Inducing human induced pluripotent stem cell differentiation through embryoid bodies: A practical and stable approach
Abstract
Human induced pluripotent stem cells (hiPSCs) are invaluable resources for producing high-quality differentiated cells in unlimited quantities for both basic research and clinical use. They are particularly useful for studying human disease mechanisms in vitro by making it possible to circumvent the ethical issues of human embryonic stem cell research. However,significant limitations exist when using conventional flat culturing methods especially concerning cell expansion,differentiation efficiency,stability maintenance and multicellular 3D structure establishment,differentiation prediction. Embryoid bodies (EBs),the multicellular aggregates spontaneously generated from iPSCs in the suspension system,might help to address these issues. Due to the unique microenvironment and cell communication in EB structure that a 2D culture system cannot achieve,EBs have been widely applied in hiPSC-derived differentiation and show significant advantages especially in scaling up culturing,differentiation efficiency enhancement,ex vivo simulation,and organoid establishment. EBs can potentially also be used in early prediction of iPSC differentiation capability. To improve the stability and feasibility of EB-mediated differentiation and generate high quality EBs,critical factors including iPSC pluripotency maintenance,generation of uniform morphology using micro-pattern 3D culture systems,proper cellular density inoculation,and EB size control are discussed on the basis of both published data and our own laboratory experiences. Collectively,the production of a large quantity of homogeneous EBs with high quality is important for the stability and feasibility of many PSCs related studies.
Key words:Induced pluripotent stem cells; Suspension culture; Embryoid body; Early prediction; Committed differentiation; Heterogeneity; Three-dimensional culture;Scaling-up; Quality control
INTRODUCTION
The emergence of human induced pluripotent stem cells (hiPSCs) has markedlypromoted the development of regenerative medicine. These cells are reprogrammed from differentiated human somatic cells by gene integration or nonintegration methods and possess the properties ofself-proliferation and committed differentiation[1-4]. More importantly,compared to human embryonic stem cells(hESCs),the use of hiPSCs successfully avoids major immunoreactive and ethical issues[5]. As a result,hiPSCs have quickly become a critical resource for biomedical research and are expected to be used in clinical cellular transplantation,disease model establishment,and drug screening. Conventional methods,however,are usually established in flat culture systems,which impose significant limitations on cell expansion,differentiation efficiency,and multicellular 3D structure establishment.Embryoid bodies (EBs),which are cultured in a suspension system,might help to address these issues. Generally,EB is a multicellular aggregate spontaneously formed by pluripotent stem cells under suspension culture conditions,which has three germ layer structures and partially recapitulates the early embryonic development[6]. Such a multicellular 3D structure improves cell-cell contacts and intercellular communication and also enhances substance exchange[7]. Although the differentiation from iPSC to target cells is a relatively complex,time consuming,and unstable process[8],EBs have been widely used in iPSC differentiation and organoid construction because of their irreplaceable structural and functional advantages[9,10]. It has been demonstrated that a standardized EB formation procedure contributes to their high quality and improves differentiation[11,12]. Therefore,the key factors need to be carefully considered when EB-mediated differentiation is selected[9,13].
In order to understand the critical events of EB-mediated differentiation,explore better methods and solve the aforementioned problems,we recapitulated the current applications and advantages of using EBs in iPSC differentiation. Combining our own and previously published data related to EB formation and differentiation,we conducted a comparative and predictive analysis and aimed to provide a reference to create a more stable and practical way of high-quality EB generation.
APPLICATION AND ADVANTAGES OF EB USE IN IPSC DIFFERENTIATION
Scale-up of culture systems and differentiation efficiency
Clinical transplantation requires large quantities of functional target cells and most of the existing strategies are difficult to implement at a large scale or have a low differentiation efficiency,therefore posing barriers to further research. Compared to flat culture systems,EB-derived differentiation culture is kept in a relatively fixed position,which offers this method an obvious advantage in quantity and differentiation efficiency[14-16]. A variety of cell lineages have been generated from hEBs such as brain,cornea,heart,liver,and blood (Table 1). In our study,we used a suspension EB-based system to generate iPSC-derived melanocytes and achieved a significantly higher differentiation efficiency compared to that in flat culture systems and these induced melanocytes showed long-termin vivofunctionality after transplantation[17]. In short,differentiation from EB to specific cell lineages is an efficient method that is likely to yield large populations of functional cells.
Ex vivo simulation and organoids establishment
Recently,due to the features of their three germ layer structure[18],hEBs have become useful models for developing human ESC- and iPSC-derived organoids by stimulation with a cocktail of biological agents (Table 1). These EB-derived organoids not only contain some cell subtypes but also show distinct stratification which is similar to thein vivostructure of the tissues or organs developing[19,20]. For example,Joet al[21]observed an identical organization structure in 3D cultured human midbrainlike organoids (hMLOs) compared with human postmortem midbrain tissue under the electron microscope. Furthermore,these EB-derived organoids are functional.Qianet al[22]found that EB-derived midbrain organoids not only expressed a wider range of characteristic markers common to normal midbrain tissue compared with direct differentiation from iPSCs,but also demonstrated firing action potentials in response to current injection which can be used to establish a disease model of microcephaly[22]. These EB-derived organoids could be used to understand unique features of specific human organs and to gain insights into different disorders.
Early prediction of differentiation potential
There is a remarkable difference in differentiation capability in distinct iPSC lineages,and it is urgently necessary to predict the differentiation potential in an early stage of the long differentiation period to avoid unnecessary resource losses. Because EBs can potentially develop into three germ layer tissues for multi-directional differentiation[23,24],it can be hypothesized that they may be suitable candidates for predicting iPSC differentiation potential. Kimet al[25]classified hESCs-derived EBs into several types including cystic,bright cavity and dark cavity according to their morphology. By detecting the expression of specific markers,they showed that most of the cells in cystic EBs are endoderm-lineage populations and both bright and dark cavity EBs own cells from all of the three germ layers. Besides the morphology,EB size can also be used determine the differentiation direction of iPSC. For example,the larger EBs (450 μm diameter) often contained cells of the cardiovascular system,while endothelial cells were usually generated in smaller EBs (150 μm diameter) and such differentiation potentials are mainly caused by the noncanonical WNT pathway[26].Moreover,gene expression profiles of hPSCs and EBs recovered by high-throughput RNA sequencing can also be used for differentiation prediction,and the results is parallel to the germ layer developmentin vivo[27].
The novel methods described above have some limitations. Simple morphology or size-dependent prediction system is somewhat subjective,and it is also closely related to the viability of the cells inside EB[28,29],whereas high-throughput RNA sequencing is expensive and complicated. In our study,we developed a simple and practical system and identified a set of parameters combining EB formation,maintenance,and germ layer-specific gene expression in EB stage to predict the differentiation tendency of the various iPSC lines. The validity of this evaluation system was finally confirmed by differentiating these iPSC lines into melanocytes[24]. Recently,we summarized the current methods related to iPSC differentiation prediction and we also postulated that the use of EBs could be more efficient when combined with other modified detection methods such molecular probes[34].Thus,due to their special morphology and functionality,EBs have played an important role in the differentiation prediction related studies.
Multiple-cell environment and cell communication
The remarkable functionality and application prospects of EBs are a result of their unique 3D microstructure and cell communication. Frequent passage in the adherent culture system limits the cell-cell communication in both cell number and time. The suspension culture system for EBs provides extensive material exchange and increases both cell-cell interactions and cell-matrix communication in the aggregate,which is conducive to the transmission and action of cell signals[35,36]. In general,intercellular communication is increased both in space and time at the 3D level. Additionally,differentiation of EBs is an asynchronous process and 3D culture produces distinct layers of spheroids (EBs) as a result of concentration gradients in the culture medium[37]. The differentiation of the outer endoderm of EB caused by growth factors in the medium will further induce endoderm differentiation by producing signals[38].This unbalanced phenomenon also explains the heterogeneity throughout the differentiation process in EBs. Moreover,more intricate gene profiles could be expressed in a 3D culture system,reflecting original expressionin vivomore accurately[16]. Joet al[21]found that gene profiles detected by RNA sequencing analysis are analogously expressed between hMLOs and the human prenatal midbrain.However,there is a significant difference between the 2D dopamine neurons and normal tissue. The integrity of gene expression is a considerable advantage of the 3D culture system,providing a better foundation for future structural and functional studies. Meanwhile,for the hiPSC-based differentiation,the cadherin-β-catenin-Wnt pathway was found to be involved in the development of EBs by intercellular adhesion[39]. In addition,the activation or inhibition of relevant signaling pathways can yield a definitive lineage differentiation[22,40,41]. Therefore,controlling the dose and duration of cytokines regulates the differentiation of iPSCs by activating various pathways.
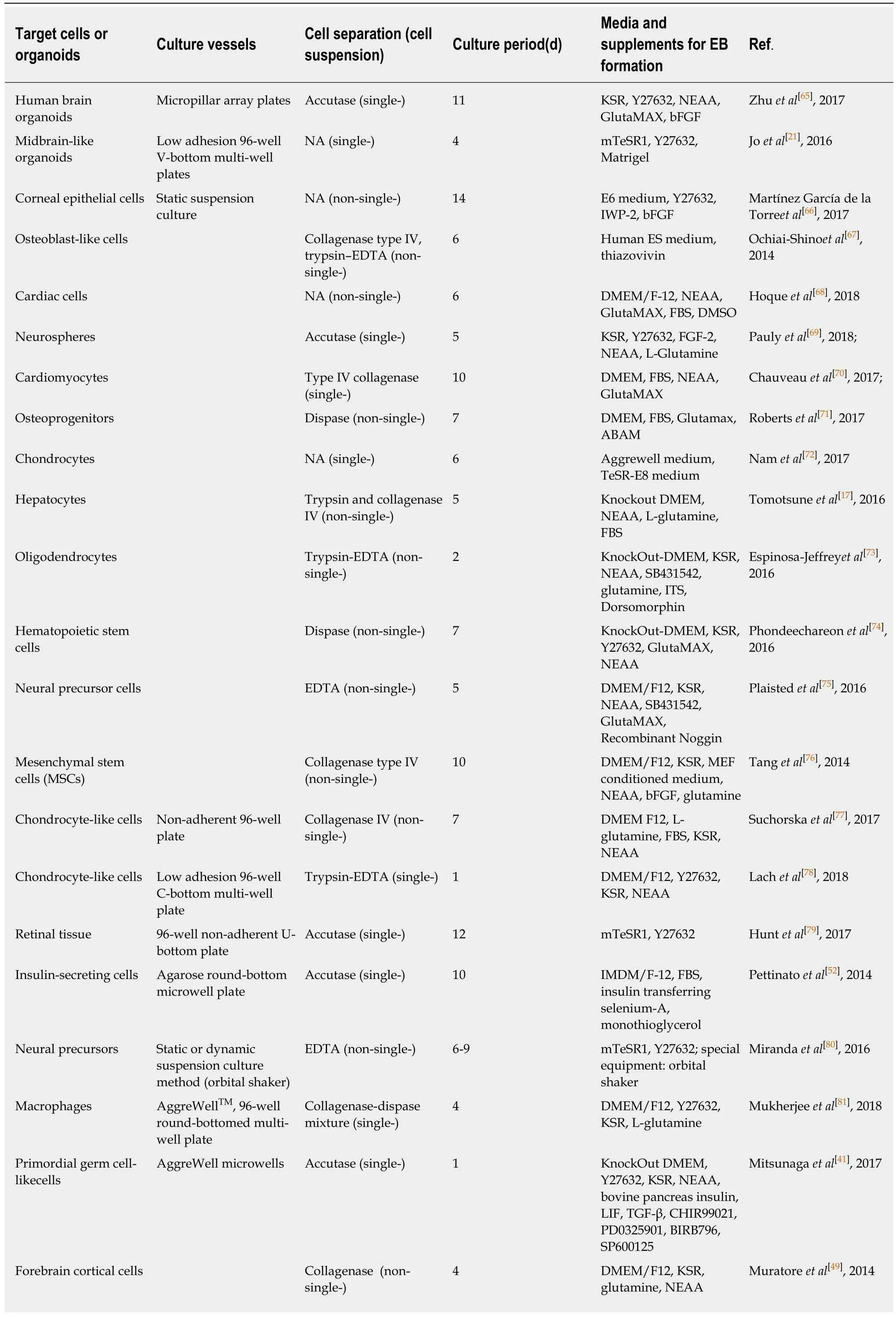
Table 1 Updated summary of the formation of human embryoid bodies

“NA” means enzyme deficiency or mechanical dissection of the iPSC clones. E6 medium: Essential 6 medium; IMDM/F-12: Iscove's Modified Dulbecco's Medium/Nutrient Mixture F-12; DMEM/F-12: Dulbecco's Modified Eagle Medium/Nutrient Mixture F-12; mTeSR1: Standardized medium for the feederindependent maintenance of hESCs and hiPSCs; TeSR-E8 medium: A feeder-free,animal component-free culture medium for hESCs and hiPSCs; MEF:Mouse embryonic fibroblast; FBS: Fetal bovine serum; LIF: Leukemia inhibitory factor; TGF-β: Transforming growth factor-beta; bFGF: Basic fibroblast growth factor; Y27632: Rho-associated kinas inhibitor; KSR: Knockout serum replacement; NEAA: Non-essential amino acids; DMSO: Dimethyl sulphoxide; IWP-2: Inhibitor of the WNT pathway; ITS: Insulin-Transferrin-Selenium; SB431542: Inhibitor of the activin type I receptor ALK4 and the nodal type I receptor ALK7; CHIR99021: Inhibitor of glycogen synthase kinase 3β (GSK3β); PD0325901: Inhibitor of the mitogen-activated protein kinase (MEK)pathway; BIRB796: Inhibitor of the mitogen-activated protein kinases (MAPK); SP600125: Inhibitor of c-Jun N-terminal kinase (JNK).
Compared with a flat culture,microgravity has been found to be a subsidiary factor that can regulate cell proliferation and survival in a 3D culture system[29-32]. Therefore,bioreactors and random positioning machines have been used to simulate states of microgravity and weightless condition for EB-derived differentiation[42,43].
In conclusion,EBs have shown great application potential in the generation of various functional cells and organoids at a large scale and also in the prediction of iPSC differentiation capability. These EB-derived cells and organoids highly resemble thein vivostatus in both gene expression and function display as a result of the structure and microenvironment in the EB.
QUALITY CONTROL OF EB
Considering the wide range of applications of EBs in iPSC differentiation and their potential advantages,a comprehensive quality control of the EB formation process would help to improve the stability and feasibility of their application.
Pluripotency maintenancein iPSCs
To generate high quality EBs,the initial step is the pluripotency maintenance in iPSCs where the addition of various cytokines and inhibitors is essential. Two studies[15,44]demonstrated that basic fibroblast growth factor (bFGF) induced hES cell derived fibroblast-like cells produce insulin-like growth factor II and transforming growth factor β family of factors and indirectly establish a balanced stem cell niche of hESCs,contributing to maintain the undifferentiated state of hESCs. In addition,Y-27632 was confirmed to benefit the expression of POU class 5 homeobox 1 (OCT4) in EB formation[45-48]. Compared with the conventional methods,single cell culture system can reduce the complexity caused by feeder cells. However,it is more difficult to maintain undifferentiated status of iPSC when single-cell passage is used. Tsutsuiet al[45]developed a unique combination of inhibition of Rho-associated kinase,glycogen synthase kinase and mitogen-activated protein kinase with bFGF for hES cells maintenance and they found these cells to present good pluripotency and genetic integrity in long-term maintenance (> 20 passages).
Formation and uniform morphology
In general,EBs can be spontaneously generated with hiPSC clusters[8,46]or single cells[41,49]in 3D culture systems using EB-specific medium. When conventional methods such as static suspension culture and the hanging drop method[50,51]are chosen,EB-derived differentiation seems unstable,which is in part associated with the heterogeneity of EBs[52,53]. Therefore,it is necessary to control the generation of uniform EBs for stable and reproducible differentiation. Recently,different types of micro-pattern plates have been developed and widely applied for the generation of EBs with uniform size and morphology using single hiPSCs (Table 1) and on this basis,a variety of culture systems such as the micro-space system[50,51,54],spinner flasks[22],and the National Aeronautics and Space Administration developed rotary cell culture system (NASA rotary system)[55]have also been designed for scale-up.
Cellular density and EB size
Besides morphological uniformity,the efficiency and direction of differentiation are also affected by EB size[56-58]and which size is more appropriate depends on the specific cell lineages. It has been demonstrated that a larger amount of cardiomyocytes was found in hESC-derived differentiation using EBs with100 μm diameter when compared to larger ones (300 μm)[59]. However,we found that 300-400 μm might be a more suitable diameter for melanocytes differentiation compared to those bigger or smaller. Since the size and quality of EB during culture maintenance are affected by the number of inoculated cells,micro-pattern plates can be used to control EB size by adjusting cellular density. However,inoculated cell density also depends on the EB culture plate type and culture duration before differentiation. In a study of iPSC differentiation into cardiomyocytes,input of 1.5 × 104to 4.0 × 104cells per EB was determined to be the proper density to form homogenous and synchronized EBs. By contrast,we found that 5.0 × 102to 1.0 × 103cells were enough for each EB generation using an ElplasiaTMmicro-well plate and this density tended to yield stable and intact EBs (Figure 1) which could be maintained over a relatively long term for differentiation. Overly high cell density (2.0 × 103cells/EB) will lead to breakage or fusion in a short time (Figure 1). In short,EBs within a certain size range are more conducive to differentiation which can be modified by cell density adjustment. However,size usually varies across different differentiation assays and needs to be optimized in accordance to the specific conditions.
FUTURE PERSPECTIVES AND CONCLUSION
EBs can effectively mimic thein vivodifferentiation process and have shown excellent advantages in the field of iPSC-derived differentiation,especially in organoid establishment. However,a gap between these products and normal organs is still inevitable because the inclusion of a cocktail of factors and additives only provides a somewhat analogous survival environment rather than completely simulating thein vivoconditions and this difference generally leads to a temporal and spatial distinction[60,61]. To reduce these differences as much as possible,many studies have been carried out with the aim to optimize the culture system,including culture duration,plate type,dose of growth factors and small molecules,matrix proteins and even the order of the additives[35,36]based on research aims and differentiated cell types. In terms of iPSC-derived EB formation,the initial inoculation density is more critical than additives and centrifugation (forcing cell aggregation),and more of such insights can likely be obtained from tumor sphere related studies[62]. For example,magnetic levitation,a new method for spheroid formation,has been used to establish a levitation profile for distinct tumor cells[63]. Whether this novel technology can also be adopted for EB related differentiation and cell purification remains to be explored.
Overall,an EB mediated culture system is more amenable to improvement in differentiation efficiency and simulation of the human microenvironment compared to 2D differentiation. Cell proliferation,cell-cell interaction,and material metabolism partially undergo the influence of microgravity,achieving more intricate gene expression and epigenetic profiles. More importantly,most of the iPSC related studies require a relatively long observation time which inevitably leads to instability. EBs with good quality control could provide a stable and feasible system to better present the functional integrity of tissues and organs[64],underlining their potential in the exploration of promising drugs and precision medicine where classical 2D cell assays might fail.
Significantly,EBs are also expected to reveal the development of early human embryos,avoiding ethical issues. EB formation and lineage commitment have become the focus of research in terms of the structure and composition of differentiated cells.RNA sequencing of single EB cells will help to clarify the evolution or/and unknown event of early embryos and the fate development of individual cells. In conclusion,the production of a large quantity of homogeneous EBs with high quality is important for many PSCs related studies,including scaling up culturing,organoid formation,and differentiation potential prediction.
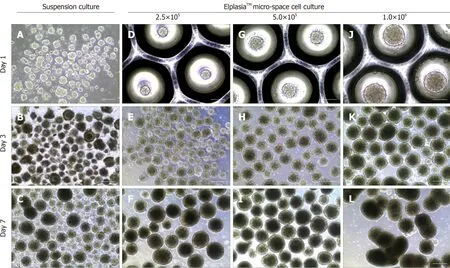
Figure 1 Static suspension culture and 24-well ElplasiaTM micro-well culture with human induced pluripotent stem cells. A-C:Induced pluripotent stem cell(iPSC)-derived embryoid bodies (EBs) formed under a suspension system and cultured with mTeSR™,respectively,on day 1 (A),day 3 (B),and day 7 (C); D-L:iPSC-derived EBs formed with single hiPSCs in micro-space cell culture plates at different cell densities: D-F: 2.5 × 105 cells/mL; G-I: 5.0 × 105 cells/mL; and J-L: 1.0× 106 cells/mL,respectively,on day 1 (D,G,and J),day 3 (E,H,and K),and day 7 (F,I,and L). Scale bars: A,D,G,and J 100 μm; resident graphs,250 μm.
 World Journal of Stem Cells2020年1期
World Journal of Stem Cells2020年1期
- World Journal of Stem Cells的其它文章
- Regeneration of the central nervous system-principles from brain regeneration in adult zebrafish
- Early therapeutic effect of platelet-rich fibrin combined with allogeneic bone marrow-derived stem cells on rats' critical-sized mandibular defects
- Sphere-forming corneal cells repopulate dystrophic keratoconic stroma: Implications for potential therapy
- Adipose stromal/stem cells in regenerative medicine: Potentials and limitations
- Generation of induced secretome from adipose-derived stem cells specialized for disease-specific treatment: An experimental mouse model
- HIF-2α regulates CD44 to promote cancer stem cell activation in triple-negative breast cancer via PI3K/AKT/mTOR signaling
