Auto-ignition Characteristics of Gasoline and Diesel Fuel Blends:A High-Pressure Ignition Delay and Kinetic Modelling Study
LI Yang
(1.Science and Technology on Combustion,Internal Flow and Thermo-structure Laboratory,School of Astronautics,Northwestern Polytechnical University,Xi'an 710072,China;2.Clean Combustion Research Centre,King Abdullah University of Science and Technology,Thuwal,Saudi Arabia)
Abstract:The ignition delay times(IDTs)of two different certified gasoline and diesel fuel blends are reported.These measurements were performed in a shock tube and in a rapid compression machine over a wide range of experimental conditions(φ= 0.5-2.0,T=700-1400 K and p=10-20 bar)relevant to internal combustion engine operation.In addition,the measured IDTs were compared with two relevant gasoline fuels:Coryton gasoline and Haltermann gasoline systematically under the same experimental conditions.Two different gasoline surrogates a primary reference fuel(PRF)and toluene PRF(TPRF)were formulated,and two different gasoline surrogate models were employed to simulate the experiments.Typical pressure and equivalence ratio effects were obtained,and the reactivity of the four different fuels diverge in the negative temperature coefficient(NTC)regime(700-900 K).Particularly at 750 K,the discrepancy is about a factor of 1.5-2.0.For the high Research Octane Number(RON)and high-octane sensitivity fuel,the simulation results obtained using the TPRF surrogate was found to be unreasonably slow compared to experimental results,due to the large quantity of toluene(77.6% by volume)present.Further investigation including reactants'concentration profile,flux and sensitivity analyses were simultaneously carried out,from which,toluene chemistry and its interaction with alkane(n-heptane and iso-octane)chemistry were explained in detail.
Key words:Gasoline and diesel;Shock tube;Rapid compression machine;Ignition delay time;Gasoline surrogate
1 Introduction
Commercial transportation grade gasoline and diesel are the most widely used light- and heavy-duty transportation fuels and are complex mixtures of hundreds of hydrocarbons including linear and branched paraffins,naphthenes,olefins,and aromatics[1-2].The co-optimization of fuel/engine systems requires an in-depth knowledge of the autoignition behavior of the fuel.Particularly for higher efficiency spark ignition engines, knocking of the end-gas is fundamentally related to the fuel's autoignition(combustion kinetics)characteristics[3-4].
Recently,there have been very limited experimental autoignition measurements of gasoline and/or diesel fuels in shock tubes(ST)and rapid compression machines(RCMs) in the literature.To the best of our knowledge,the only ignition delay time(IDT)measurements of diesel fuel in a RCM were performed by Kukkadapu et al.[5],in which the IDT measurements of two well-characterized reference diesel fuels,namely commercial grade ultra-low-sulfur diesel(ULSD#2) and Fuels for Advanced Combustion Engines research diesel(FD9A).The experimental results showed that diesel blends with similar cetane ratings and different compositional makeups exhibited varying ignition propensities over different temperature regimes,thereby demonstrating the effect of molecular composition on autoignition characteristics.In particular the difference in ignition propensities was observed at temperatures at which the low temperature branching reactions are active.We have summarized the literature studies that have fo-cused on autoignition of diesel fuels in Table S1 in the Supplementary Material 1,including the facilities used and the conditions of pressure,temperature,equivalence ratio,and dilution studied in the experiments.
Lee et al.[6]investigated the autoignition behavior of two oxygenated certification gasoline fuels:Haltermann Gasoline(RON=91)and Coryton gasoline(RON=97.5)in both a high-pressure shock tube and in a rapid compression machine at pressures of 10,20 and 40 atm,and equivalence ratios of 0.45,0.9 and 1.8.The experimental results were simulated using three different gasoline surrogate models.It was found that the effects of fuel octane number and fuel composition on ignition characteristics are strongest in the intermediate temperature(negative temperature coefficient)regime.Also,it was shown that more complex surrogate mixtures are needed to emulate the reactivity of gasoline with higher octane sensitivity(S=RON-MON).
As a succeeding investigation of the above study from Lee et al.[6],the objectives of this study are three fold:1) To investigate the autoignition characteristics of two oxygenated gasoline/diesel fuel blends over a range of pressures(10-20 bar),temperatures(700-1400 K)and equivalence ratios in a well heated ST and a RCM;2)To formulate two different gasoline surrogates;a PRF and a TPRF,and simulate the experimental results using two different gasoline surrogate models from Lawrence Livermore National Laboratory (LLNL)[7]and King Abdullah University of Science and Technology(KAUST)[8];3)To systematically compare the reactivity of the two gasoline fuels studied by Lee et al.[6]and the two gasoline/diesel fuel blends developed in this study.
2 Experimental method and conditions
The two certification gasolines/diesel fuel blends used in this study were supplied by Coryton Advanced Fuels,and the fuel compositions were determined using detailed hydrocarbon analysis(DHA)at Saudi Aramco's Research and Development Center as described by Lee et al.[6].
The compositions of Coryton gasoline and diesel were summarized in Table S2 and S3 in the Supplementary Material 1 respectively,using the above detection method.The two Coryton gasoline/diesel fuel blends used in this study were blended using 75/25 and 50/50 (% by volume) gasoline/diesel,respectively,as shown in Table 1.

Table 1 The two Coryton gasoline/diesel fuel blends used in this study
Table 2 summarized the experimental conditions investigated in this work,notably,experiments of Coryton Gasoline and Haltermann Gasoline were carried in the paper by Lee et al.[6].These are condi-tions of direct relevance to gasoline, diesel,and low-temperature combustion(LTC)engine technologies.In this way,for each individual fuel,the pressure and equivalence ratio effects on IDT can be systematically evaluated,and at an equivalence ratio of 0.5,1.0 and a pressure of 10,20 atm the reactivity can be consistently compared.
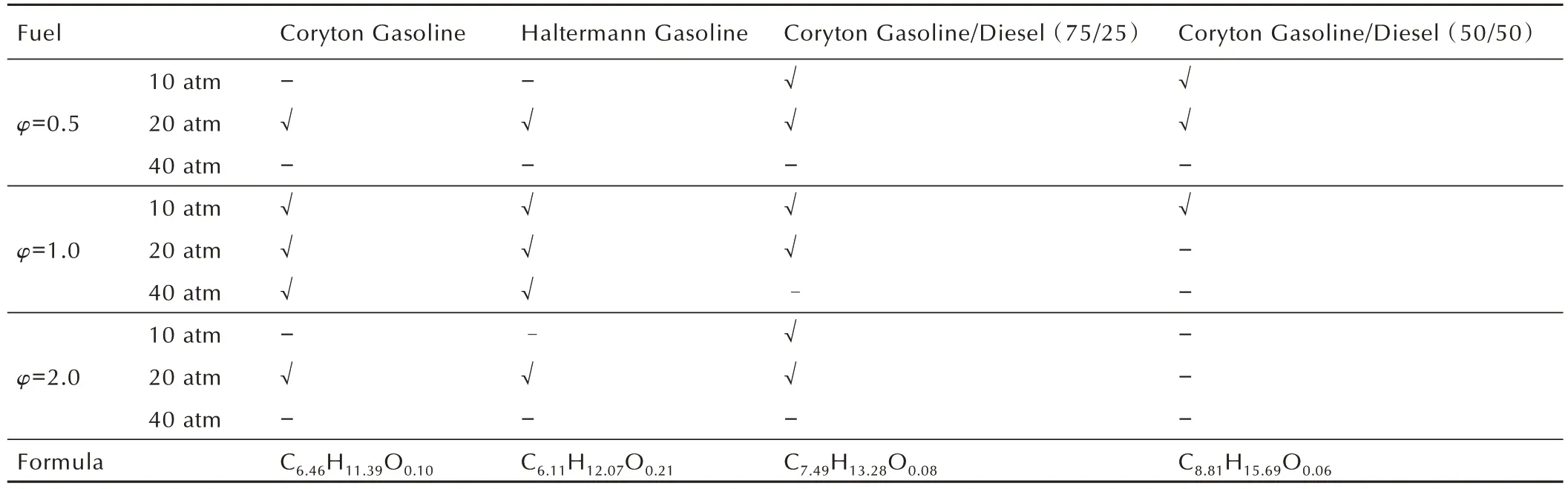
Table 2 Experimental conditions of four gasoline and diesel fuels and fuel blends.
All experiments were performed in the NUI Galway HPST and RCM facilities as described previously by Lee et al.[6].Detailed uncertainty analysis of the measurements was introduced by Li et al.[9].It is worth nothing that,to perform experiments on these two extreme,low-vapor pressure gasoline/diesel fuel blends,the heating system of the RCM was systematically upgraded,and the initial temperatures of the HPST and RCM were maintained at 120 ℃and 150 ℃,respectively.
Furthermore,to confirm that these two low-vapor pressure gasoline/diesel fuel blends did vaporize,an optically accessible test cell was constructed to measure the IR absorption coefficients of the fuel mixture at 3.39 µm,and the measurement was carried out on the preheated HPST as well.Using this arrangement,a two-point transmitted light intensity measurement yields the test gas absorption coefficient in accordance with the Beer-Lambert law:

whereAis light absorbance,εis the molar absorption coefficient,lis the optical path length andcis concentration.I0andITare the incident and transmitted light intensities.
Figure 1 shows good agreement between the absorbance measurements as a function of partial pressure for the Coryton gasoline/diesel 75/25 fuel blends injected into the test cell and fuel/air mixture prepared in the HPST.The measurements in the HPST cover the upper limit of total pressure used in the experiments(≈1500 mbar),and the molar absorption coefficientεfor this gasoline/diesel blend has been calculated to be 9.62 m2·mol-1.
In addition,a simple injection test was also performed simultaneously.By injecting 0.5-1.0 ml volume of fuel into a vacuumed fuel tank at one time,the pressure was monitored instantly.Figure 2 shows the testing results,in which a linear relation between the monitored pressure and injected volume of fuel was observed,this again testified the complete vaporization of the gasoline and diesel blends.
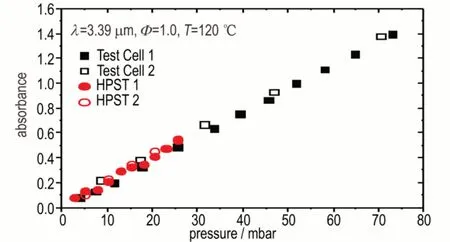
Fig.1 Fuel concentration measurements by 3.39 µm He-Ne laser absorption(Coryton gasoline/diesel 75/25 fuel blends)

Fig.2 Fuel injection test:monitored pressure as function of injected volume of fuel
3 Surrogate model simulation
Octane number(ON)is an important indicator of the anti-knock quality of commercial gasoline fuels.It is experimentally measured in a Cooperative Fuel Research(CFR)engine under two standard operating procedures: ASTM D2699[10]and ASTM D2700[11],resulting in the research octane number(RON)and motor octane number(MON)respectively.Primary reference fuels(PRF)is the simplest surrogate used for representing a real gasoline fuel,which are binary blends of iso-octane andn-heptane.By definition,the RON and MON values assigned for iso-octane and n-heptane are both 100 and 0 respectively.In addition,the difference and average values between the two properties are defined as octane sensitivity(S=RON-MON)and anti-knock index(AKI=(RON+MON)/2)respectively.In order to match the octane sensitivity of a commercial fuel,toluene primary reference fuel(TPRF) is another commonly used simple gasoline surrogate,which is a ternary mixture of PRF-components and toluene.Toluene is a highly unsaturated hydrocarbon or aromatic hydrocarbon,with the RON and MON values of 120 and 109 respectively[12].
Table 3 summarized the key properties of investigated in this work and from that of Lee et al.[6],which include the research octane number(RON),motor octane number(MON),octane sensitivity and anti-knock index(AKI)values.In general,these properties all decrease as we move from the pure Coryton gasoline fuel to the(50/50)Coryton gasoline/diesel blend.Based on these properties,two different gasoline surrogates(a PRF and a TPRF blend)were formulated by emulating the AKI,RON and octane sensitivity[12-13],as shown in Table 4.

Table 3 Comparison of the key properties of four different fuels
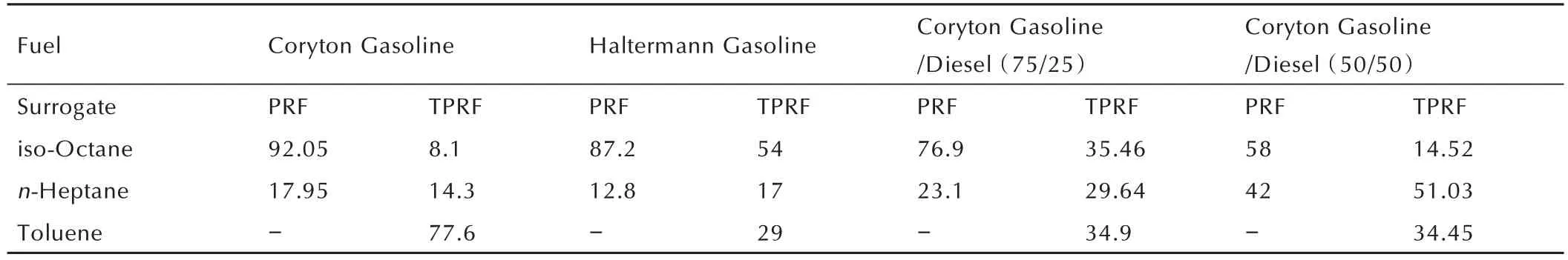
Table 4 Gasoline surrogate formulation for four different fuels
Two different gasoline surrogate models,one from Lawrence Livermore National Laboratory(LLNL)[7]and the other from King Abdullah University of Science and Technology(KAUST)[8],were employed to simulate the experimental data using the two gasoline surrogates formulated above:
⋅LLNL(1389 species and 5935 reactions)
⋅KAUST(2768 species and 9236 reactions)
4 Results and Discussion
Atφ= 1.0 andp= 10 atm,the IDTs of the four different fuels are compared,Figure 3.At intermediate to high temperatures(900-1400 K),the four fuels have the same reactivity(solid symbols),while in NTC regime(700-900 K),IDTs decrease with the pure Coryton fuel having the longest ignition times and the 50/50 Coryton gasoline/diesel fuel having the fastest ignition times(open symbols).Particularly at around 750 K,the IDT differ by about a factor of 2.0 between the two different(75/25 and 50/50)Coryton gasoline/diesel fuel blends.
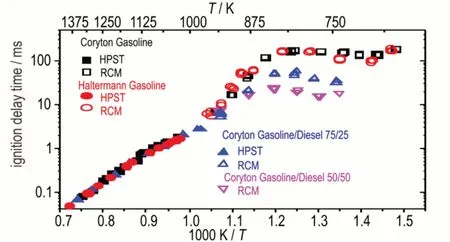
Fig.3 Fuel reactivity comparison at φ = 1.0 and p = 10 atm
Figure 4 presents the pressure effect on IDTs for the Coryton gasoline/diesel 75/25 fuel blend for data measured in both the HPST and in the RCM atφ=1.0,p=10 and 20 atm.In addition,simulation results generated using KAUST model and two different gasoline surrogates are also systematically compared.Similarly,the equivalence ratio effect on IDTs for the Coryton gasoline/diesel 50/50 fuel blend is shown in Figure 5 for experimental results obtained in the RCM atφ=0.5 and 1.0,p=10 atm.Figure 5 also include simulated ignition times using KAUST model and two different gasoline surrogates.Note,because of the limitation of the length of this paper,the simulation results generated using LLNL model and two different gasoline surrogates were presented and systematically compared in supple-mentary material.All these simulations have taken the facility effects into account(heat loss of RCM),and the volume-time histories have also been provided as supplementary material.

Fig.4 IDT simulation for Coryton gasoline/diesel 75/25 fuel blend using two different gasoline surrogates at φ=1.0,p=10 and 20 atm
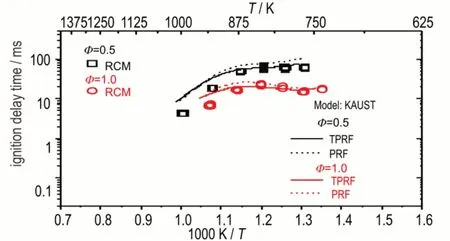
Fig.5 IDT simulation for Coryton gasoline/diesel 50/50 fuel blend using two different gasoline surrogates at φ=0.5 and 1.0,p = 10 atm
The experimental results show a clear negative temperature coefficient(NTC)behavior in the temperature range 750 to 900 K,it is worth nothing that because of the high initial temperature(150 ℃)setting,even by using 100% CO2as diluent gas,the lowest compressed gas temperature achieved here is approximately 750 K.The reactivity increases with increasing pressure and equivalence ratio.The divergence becomes more pronounced in the NTC regime,due to an increased concentration of fuel,since fuel radical chemistry dominates the fuel's reactivity in this temperature regime.In general,both gasoline surrogates can capture well the experimental IDTs over a wide range of temperatures,equivalence ratios and pressures.The TPRF surrogate performs better than the PRF surrogate as we expected,and the PRF surrogate shows a more pronounced NTC behavior than the TPRF surrogate.
Moreover,to systematically compare the reactivity of the four different fuels and eliminate the effect of the limited quantity of experimental data presented in Table 2 in addition to facilities effects,constant volume simulations have been performed for these four different fuels,using two different gasoline surrogates and two kinetic models,at three different equivalence ratios and two different pressures.Here,simulation results atφ=1.0 andp=10 atm were selected as representatives,shown in Figure 6.The reactivity of four different fuels were compared as a function of different gasoline surrogates and kinetic models.At intermediate to high temperatures(900-1400 K),all four fuels present the same reactivity,which indicates the dominance of oxygen chemistry over this temperature regime.In the NTC regime(700-900 K),IDTs decrease from the fuel with the highest octane number(pure gasoline) to that with the lowest (50/50 gasoline/diesel).Particularly at 750 K,the discrepancy is about a factor of 1.5-2.0.This discrepancy corresponds to the experimental data shown in Figure 3.In general,the LLNL model shows a more pronounced NTC behavior than the KAUST model,and the PRF surrogate shows a more pronounced NTC behavior than the TPRF surrogate.It is worth noting that the simulation results of the high RON and high sensitivity fuel(Coryton gasoline) using the TPRF surrogate are unreasonably slow which has been highlighted in Figure 6,this will be further investigated in the next section.
5 TPRF surrogate chemistry
In this section,we aim to investigate the TPRF surrogate chemistry for Coryton gasoline.Given that the IDT of Coryton gasoline was over predicted by TPRF surrogate using the LLNL model[7]and KAUST model[8](shown in Figure 6),one more recently updated gasoline surrogate model developed from LLNL[14-15]is used in this section,which has been named as“LLNL_New”.The major difference between the LLNL and LLNL_New model is the toluene sub-mechanism,LLNL_New model has adopted an updated toluene model developed by Zhang et al[16],which has been well validated.Figure 7 shows the comparison of toluene IDT predictions using four different models(in different colors),it can be seen that Zhang and LLNL_New models predict identical results,so do LLNL and KAUST models.Meanwhile,the update toluene chemistry shows less reactivity than the old ones.
Fig.6 Constant volume IDT simulation for four different fuels at φ = 1.0 and p = 10 atm
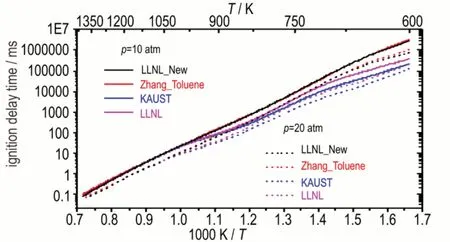
Fig.7 Comparison of toluene IDT predictions using four different models at φ = 1.0,and p = 10 and 20 atm
Figure 8 shows the IDT simulation based on TPRF surrogate for four different fuels using LLNL_New and KAUST models atφ=1.0 andp=10 atm.For the Coryton gasoline simulation in black color,LLNL_New model predicts much shorter IDTs than KAUST model towards low temperature(T<800 K),indicating a more pronounced NTC behavior.Interestingly,with large quantity of toluene(77.6% by volume)in the TPRF surrogate,such reactivity difference is opposite to that shown in Figure 7.Therefore,a more detailed investigation was carried out including the reactants'concentration profile,flux and sensitivity analyses,all simulation methods and approaches can be found in our recent publications[17-18].
Figure 9 shows the reactants'concentration and pressure profiles for Coryton gasoline oxidation using TPRF surrogate and LLNL_New model atφ=1.0,p=10 atm andT=750 K.In the figure,different line colors and types stand for different components and models respectively,and the pressure traces are in magenta color corresponding to the y-axis at right side.A clear two-stage ignition event was observed for simulation using LLNL_New model,which can be attributed to the low-temperature oxidation chemistry of saturated alkyl radicals from iso-octane andn-heptane.
Figure 10 shows the flux analysis for the TPRF surrogate blend atφ=1.0,p=10 atm,T=750 K,and at the timing of the rapid decay of reactants after the first stage ignition,which is around 30 ms.In order to make the results more visualized,only the qualitative or generalized reaction pathways were exhibited in the figure,while the quantitative values for the flux of all reactions have been gathered in the Supplementary Material 2.As can be seen,both iso-octane andn-heptane exhibit typical alkyl radical low temperature chemistries,the competition between the HO˙2radical concerted elimination from RO2and RO2isomerization to QOOH leads to the NTC behavior[19-20].
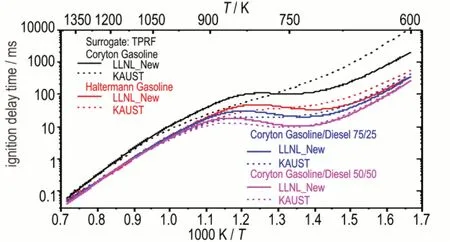
Fig.8 IDT simulation based on TPRF surrogate for four different fuels using LLNL_New and KAUST models at φ=1.0 and p=10 atm

Fig.9 Reactants' concentration and pressure profiles for Coryton gasoline oxidation using TPRF surrogate and LLNL_New model at φ = 1.0,p = 10 atm and T = 750 K
Figure 11 shows the sensitivity analysis for pure toluene and TPRF surrogate blend atφ= 1.0,p= 10 atm andT= 750 K,it can be seen that,the most inhibiting reaction is the fuel initiation chemistry:H-atom abstraction from the allylic H-atom on the methyl group by O˙H radical forming the benzyl radical(C6H5CH2).Thereafter,the reactions between the formed benzyl radical(C6H5CH2)and HO˙2radical on the potential energy surface (PES) of C6H5CH2OOH took over the dominance,the association reaction forming benzyl-peroxy (BZCOOH)and chemical activation reaction forming benzoxy radical(C6H5CH2O˙) plus O˙H radical became the top reactivity inhibiting and promoting reactions respectively.Of interest is that sensitivity analysis results shows that by mixing toluene into then-heptane and iso-octane,the main promoting reaction,namely allylic H-atom abstraction by molecular oxygen (C6H5CH3+O2↔ C6H5CH2+HO2) turned into an inhibiting reaction,as highlighted in red rounded rectangles.The flux analysis shows that,this abstraction reaction actually occurs in the reverse direction.To summarize,by blending toluene with PRF,HO˙2radical can be sourced from the concerted elimination reaction of RO2radicals from the iso-octane and n-heptane oxidation.Then it reacts with benzyl radi-cal(C6H5CH2) via association or chemical activation reactions on the C6H5CH2OOH PES,or reacts reversely to give initial reactants(toluene and molecular oxygen).With more accurate toluene chemistry,LLNL_New model was able to predict above complex interaction,which results in more reasonable IDT results as shown in Figure 8.
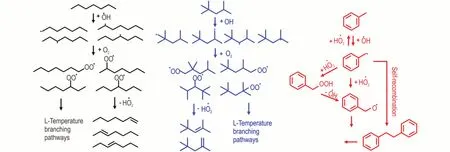
Fig.10 Flux analysis for the TPRF surrogate blend at φ = 1.0,p = 10 atm and T = 750 K
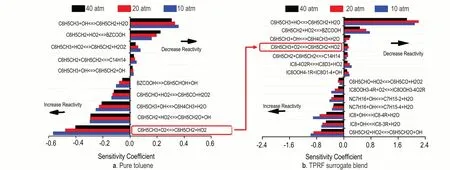
Fig.11 Sensitivity analyses for pure toluene and TPRF surrogate blend at φ = 1.0,p = 10 atm and T = 750 K
6 Conclusions
This work represents an ignition delay study of two Coryton gasoline/diesel fuel blends(75/25 and 50/50)oxidation at elevated pressures in a HPST and in a RCM over a wide range of pressures,temperatures and equivalence ratios.The results presented provide ignition delay time(reactivity)of Coryton gasoline/diesel fuel blends at engine relevant conditions.It was found that increasing pressure resulted in shorter measured ignition delay times(higher reactivity)for all equivalence ratios investigated,which is typical of the influence of pressure on fuel reactivity.The effect of equivalence ratio on ignition delay times depended on the temperature regime of the experiment,whereas all mixtures had similar reactivity at higher temperatures(>900 K),fuel-rich mixtures are most reactive at lower temperatures(<900 K).NTC behavior was observed in the low temperature regime(700-900 K).
In addition,the reactivity of four different gasoline and diesel fuels:Coryton gasoline,Haltermann gasoline,Coryton gasoline/diesel 75/25 and Coryton gasoline/diesel 50/50 fuel blends were systematically compared under the same experimental conditions.It was found that the lower RON fuel has shorter IDT than that of higher RON fuel,and this phenomenon becomes more pronounced the richer the fuel blend(φ=2.0),particularly in the NTC regime;at 750 K the discrepancy is about a factor of 1.5-2.0.
It was found that TPRF surrogate simulation results for high RON and high octane sensitivity fuel(Coryton gasoline) are unreasonably slow using both LLNL and KAUST models,and this is mainly due to the large quantity of toluene(77.6% by volume)in the surrogate.A detailed investigation was then carried out using a recently published LLNL_New model which contains an updated toluene sub-mechanism.Reactants'concentration profile,flux and sensitivity analyses were simultaneously performed,from which,toluene chemistry and its interaction with alkane(n-heptane and iso-octane)chemistry were discovered.In toluene oxidation,HO˙2radical was only involved with the reaction with the benzyl radical(C6H5CH2),however,three different reaction types were found to control the reactivity comprehensively:(a) association reaction forming the benzyl-peroxy(BZCOOH);(b)chemical activation reaction forming the benzoxy radical(C6H5CH2)plus O˙H ;(c) reverse reaction of H-atom abstraction from methyl group by molecular oxygen(C6H5CH3+ O2↔ C6H5CH2+ HO2).

