Theoretical Study on Ignition Delay Time of Thermally Cracked n-Decane
WANG Hong-yan,PEI Shan-shan,WANG Li,ZHANG Xiang-wen,LIU Guo-zhu
(School of Chemical Engineering and Technology,Key Laboratory for Green Chemical Technology of Ministry of Education,Collaborative Innovation Center of Chemical Science and Engineering(Tianjin),Tianjin University,Tianjin 300072,China)
Abstract:Endothermic hydrocarbon fuels undergo thermal cracking before entering the combustion chamber and can produce a mixture of unreacted fuels and pyrolysis products(i.e.cracked fuels).The objective of this work is to investigate the effects of pyrolysis conversions,pyrolysis pressures,ignition pressures and free radicals on ignition characteristics of cracked n-decane over temperature of 1300-1800 K,pressure of 0.1-3.0 MPa and equivalence ratio of 1.0.Components of the thermally cracked n-decane at 3.0 and 5.0 MPa in a flow reactor were calculated theoretically using an accurately combined mechanism,which are in good agreement with the experimental results in literature.The results showed the conversion rates of n-decane cracking at 3 and 5 MPa are 46.2% and 58.8%,respectively.The distribution of cracking products is consistent,but the ethylene content decreases with the increase of pressure,while the alkane content increases with the increase of pressure.Meanwhile,the content of free radicals at 3 MPa is slightly higher than that at 5 MPa,but the content of free radicals is very low.Ignition delay time increases with the decreasing of n-decane conversion and pyrolysis pressure,while higher ignition pressure can shorten it significantly.Furthermore,the presence of free radicals in cracked n-decane could accelerate the ignition process with ignition delay time shortening more than 15% when the conversion was less than 40%,compared with that of cracked n-decane without radicals.
Key words:ignition delay time;cracked fuels;n-decane;free radicals;sensitivity analysis
1 Introduction
Endothermic hydrocarbon fuels are proposed as one of the key candidates to solve the thermal management problems in advanced aircrafts,due to their excellent endothermic capacities and capacities to provide extra heat sinks by decomposition reactions[1-3].In advanced aircraft engines,fuels will undergo thermal cracking in heat exchanger prior to combustor,producing a mixture of smaller hydrocarbons,hydrogen and a small amount of free radicals(e.g.methyl,ethyl,allyl).Then the mixture of cracking products(including radicals) and unreacted fuels that is marked as cracked fuel undergoes the combustion in the combustor[4-6].Therefore,it is significant to study the ignition characteristics of cracked fuel to design and improve the engine in advanced aircraft.
Nowadays,the researches in literature mainly focus on the measurement of the ignition delay time of single or binary fuels,while the reports on the ignition delay time of cracked fuel are relatively few.Puri et al.[7]theoretically studied ignition delay time of two different components of cracked JP-7 by different kinetic models,concluding that the addition of C3 species could reduce the ignition delay time with a limit.Colket and Spadaccini[4]conducted shock tube experiments to investigate the effect of cracking on ignitability over the range of temperature of 1201-1455 K,equivalence ratio of 0.5-1.0,and pressure of 0.7 MPa.0.3CH4/0.6C2H4/0.1C7H16(baseline-fuel) was chosen as the surrogate of cracked products,with addition of heptane into baseline-fuel to simulate low conversion (80%C7H16)and addition of hydrogen for dehydrogenation (10%H2).They concluded that all these mixtures ignited more easily than heptane at present experiments.In addition, addition of heptane increased ignition delay time while addition of hydrogen decreased it but with small amount.Castaldi et al.[8]studied theoretically about how pyrolysis influenced on ignition delay time of JP-8 at stoichiometric ratios,based on calculated pyrolysis products which contained 17 pyrolysis product components and 35% unreacted fuels.Ignition delay time calculations of three modified fuels(propene converted to propane,toluene to methylcyclohexane,methane to ethane and hydrogen)were compared with JP-8 at 1000 K and 0.1-0.3 MPa.They found that pyrolysis products ignited faster than JP-8 at 0.1 MPa while slower than JP-8 at 0.3 MPa.The process of re-hydrogenating the unsaturated products would inhibit ignition,while methane converting to ethylene and hydrogen enhanced ignition,from 121 ms to 107 ms at 0.1 MPa.More recently,our previous work[9]measured ignition delay times ofn-decane,cracking gas and crackedn-decane by using shock tube at 1296-1915 K, 0.1-0.2 MPa.0.23CH4/0.19C2H6/0.32C2H4/0.07C3H8/0.19C3H6(marked as cracking gas)was chose as surrogate of cracking products ofn-decane.Experiment results showed thatn-decane and all these crackedn-decane ignited faster than cracking gas.Furthermore,crackedn-decane obtained atT> 1480 K forx= 37.97%,17.61% and atT<1480 K forx= 62.15% showed shorter ignition delay time thann-decane,indicating that thermal cracking could improve ignitability with a limited degree.It should be noted that these studies only focus on surrogate of cracked fuel and very few calculated points for cracked fuels neglecting other complex components and free radicals of products.The complex free radicals produced by pyrolysis maybe influence the ignition process significantly for their active characteristics.However,the complex diversity of products and the difficulty in capturing free radicals make it very difficult to measure the ignition delay time of cracked fuels.Therefore,it is necessary to investigate the ignition characteristics by theoretical simulation to make it clear about the effects of free radicals on ignition of cracked fuels.
Considering aviation kerosene is a complex mixture of different hydrocarbons[10],it is necessary to uses surrogate to simply the problems[9,11-12].n-Decane has been used to be the surrogate of kerosene for its ignition delay time and properties similar to Jet fuels[12-14].Thus,n-decane was selected to study the ignition characteristics of cracked fuel in this work.Firstly,components of cracking products ofn-decane are calculated at 3,5 MPa and 945 K.Secondly,the ignition delay time for crackedn-decane based on cracking products were simulated at 300-1800 K,pressure of 0.1-3.0 MPa and equivalence ratio of 1.0.Thirdly,the further effects of pyrolysis conversions,pyrolysis pressures,ignition pressures and free radicals on ignition characteristics of crackedn-decane were investigated and analyzed.
2 Kinetic Model
Due to the difficulty to capture free radicals and the complexity of products,all the ignition calculations are based on calculated pyrolysis products ofn-decane,consisting of gaseous and liquid products,and small amounts of free radicals.Some efforts were applied to develop pyrolysis mechanisms ofn-decane applicable for different conditions in recent years.Ward et al.[15]developed a unique two-dimensional computational fluid dynamics model to study the effects of pressure on flowing mild-crackedn-decane with low conversion.Malewicki et al.[16]revised and extended the revised 1st Generation Surrogate model,basing on pyrolysis and oxidation ofn-decane in the high-pressure single pulse shock tube.The additional revised mechanism showed improvements in predicting 1-olefin species profiles during the pyrolysis and oxidation ofn-decane.Jia et al.[17]investigatedn-decane pyrolysis under supercritical pressures(3-5 MPa)and developedn-decane pyrolysis mechanism containing 164 species and 842 reactions,which was validated by electrically heated tube test with products distributions and the chemical heat sink.Zeng et al.[18]conducted pyrolysis experiments ofn-decane in the flow reactor at 0.0007-0.1 MPa and developed a mechanism with 234 species and 1452 reactions.In this work,the mechanism proposed by Jia et al.[17]was chosen as the basis of calculation forn-decane pyrolysis,because the validated experiments under the supercritical condition make it closer to real conditions in heat exchanger.
For oxidation mechanism of crackedn-decane,it is worth noting that the mechanism should perform good simulation for both light hydrocarbons andn-decane,since cracked fuels contain not only unreactedn-decane but also light hydrocarbons and some free radicals.Therefore,in current study,the reaction mechanism of crackedn-decane oxidation takes the merged mechanism(i.e.Combined mechanism)in our previous paper[9],which was validated by predicting ignition delay time of bothn-decane and light hydrocarbon (CH4, C2H6, C2H4, C3H8,and C3H6)at 0.1 MPa.
The simulation ofn-decane pyrolysis was performed using Plug Flow Reactor of Chemkin-pro package[19],with measured axial temperature[17].The rationality of using one-dimensional homogeneous flow tube reactor has been verified in the Supporting Information.The calculations of ignition delay time of crackedn-decane were conducted in a closed homogenous batch reactor using Chemkin-pro package by assuming constant volume and adiabatic conditions.The ignition delay timeτigwas defined as the time interval from the starting point of simulation to the maximum rate of temperature rise(max dT/dt)[4,20].
3 Results and Discussion
3.1 Pyrolysis Products Distribution of n-Decane
Calculation of pyrolysis ofn-decane was performed by Chemkin-pro using the Plug Flow Code at 3-5 MPa(pyrolysis pressure marked aspp),with outlet temperature of 945 K and a flow rate of 1.0 g·s-1.The flow tube length is 1 m with internal diameter of 1.0 mm and the temperature distribution profiles along the reactor is taken from the measured data in literature[17].All the experimental data ofn-decane pyrolysis were shown in Figure 1,including major gaseous and liquid species and the major free radicals in cracking products.The exiting conversions of pyrolysis ofn-decane at 3 and 5 MPa reach to 46.2% and 58.8%,respectively.Figures 1a and 1b show more ethene and less alkane produced at 3 MPa than 5 MPa,consistent with the reported results in the literature[15,21].The behavior could be better understood by free radical chain reactions[22],which describe that n-alkane chain firstly experiences decomposition by a carbon-carbon bond fission,following with bimolecular (H-abstraction reactions) or unimolecular reactions (β-scission reaction) to produce smaller alkane or alkene,respectively.Higher pressure is beneficial for bimolecular reaction rather than unimolecular reactions,result-ing in more alkane production,while pressure decreasing is favorable to unimolecular reactions resulting in more alkene production.
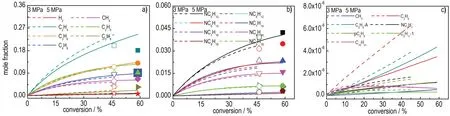
Fig.1 Simulated(lines)and experimental(dots)[17] main products distributions of thermal cracking of n-decane at 3 MPa and 5 MPa.(a)main gaseous products;(b)main liquid products;(c)major free radicals
Except stable molecular products, different kinds of free radicals were produced during the pyrolysis ofn-decane.The seven initially produced free radicals(decyl contains all five different structures)are illustrated in Figure 1c.It can be observed that as the pressure increases,the contents of all free radicals decrease,especially for vinyl radical(C2H3).Although the content of free radicals is less about 4-5 orders of magnitude than stable pyrolysis products,the presence of free radicals maybe influence the ignition delay time of crackedn-decane significantly.
3.2 Ignition Delay Time of Cracked n-Decane
3.2.1 Effects of Pyrolysis Conversions of n-Decane
Figure 2 shows the ignition delay time of stoichiometric crackedn-decane over a range of temperature 1300-1800 K at 0.1 MPa(ignition pressure marked aspiandn-decane conversion makred asx).It can be found that ignition delay times of these crackedn-decane are shorter than that ofn-decane,indicating that thermal cracking could improve the ignitability.In addition,the promoting effect increases with the increasing of conversion of thermal cracking ofn-decane.However, the results of crackedn-decane present large difference with experimental results in our previous work[9],in which thermal cracking could improve the ignitability only atT>1480 K forx=37.97%,17.61% and atT<1480 K forx=62.15% to a limited degree.The main reason for the difference between experimental and theoretical results is that the components of pyrolysis products are different.In experimental research,only five lighter hydrocarbons (0.23CH4/0.19C2H6/0.32C2H4/0.07C3H8/0.19C3H6)were proposed as the surrogate for pyrolysis products ofn-decane,to simplify the problem and increase experimental operability.While in theoretical study,except the lighter hydrocarbons like methane,ethane,etc.,the relative heavier pyrolysis products(i.e.liquid products)and a few free radicals are also considered.Furthermore,the different conditions of pyrolysis ofn-decane in both studies would lead to different products distributions,which is also accountable for the different results.Therefore, further experimental study on cracked fuels should be conducted based on more detailed components as surrogate of pyrolysis products.
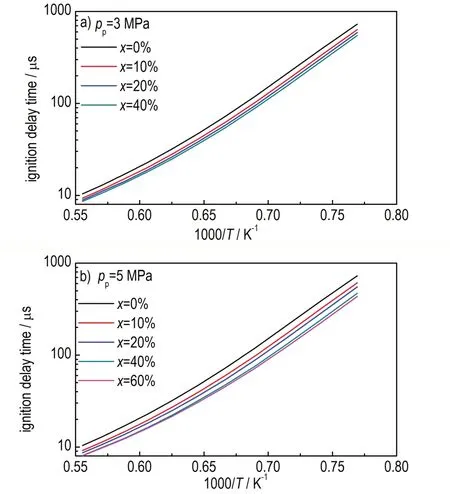
Fig.2 Predicted ignition delay times of cracked n-decane at different pyrolysis pressures with different conversions of n-decane
3.2.2 Effects of Pyrolysis Pressure of n-Decane
Different pyrolysis pressure might also influence the ignition delay time of crackedn-decane by affecting the products distribution.Figure 3 compares the ignition delay times at conversions of 10%,20%,and 40% under pyrolysis pressure of 3 and 5 MPa.At the same cracking conversion,ignition delay time of crackedn-decane at 5 MPa is slightly shorter than that at 3 MPa,and with the deepening of the cracking,the difference of ignition delay time gradually increases.As indicated in section 3.1,pyrolysis products at 3 MPa have more ethylene and relative more free radicals,but the ignition is a little more difficult than that at 5 MPa.It can be concluded that ethylene content has no significant influence in the ignition of crackedn-decane,and the ignition delay time might be more relevant to the type of free radicals but not the content.
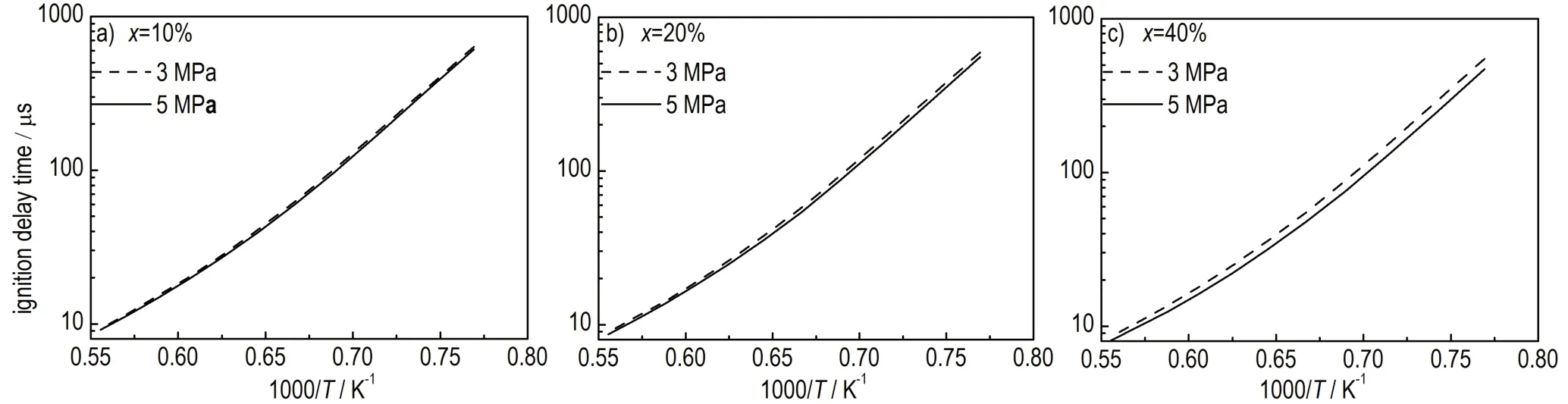
Fig.3 Predicted ignition delay times of cracked n-decane under different pressures at different conversions from 10% to 40%
Sensitivity analysis refers to the change of system characteristic quantity(such as ignition delay time)caused by the slight change of reaction rate constant of each chemical reaction under the condition of given temperature and pressure.Its value is called sensitivity coefficient,which can be calculated using equation(1).To better understand this result,sensitivity analysis was performed by perturbing the reaction rate constant by a factor of 2,using
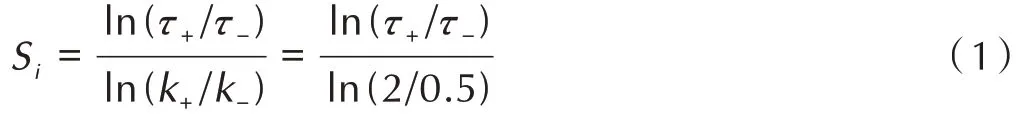
WhereSirepresents the sensitivity coefficient,k+andk-represent the perturbation on reaction rate,τ+andτ-represent the corresponding ignition delay time calculated with the increased and decreased rate coefficients,respectively.The positive coefficient indicates that the specified reaction inhibits the ignition,while negative indicates enhances ignition.
Figure 4 depicts the sensitivity analysis of ignition delay time of 40% crackedn-decane obtained under pyrolysis pressures of 3,5 MPa,atpi=0.1 MPa,T= 1500 K,andφ= 1.0.It shows that the sensitive reactions for ignition of crackedn-decane are same and the mostly sensitive reactions are associated to C0—C3.It can be noted that R1:H + O2= OH + O has the largest sensitivity coefficient to promote ignition because it is the most important chain branching reaction of hydrocarbons at high temperatures[23-25].Therefore, reactions producing H-atom have negative coefficients, such as R5 and R6,while those reactions consuming H-atom have positive coefficients,e.g.R25 and R26.Moreover,the formation of vinyl radical(C2H3) has a positive effect on ignition,such as the H-abstraction reaction of C2H4by OH to form C2H3(R3).The formed vinyl radical then reacts with oxygen,leading to another branching reaction(R4),resulting in positive improvement for ignition.In addition,the reaction producing resonance stabilized allyl radical(C3H5-A)[26]and inactive methyl radical (CH3) followed by chain termination reaction would inhibit the reactivity,such as R17,R27,and R21.Meanwhile,the chain termination reactions inhibit ignition by consuming active radicals to form stable structure,R14 and R26 for instance.
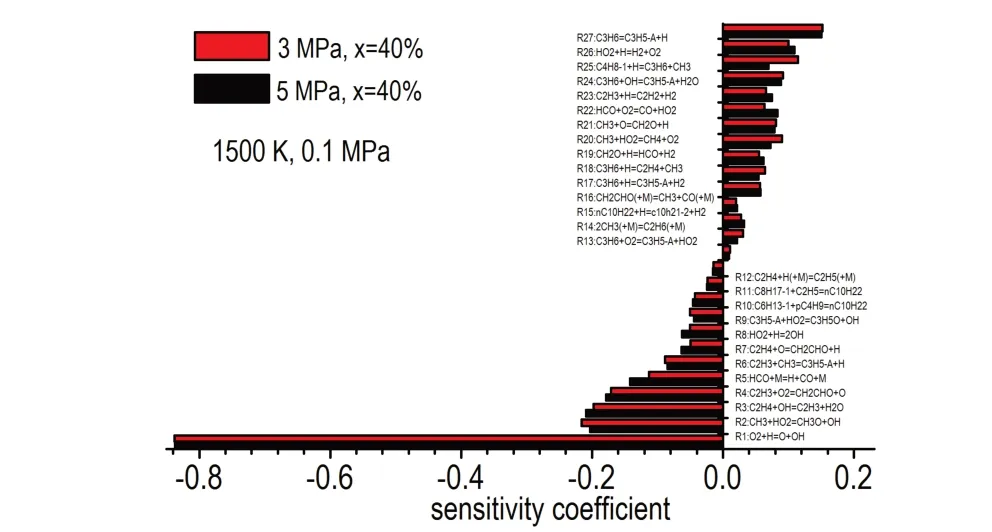
Fig.4 Sensitivity analysis for ignition delay times of two cracked n-decane under different pyrolysis pressures at 0.1 MPa,1500 K,φ=1
The major free radicals(H,O,CH3,C2H5,C2H3,C3H5-A,etc.) mole fractions profiles are described in Figure 5,in which C2H5,C2H3molar fractions were enlarged five times for their low concentrations during the ignition process.As can be noted,the extremely sharp increases of H-atom and O-atom indicate the ignition of cracked fuel.Crackedn-decane atpp= 5 MPa produces more vinyl radical(C2H3) and less methyl radical and allyl radical(C3H5-A)than those atpp=3 MPa.
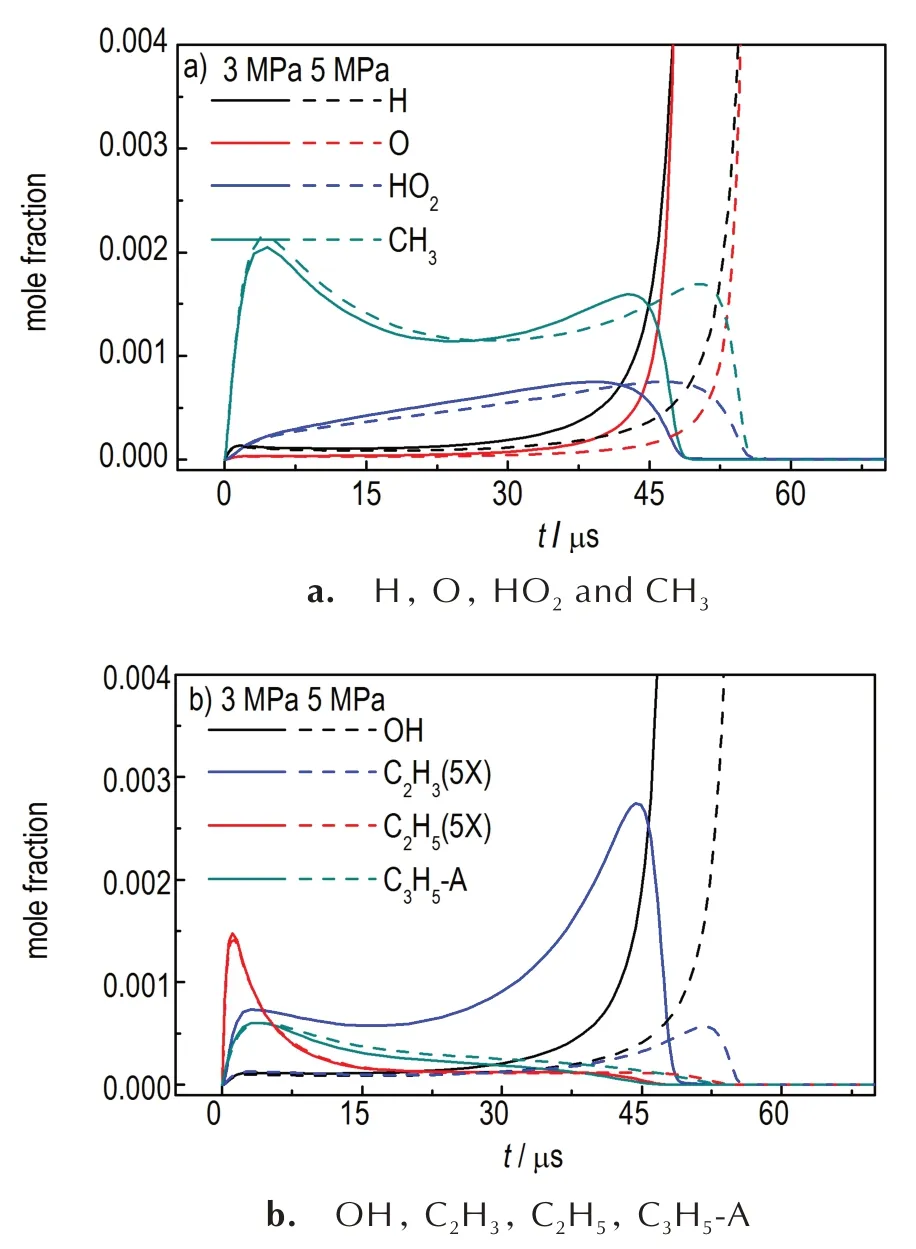
Fig.5 At 1500 K,0.1 MPa and φ=1,predicted profiles of major radicals of two cracked n-decane under different pyrolysis pressure
3.2.3 Effect of Free Radicals in Pyrolysis Products
Free radicals could react with fuels directly by H-abstraction reactions,which might enhance the ignition progress by accelerating initial stage of fuel decomposition.Therefore,ignition delay time of cracked fuels with or without radicals were simulated at the temperature of 1300-1800 K and pressure of 0.1 MPa,as described in Figure 6.It can be noted that the ignition delay times of crackedn-decane with free radicals are shorter than that of crackedn-decane without radicals,and the ignition delay time become shorter with the conversions increasing.
For better understanding this result,Figure 7 ranks the sensitivity coefficients of stoichiometric crackedn-decane with or without free radicals in air ofpp=5 MPa,x=40%,atT=1500 K,φ= 1.0 and 0.1 MPa.Consistent with section 3.2,most C0—C3 reactions are sensitive to crackedn-decane ignition,except R10 and R15.Similarly,chain branching reactions and reactions producing H-atom and C2H3radical express negative sensitivity coefficients,while chain termination reactions,including reactions forming CH3and C3H5-A and consuming H atom show positive coefficients on ignition delay time.
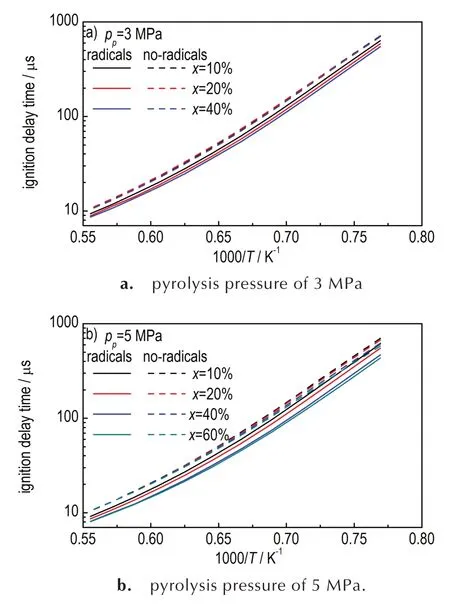
Fig.6 Comparison for ignition delay times of two different cracked n-decane with or without radicals at 0.1 MPa,φ = 1.(a) pyrolysis pressure of 3 MPa,(b) pyrolysis pressure of 5 MPa
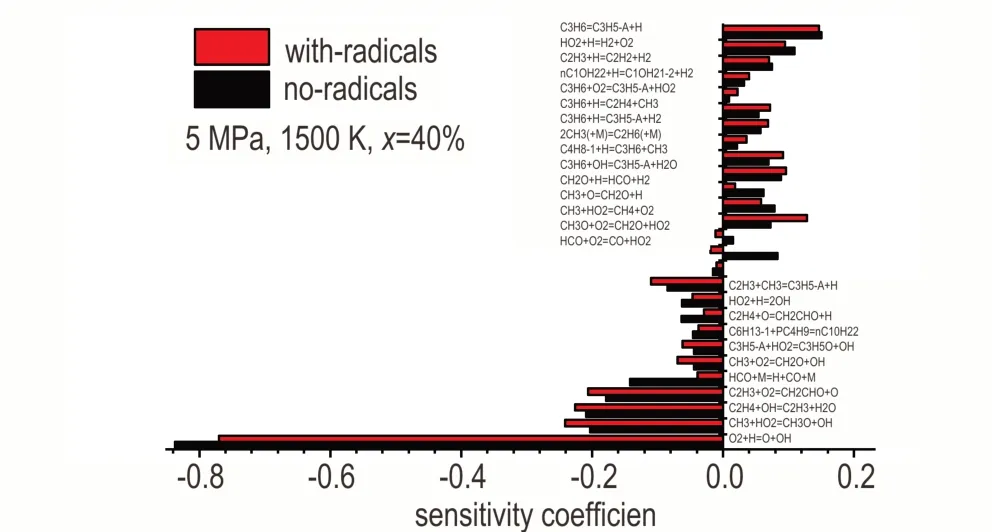
Fig.7 Sensitivity analysis for ignition delay times of two cracked n-decane with or without radicals at 0.1 MPa,1500 K,φ=1
Similarly,the profiles of the major free radicals(H,O,CH3,C2H5,C2H3,C3H5-A,etc.)are performed to analyze how the radicals change during the ignition process,shown in Figure 8.As discussed in Section 3.2.2,the sudden increases of H-atom and O-atom indicate the ignition sign of cracked fuels.It also can be found that there has obvious difference of the amounts of major radicals between crackedn-decane with or without free radicals in air.Both less methyl radical and allyl radical(which would hinder the ignition process),along with more vinyl radical(C2H3) that helps the ignition,would result in shorter ignition delay time for cracked fuels with radicals.
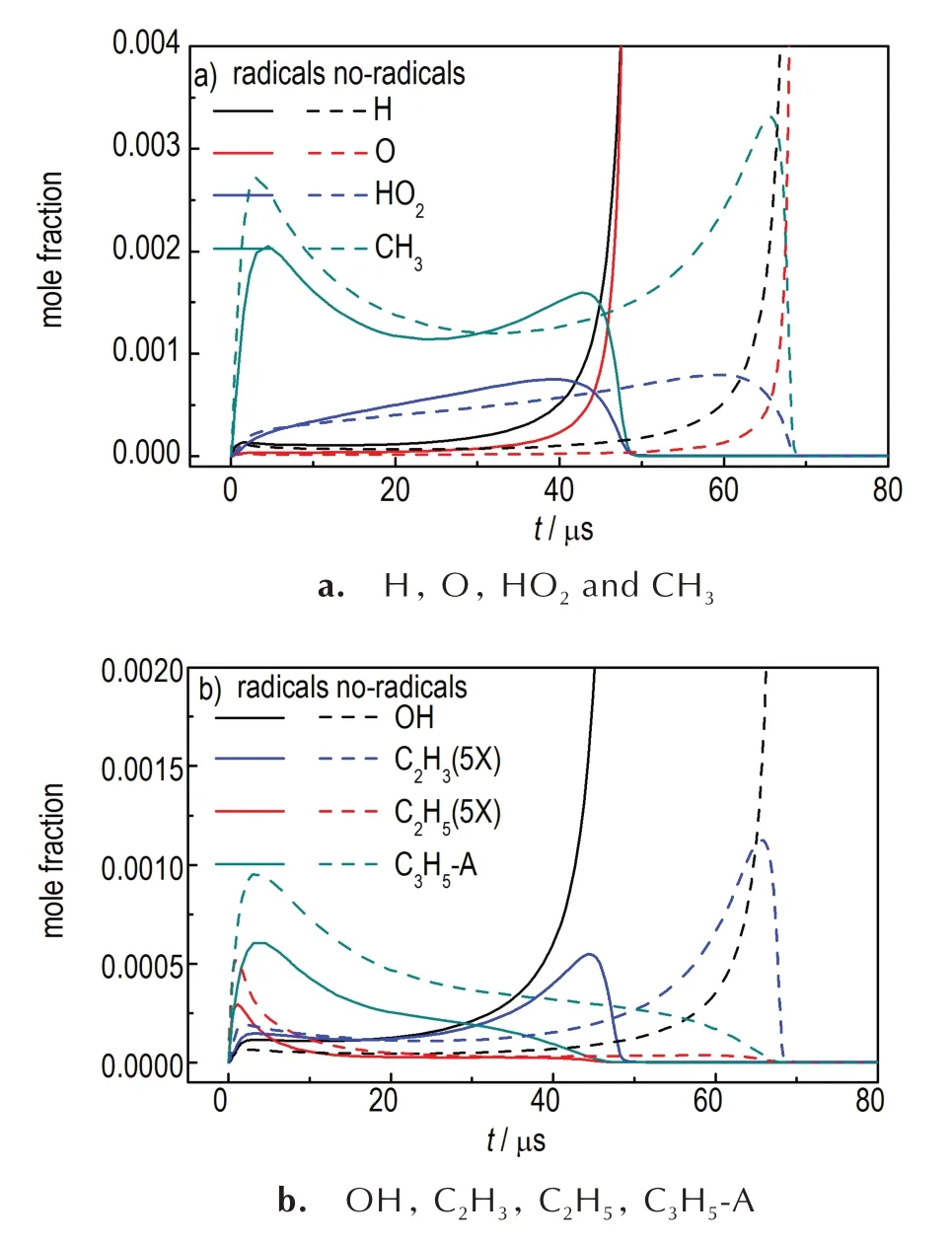
Fig.8 Predicted profiles of major radicals of two cracked n-decane with or without radicals at 0.1 MPa,1500 K,φ=1
3.2.4 Effect of ignition pressures
The impact of ignition pressures on ignition delay times of crackedn-decane were also studied.Figure 9 shows the ignition delay time of crackedn-decane obtained under 5 MPa andx= 40% at temperatures from 1300 K to 1800 K,pressures from 0.1 MPa to 3.0 MPa.It could be noted that higher ignition pressures result in much shorter ignition delay times,resulting from higher concentration of fuels at higher pressures.
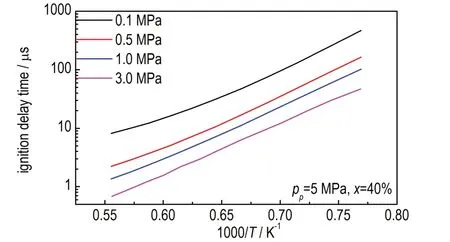
Fig.9 Simulated ignition delay times of cracked n-decane under 5 MPa and x = 40% at different ignition pressures of 0.1-3.0 MPa
4 Conclusion
In advanced aircraft,endothermic hydrocarbon fuels firstly undergo thermal cracking to produce complex pyrolysis products and small amounts of free radicals prior to combustion.In this work,theoretical calculation was conducted to investigate the ignition delay time of cracked fuels(composed of unreacted fuels and pyrolysis products) by usingn-decane as the surrogate of endothermic hydrocarbon fuels.The results showed the conversion rates ofn-decane cracking at 3 and 5 MPa are 46.2% and 58.8%,respectively.The distribution of cracking products is consistent,but the ethylene content decreases with the increase of pressure,while the alkane content increases with the increase of pressure.Meanwhile,the content of free radicals at 3 MPa is slightly higher than that at 5 MPa,but the content of free radicals is very low.Ignition delay time increases with the decreasing ofn-decane conversion and pyrolysis pressure,while higher ignition pressure can shorten it significantly.Furthermore,the presence of free radicals in crackedn-decane could accelerate the ignition process with ignition delay time shortening more than 15% when the conversion was less than 40%,compared with that of crackedn-decane without radicals.Sensitivity and rate of production analysis of major free radicals were conducted to try to analyze the above results,showing that most of sensitivity reactions are associated to C0—C3 reactions,and more vinyl radical(C2H3)and a little less methyl radical and allyl radical (C3H5-A) formed during the ignition process could be helpful in the ignition stage of crackedn-decane.
Pyrolysis of fuels has a positive impact on ignition of cracked fuels in this theoretical work,exhibiting different results compared with experimental results.It is noteworthy that free radicals in cracked fuels display significant importance in ignitability of cracked fuels.Further efforts should be focused on experimental to study how radicals impact on the ignition delay time,to modify the mechanism and provide guidance for endothermic hydrocarbon fuels used in advanced aircraft.

