Mass acquisition of human periodontal ligament stem cells
Hidefumi Maeda, Department of Endodontology and Operative Dentistry, Division of Oral Rehabilitation, Faculty of Dental Science, Kyushu University, 3-1-1 Maidashi, Fukuoka 8128582, Japan
Abstract The periodontal ligament (PDL) is an essential fibrous tissue for tooth retention in the alveolar bone socket.PDL tissue further functions to cushion occlusal force,maintain alveolar bone height, allow orthodontic tooth movement, and connect tooth roots with bone.Severe periodontitis, deep caries, and trauma cause irreversible damage to this tissue, eventually leading to tooth loss through the destruction of tooth retention.Many patients suffer from these diseases worldwide, and its prevalence increases with age.To address this issue,regenerative medicine for damaged PDL tissue as well as the surrounding tissues has been extensively investigated regarding the potential and effectiveness of stem cells, scaffolds, and cytokines as well as their combined applications.In particular, PDL stem cells (PDLSCs) have been well studied.In this review, I discuss comprehensive studies on PDLSCs performed in vivo and contemporary reports focusing on the acquisition of large numbers of PDLSCs for therapeutic applications because of the very small number of PDLSCs available in vivo.
Key Words:Induced pluripotent stem cells; Mesoderm specific transcript; Periodontal ligament stem cells; Periodontal tissue; Regenerative medicine; Semaphorin 3A
HOW ARE REGENERATIVE TREATMENTS OF PERIODONTAL DEFECTS PERFORMED USING CELL-RELATED THERAPY?
Stem cell-based therapy (Figure 1)
In humans, autologous transplantation of cultured periosteum sheets[1], periodontal ligament (PDL) progenitor cells[2], PDL cell sheets[3], dental pulp stem cells[4,5], gingivaderived cells[6], and tissue-engineered bone constituted by bone marrow mesenchymal stem cells (BMMSCs)[7]into patients with periodontal defects have been reported.All of the cited studies clinically verified the potency of stem cells for periodontal regeneration.In contrast, Chenet al[8]transplanted PDL stem cell (PDLSC) sheets and found no significant improvement compared with the control group.However, these authors targeted very small periodontal defects and applied scaffolding materials as controls, possibly making it difficult to detect significant differences.
In animal periodontal defect models, Iwasakiet al[9]reported no significant advantage of spheroid formation by human PDLSCs in rats, despite increased expression of genes related to angiogenesis and anti-inflammation[9].Nevertheless, a recent study demonstrated that coculture of human PDLSC spheroids with vascular endothelial cells promoted rat periodontal regeneration[10].In addition, a previous study revealed that pellets of cultured human PDLSCs showed periodontal regeneration capacity in mice[11].Meanwhile, application of other immature cells,including adipose-derived stem cells[12], stem cells from human exfoliated deciduous teeth[13], dental pulp stem cells[14], dental follicle cells[15], induced pluripotent stem (iPS)cells[16], and iPS-derived mesenchymal stem cells (MSCs)[17]was shown to induce periodontal regenerationin vivo.These reports suggest that the indicated cell sources may have potential for clinical use.
Gene/noncoding RNA modified cell therapy
No clinical studies on gene or noncoding RNA modified cell therapy for the treatment of patients with periodontal disease have been reported because of the associated safety issues.However, there have been some reports involving animal models with experimentally produced periodontal defects.Osteoprotegeringene-transferred rabbit PDLSCs andplatelet-derived growth factor-BB-transduced human PDLSCs exhibited increased bone formation in periodontal defects[18,19].
The development and characterization of other tissue-derived cells with gene transduction have been reported.Specifically,bone morphogenetic protein 2-transfected canine BMMSCs[20],fibroblast growth factor 2-transduced canine BMMSCs[21],hepatocyte growth factor-transduced human dental pulp cells[22], and leptin-transduced rat BMMSCs[23]were able to restore periodontal defectsin vivo.
Modification of PDLSCs using noncoding RNA, including microRNA, long noncoding RNA, and circular RNA have been reported to induce their osteogenic differentiation, suggesting the application of these cells to bone defective in periodontitis[24].
Although the above studies indicated the potential of novel therapies for repair of severe periodontal defects, further basic studies are indispensable for future clinical trials.
Cell culture conditioned medium and exosomes
Conditioned medium from cultured cells and extracellular vesicles secreted from stem cells have various effects, including tissue regeneration, cell proliferation, chemotactic and metabolic activities, anti-inflammation, and cell-cell communication[25].Because these cells and vesicles possess great potential, researchers have examined their roles in periodontal regeneration studies, but related clinical trials have not been reported.
There have been some studies on the effects of conditioned medium or exosomes from PDLSCs as well as other tissue-derived stem cells in animal models.Recently,Nagataet al[26]demonstrated periodontal regeneration activity of conditioned medium from cultured human PDLSCs injected into rat periodontal defects.Another study showed the capability of conditioned medium from human gingival stem cells as well as human cultured human PDLSCs for periodontal regeneration[27].Furthermore,conditioned medium from human BMMSCs was clearly able to repair canine and rat periodontal defects[28,29].A recent report applying exosomes from human BMMSCs to rat periodontal defects showed induction of newly-formed bone and PDL tissue[30].
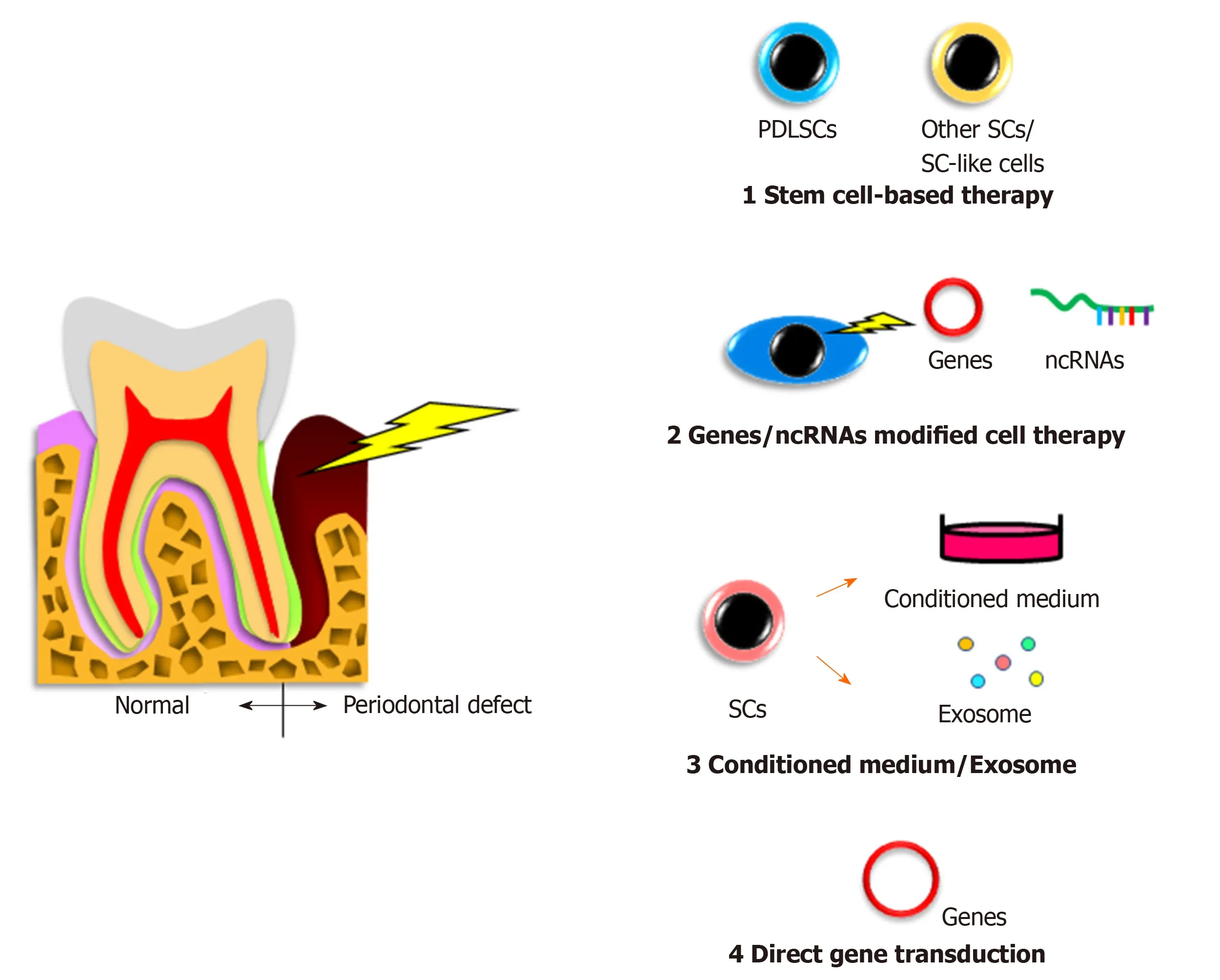
Figure 1 Cell-related therapies for periodontal regeneration.
Although the above effects do not reflect direct contributions of stem cells to treatment, these indirect effects of stem cells may deserve further consideration as treatment options.
Gene therapy
Directin vivogene transfer of thebone morphogenetic protein 2/7[31]orplatelet-derived growth factor[32]genes to rat periodontal tissue promoted bone growth or bone regeneration and cementum formation, respectively.Meanwhile, another group directly transferred thebone morphogenetic protein 4gene to rat PDL tissue by electroporation but did not detect any obvious bone augmentation[33].Similarly,embedding of aplatelet-derived growth factor-Bplasmid with collagen gel into alveolar bone defects in rats had no significant effects[34].
The gene therapy method has not been investigated in human patients, and its effectiveness needs to be fully verified before it can be used as a relatively easy therapeutic modality.
WHAT ARE PDLSCs?
Periodontal tissue (periodontium) is a complex tissue mainly composed of two hard tissues (alveolar bone and cementum coating tooth root surfaces) and two soft tissues(PDL tissue and gingival tissue)[35].In particular, PDL tissue has crucial roles in supporting the tooth and integrating the tissues.
PDLSCs are somatic stem cells localized in PDL tissue[36]and derived from cranial neural crest cells[37,38].PDLSCs have similar features to BMMSCs and exhibit selfrenewal capacity and multipotency[39].These cells have the potential to undergo triploblastic differentiation with the ability to differentiate into not only osteoblasts,adipocytes, chondrocytes, cementoblasts, and tendon/ligament fibroblasts[40,41]but also myocytes[42], neural cells[43], retinal cells[44], endothelial cells[45], pancreatic islet cells[46],and hepatic cells[47].In addition, PDLSCs express cell surface markers such as STRO-1,CD146/MUC18[36], CD44, and CD90 (markers associated with stromal cells), CD105 and CD166 (markers associated with stromal cells and endothelial cells)[48], and CD10,CD26, CD29, CD73, and CD349/FZD9[49]but do not express hematopoietic cell surface markers such as CD31 and CD45, similar to BMMSCs[50].
PDLSCs also express embryonic stem cell-related transcription factors like NANOG and OCT-4 and embryonic stem cell antigens like stage-specific embryonic antigen-1(SSEA-1)/CD15, SSEA-3, SSEA-4, TRA-1-60, TRA-1-81, alkaline phosphatase, and REX1/ZFP42[49,51].However, PDLSCs from aged people exhibit decreased capacities for proliferation, migration, and multiple differentiation with reduced SSEA-4 expression[52].
Finally, PDLSCs possess immunomodulatory properties[48], among which reactive oxygen species production may be interestingly regulated by dual mechanisms depending on the degree of inflammation[53].
WHERE ARE PDLSCs REQUIRED?
Severe periodontitis, deep caries, and trauma cause irreversible damage to PDL tissue as well as the surrounding tissues such as alveolar bone, gingiva, and cementum,eventually resulting in tooth loss.The 8020 Promotion Foundation survey for causes of tooth loss in Japan (https://www.8020zaidan.or.jp/english/) performed in 2018 reported that periodontitis, caries, and tooth fracture accounted for 84.0% of all causes of tooth loss.The Global Burden of Disease 2015 study suggested that 7.4% of people worldwide suffered from severe periodontitis[54].Meanwhile, the National Survey of Dental Diseases in Japan performed in 2016 (https://www.mhlw.go.jp/toukei/list/62-28.html) reported that Japanese people with periodontal health (< 4 mm periodontal pocket depth) comprised less than 37.0% of people aged ≥ 50 years.
To date, transplantation of PDLSCs has led to successful periodontal regeneration in experimentally produced periodontal defects in dogs[55,56], rats[57], and pigs[58].Furthermore, Yanet al[59]performed a systematic review and meta-analysis and decisively stated that cell-based therapy is an effective therapy for regeneration of lost periodontal tissue.
PDLSCs have been proposed as the most promising cells for regeneration of severely damaged PDL tissue, among other stem cells such as dental pulp stem cells,stem cells from human exfoliated deciduous, dental follicle stem cells, stem cells from apical papilla[60], BMMSCs, and alveolar periosteal cells[61].
Interestingly, the fate of transplanted PDLSCs was examined in a rat periodontal defect model[62].The findings revealed that PDLSCs contributed to periodontal repair but did not become markedly engrafted, suggesting a supportive role of PDLSCs for activating the regenerative capability in the damaged periodontium.Considering the critical role of PDLSCs in periodontal regenerative therapy, PDLSCs themselves may be difficult to engraft into defect sites, but the use of 2D or 3D construction methods combined with extracellular matrices may be effective.
WHY ARE LARGE AMOUNTS OF PDLSCs NEEDED?
In human PDL tissue, STRO1+/CD146+cells, regarded as candidate PDLSCs[36,63], were reported to comprise only about 0.07% of the total cells[64].Another study described that PDLSCs comprised 2.4% of the total cells[63].Regardless of the actual numbers,both studies indicated that very few PDLSCs are present in PDL tissue.
While the defect volumes in cases reported in human clinical studies have been very limited as described above, the defect areas in severe cases leading to tooth loss can vary across a wide range.Therefore, clinical application of PDLSCs to regenerative therapy of periodontal defects in humans will require the acquisition of large numbers of PDLSCs.Meanwhile, delivery of autologous PDLSCs to patients will necessitate the patients to undergo surgically invasive procedures.In addition, the subsequent expansion of small numbers of PDLSCsin vitrocould lead to loss of their stemness.In this regard, it is of concern that large amounts of PDLSCs are needed for regenerative treatment.To address this issue, methods to acquire large numbers of PDLSCs have been explored.
HOW TO SOLVE THE INSUFFICIENCY OF PDLSCs?
Reprogrammed cells (Figure 2)
We have reported unique methods for conversion of PDL cells to PDLSCs by gene transduction[65,66].In a previous study, semaphorin 3A-transduced human PDL cells were converted into stem-like cells that showed multipotency and expressed both embryonic stem cell and MSC markers[65].Furthermore, we recently demonstrated that an unexplored gene,mesoderm-specific transcript, was expressed in PDLSCs and that human PDL cells transduced with themesoderm-specific transcriptgene acquired PDLSC properties similar to semaphorin 3A-transduced cells[66].In addition, the transduction changed the spindle shape of PDL cells to a stem cell-like round shape.Therefore, although the safety of these cellsin vivoneeds to be confirmed for clinical use, cell transformation with these genes is a potential method for mass acquisition of PDLSCs.
iPS cells
Our group was the first to report the development of PDLSC-like cells from human skin fibroblast-derived iPS cells[67].Our study indicated that iPS cells themselves were unable to directly differentiate into PDLSCs, whereas neural crest-like cells developed from iPS cells attained PDLSC properties when cultured on extracellular matrix secreted from human primary PDL cells.We believe that this method may have great potential to solve the issue of insufficient numbers of PDLSCs.In addition, a recent study produced human leukocyte antigen homozygous iPS cells by gene modification,which have immune compatibility[68].This development will enable the clinical use of iPS cell-derived PDLSCs benefiting many patients with severe periodontal defects.However, the issue of cost needs to be solved.
CONCLUSION
Many researchers have attempted to develop innovative and critical methods for periodontal therapy from various angles to support people’s health and life and address the aging society.PDLSC-based therapy is one of these methods, and we believe that it has the potential to deliver sustainable oral health to people around the world.
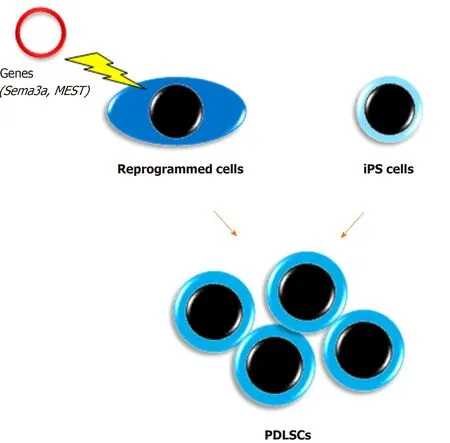
Figure 2 Acquisition of a large number of periodontal ligament stem cells.
ACKNOWLEDGEMENTS
The author thanks Drs.Tomokiyo, Hamano, Hasegawa, Sugii, Yoshida, and Itoyama for their great support in the preparation of this review.
 World Journal of Stem Cells2020年9期
World Journal of Stem Cells2020年9期
- World Journal of Stem Cells的其它文章
- Dental stem cells:The role of biomaterials and scaffolds in developing novel therapeutic strategies
- Inflammatory niche:Mesenchymal stromal cell priming by soluble mediators
- Role of the CXCR4-SDF1-HMGB1 pathway in the directional migration of cells and regeneration of affected organs
- Mechanotransduction of stem cells for tendon repair
- Senescent mesenchymal stem/stromal cells and restoring their cellular functions
- Mechanisms of action of neuropeptide Y on stem cells and its potential applications in orthopaedic disorders
