), species ofTetraodonclustered into three distantly associated groups, thus indicating the non-monophyletic nature of the genus.Kottelat (2013) presented a re-classif ication forTetraodonas traditionally diagnosed. His taxonomic classif ication was actually based on Yamanoue et al.(2011) and Igarashi et al. (2013) molecular trees,Dekkers’ (1975) morphological data and Tyler’s(1980) morphological descriptions, color pattern and its ontogeny (pers. comm., Kottelat, October, 2017).He restrictedTetraodons.s. to the African freshwater group composed of six species:T.duboisi,T.lineatus,T.mbu,T.miurus,T.pustulatus, andT.schoutede.Nevertheless, molecular evidence for the monophyletic nature of this genus was strong but not totally conclusive. Only two species of African puff erf ish (T.mbuandT.miurus) formed a lineage in Yamanoue et al.’s (2011) phylogenetic trees inferred from complete mitogenome. Unfortunately, the type species of the genus,T.lineatus, was not sampled.Five African puff erf ish species were clustered together in Santini et al.’s (2013) phylogenetic analysis based on COI, Cytband six nuclear genes for the family Tetraodontidae. A monophyletic lineage formed by these f ive species was also recovered in Igarashi et al.’s (2013) molecular phylogenetic trees of the genusTetraodons.l. based on 16S rRNA and Cytbgene.However, no information about the precise collecting locality of the voucher specimen ofT.lineatuswas provided. This species has a much wider distribution from the Senegal and Volta River basins to the Niger and Nile River basins and Lake Turkana. From taxonomists’ point of view, the identif ication of a given species is doubtful if comparisons with its type or topotypic specimens are not made. In particular,COI gene comparisons found that the puff erf ish sample from Lake Turkana was identical to those of the Nile River basin, the type locality ofT.lineatus.In phylogenetic trees inferred from COI, Cytband 16SrRNA gene (Fig.4b-d), the sample of Lake Turkana clustered with the samples of the species under the name ofT.lineatusdetermined by Yamanoue et al. (2011), Santini et al. (2013) and Igarashi et al.(2013), into a monophyletic lineage which truly represents the species. The mitogenome amplif ied in this study for the puff erf ish sample from Lake Turkana here recognized with conf idence asT.lineatuscan be employed as reference genome to clear up ambiguity in identif ication of this species in previous molecular studies and thus provide conclusive molecular evidence for the monophyletic nature ofTetraodons.s.
5 CONCLUSION
The complete mitogenome of the puff erf ish specimen from Lake Turkana in Kenya supported the morphology-based recognition of the specimen asT.lineatus. Mitogenomic comparison and phylogenetic analysis provides conclusive molecular evidence for the monophyletic nature ofTetraodons.s. and also for the identif ication of the Chinese specimen recently recorded asT.lineatusand its African source.
6 DATA AVAILABILITY STATEMENT
The complete mitochondrial genome presented here is deposited in GenBank (accession No.MG913990).
7 ACKNOWLEDGMENT
We would like to express thanks to the National Museums of Kenya (NMK) and the Kenya Wildlife Service (KWS) for facilitated surveys and issuance of research permits. Our thanks are also given to Joseph Gathua, Julius Kioko, and Brian Okwiri of Ichthyology Section, NMK for assistance with f ield work.
 Journal of Oceanology and Limnology2020年2期
Journal of Oceanology and Limnology2020年2期
- Journal of Oceanology and Limnology的其它文章
- Contribution of surface wave-induced vertical mixing to heat content in global upper ocean*
- Upper ocean response to typhoon Kujira (2015) in the South China Sea by multiple means of observation*
- Inf luence of simulating deep-sea environmental factors on cathodic performance of seawater battery*
- Adsorption characteristics of chitooligosaccharides onto activated charcoal in aqueous solutions*
- Eff ects of hypoxia on survival, behavior, and metabolism of Zhikong scallop Chlamys farreri Jones et Preston 1904*
- Distinct inf luence of trimethylamine N-oxide and high hydrostatic pressure on community structure and culturable deep-sea bacteria*
