Isoflavones and inflammatory bowel disease
Ze-Yu Wu, Li-Xuan Sang, Bing Chang
Ze-Yu Wu, Bing Chang, Department of Gastroenterology, The First Affiliated Hospital of China Medical University, Shenyang 110001, Liaoning Province, China
Li-Xuan Sang, Department of Geriatrics, The First Affiliated Hospital of China Medical University, Shenyang 110001, Liaoning Province, China
Abstract
Key words: Isoflavone; Metabolism; Effects; Inflammatory bowel disease; Structure;Treatment
INTRODUCTION
Inflammatory bowel disease (IBD) is a chronic recurrent inflammatory disease. There are three forms of IBD: Ulcerative colitis (UC), Crohn’s disease (CD), and indeterminate colitis[1,2]. The etiology is not very clear, and this disease has a close relationship with genes, immunity, intestinal flora,etc[3]. The incidence of IBD is increasing[4,5]. IBD has become a global health problem[6]. Studies found that diet can affect the intestinal flora of the human body and then affect human immunity[7-9].Therefore, diet plays an important role in the occurrence and development of IBD.
A Westernized diet can promote the occurrence and development of IBD[10]. A large intake of total fats, polyunsaturated fatty acids, meat, and omega-6 fatty acids increased the risk of UC and CD, while a high intake of dietary fiber, fruits, and vegetables can reduce the risk[11]. Some foods, such as omega-3 fatty acid-rich fish,monounsaturated fat, fruits, and vegetables, are recommended as part of the diet of IBD patients[7]. The Asian population has its own dietary structure and soy products are an integral part, thus the intake of isoflavones in Asians is high[12]. Isoflavones are a class of phytoestrogens, the structure of which is similar to that of estrogen[13], and they were reported to have effects on many diseases, including osteoporosis[14],cardiovascular disease[15], breast cancer[16], colorectal cancer[17], and Alzheimer’s disease[18]. In recent years, studies have reported the effect of isoflavones on IBD. In this paper, we review the relationship between isoflavones and IBD.
ISOFLAVONE
Isoflavones constitute a class of phytoestrogens, which include genistein, daidzein,glycitein, formononetin, biochanin A, and irilone. The first three are from soybeans,and the last three are from red clover[13,19]. The content of isoflavones is the most abundant in soybeans and their derivatives, which are the major source of isoflavones for humans. In addition, the content of isoflavones in different derivatives is related to the processing methods of soybeans[20,21]. There are two forms of isoflavones:Glucosides and aglycones[13,22], which are absorbed in the human body in both the small and large intestine[23]. Isoflavones in the form of aglycones are absorbed faster than glucosides, which can be hydrolyzed into bioactive aglycones in the proximal intestine for better absorption[24,25]. Isoflavones are mainly in the form of glucuronide in human blood, accounting for 75%; 24% in the form of sulfate; and ≤ 1% in the form of aglycone[26]. Isoflavones are similar to estrogen in structure, thus, isoflavones can bind to estrogen receptors[27].
METABOLISM OF ISOFLAVONES IN THE INTESTINE
Isoflavones can be metabolized into equol and O-demethylangolensin(O-DMA)[28].The metabolism for these is different in different individuals. In China, Korea, and Japan, more than 80% of the population can produce equol, while only 25% of the population in North America and Europe can produce it[25]. There are a large number of intestinal floras that play an important role in metabolism. Many intestinal floras can metabolize isoflavones. Some lactic acid bacteria and bifidobacteria with βglucosidase activity can transform glycoside into aglycone isoflavone[29]. Lactic acid bacteria and bifidobacteria prefer to transform genistin into genistein[30]. However, not all isoflavones can be metabolized by intestinal floras. Irilone, a red clover isoflavone,is difficult for intestinal bacteria to degrade[19].
Eubacterium limosumis an absolute anaerobic bacterium in the human intestinal tract, which was shown to O-demethylate isoflavones and convert biochanin A,formononetin, and glycitein into genistein, daidzein, and 6,7,4’-trihydroxyisoflavone,respectively, but it could not further transform these three products[31].Bifidobacteriumwas demonstrated to convert daidzin into daidzein, but not into equol[32].Escherichia coliHGH21 and a Gram-positive (G+) strain HGH6 transformed daidzin and genistin into daidzein and genistein, respectively[33].Clostridium sp. strainHGH 136, a G+anaerobic bacterium in the gut, transformed daidzein into O-DMA by cutting off the C-ring of isoflavones[34].Eubacterium ramulusalso converted daidzein to O-DMA[35].Strain SY8519, a strain belonging toClostridiumrRNA cluster XIVa in the human intestine, produced O-DMA from daidzein[36].
Some intestinal microfloras were shown to convert daidzein and genistein into dihydrodaidzein and dihydrogenistein, two intermediate metabolites, respectively[29,37]. Tamuraet al[38]isolated aClostridium-like bacterial strain, TM-40, from the feces of a 7-year-old boy. It could transform isoflavones both daidzein and daidzin into dihydrodaidzein but did not produce equol. The strain was 93% similar toCoprobacillus catenaformis[38].Enterococcus faecalisINIA P333 andLactobacillus rhamnosusINIA P540 converted daidzein into DHD[29]. In an anaerobic environment, the G+strain HGH6 further converted daidzein and genistein into dihydrodaidzein and dihydrogenistein, respectively, but HGH6 could not further convert dihydrodaidzein and dihydrogenistein[33].
Certain bacteria can convert isoflavones into equol. The rod-shaped Gram-negative bacterium,Eggerthellastrain Julong 732, in the human intestine, was demonstrated to transform dihydrodaidzein into S-equol in an anaerobic environment. Full 16S RNA gene sequence analysis indicated that the bacterium was 92.8% similar toEggerthella hongkongenisHKU10[39,40].Eggerthella sp. YY7918, a bacterium that is 93.3% similar toEggerthella hongkongenisHKU10, also converted daidzein and dihydrodaidzein into equol[41].Slackia isoflavoniconvertensconverted daidzein into equol and genistein into 5-hydroxy-equol[42].Slackia equolifaciens sp. nov., Slackia sp. strain NATTS, andLactococcusstrain 20-92 converted daidzein into equol[43-45]. The metabolic process of isoflavones is shown in Figure 1.
CHANGES IN IBD
UC and CD are the two main forms of IBD. UC can affect the end of the ileocecum, the whole colon, and rectum, and CD can affect the whole digestive tract, which seriously affects the quality of life for patients. The changes of IBD can occur in the intestinal mucosal barrier, the intestinal flora, and their metabolites, cytokines.
The intestinal barrier of IBD patients is abnormal, and the mucous layer and epithelial cells of the intestinal tract are changed. Mucin 2 and IgG Fc-binding protein,the main components of the mucus layer of intestinal barrier, decrease in the active UC, leading to a thinning of the mucus layer. This change occurs in the early stage of UC, even before the inflammation[46,47]. Intestinal mucosal barrier damage and increased intestinal mucosal permeability are associated with the occurrence of UC and CD[48]and abnormal intestinal tight junctions are one of mechanisms leading to these changes[49].
There are abnormalities in the cytokines of IBD patients. For example, studies found that interleukin (IL)-17A and IL-23 were increased in IBD patients, and they were increased more in UC than in CD[50]. Lena Öhmanet al[51]found that the level of serum IL-17A was related to the severity of newly diagnosed, untreated UC.Cytokines, such as the IL-1 family and IL-17, play an important role in the development of IBD[52].
An imbalance of intestinal flora is closely related to the incidence of IBD. The abundance, evenness, and diversity of intestinal flora in UC patients are reduced[53].Some gut microfloras that are very important for maintaining intestinal homeostasis show dysbiosis, such as the butyrate-producing microflora, humanRoseburia hominis,andFaecalibacterium prausnitzii(F. prausnitzii), which are reduced in UC patients[54].Even in the first-degree relatives of UC patients,F. prausnitziiis decreased[55]. In addition to UC patients, healthy people living with UC patients also have certain intestinal dysbiosis[56]. The dysbiosis in CD is more serious than that in UC[57], mainly causing a reduction of the phylum Firmicutes[58]. The dysbiosis of viruses and fungi also plays a role in the development of IBD[59,60].
An imbalance of metabolites, caused by the imbalance of intestinal flora, also affects the occurrence and development of IBD. Short-chain fatty acids (SCFAs),including acetic acid, propionic acid, and butyric acid, are produced by intestinal microflora and play an important role in the intestinal tract[61]. SCFAs were reported to promote the production of IL-10, an anti-inflammatory cytokine, by Th1 cells and to have anti-inflammatory effects[61,62]. In a colitis mouse model, it was found that butyrate, an SCFA, can maintain the balance of Th17/Treg in the intestinal tract and has an anti-inflammatory effect[63]. A decrease of SCFA can also increase the intestinal permeability[64]. Therefore, the regulation of intestinal microflora and its metabolites is of great significance to the recovery of IBD.
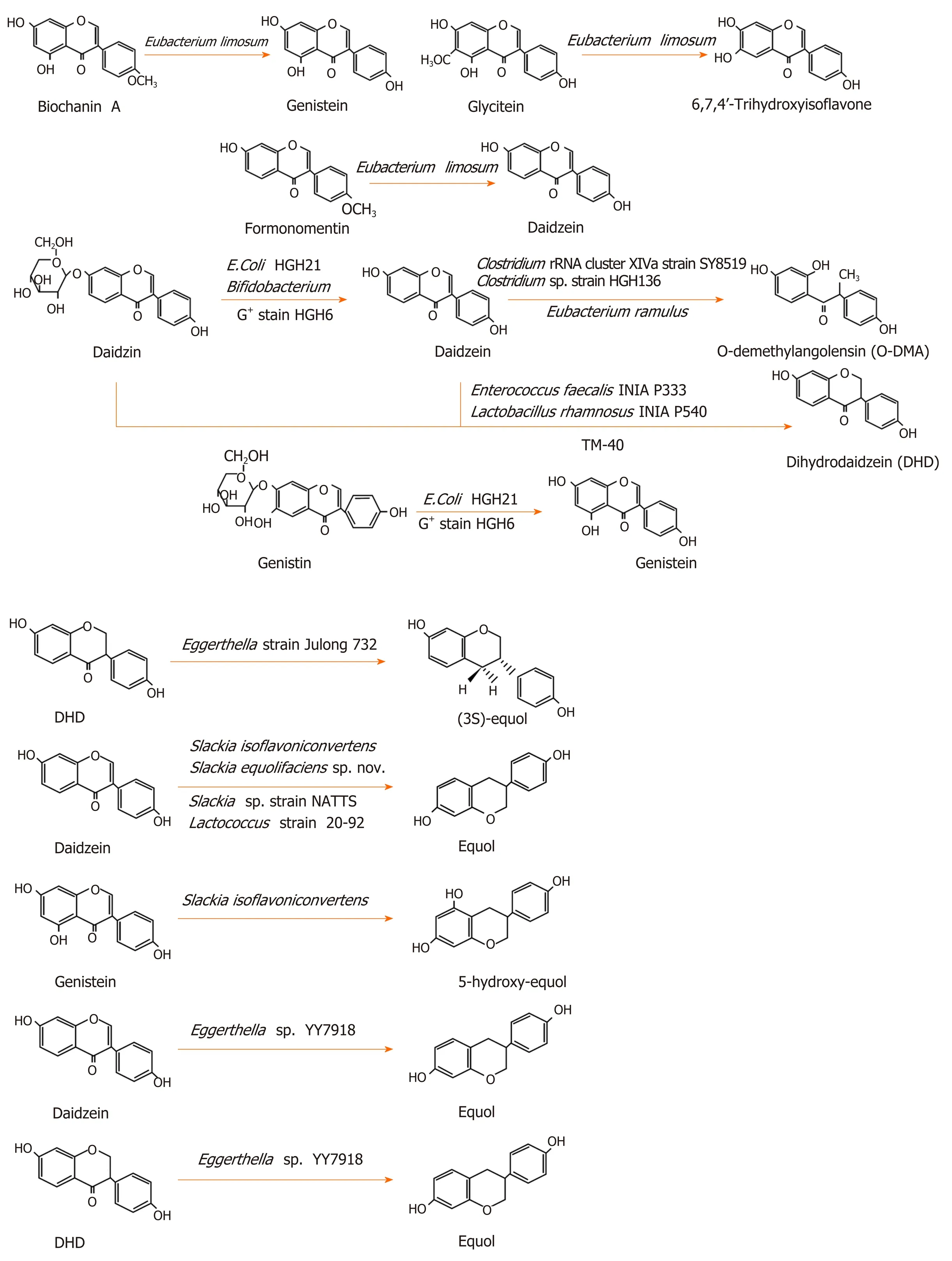
Figure 1 The metabolic process of isoflavones[13,27,29,31-36,38-45,117].
ISOFLAVONE AND IBD
In recent years, studies have reported the effects of isoflavones on IBD. In a mouse model, daidzein and glyceolins (isopentehexene isoflavone, a derivative of daidzein,produced by soybeans under stress), relieved dextran sulfate sodium (DSS)-induced colitis, therefore, they may be used in the treatment of UC[65,66]. A moderate isoflavone intake by UC patients in the remission period was beneficial[67]. Isoflavones demonstrated effects on the symptoms of UC patients in the remission period.Isoflavones intake may contribute to reducing the incidence of abdominal pain[67]. A high intake of daidzein and total isoflavones helped to reduce the mucus in the feces of UC patients; however, a high intake of daidzein alone may increase fecal pus[68]. It was reported that soybean isoflavones may have a preventive effect on CD[69].
Isoflavones have an anti-inflammatory effect[70]. After treatment with genistein, an isoflavone, inflammation was reduced in a 2,4,6-trinitrobenzenesulfonic acid-induced colitis rat model[71]. Isoflavones play a role in many aspects of inflammation. The expression and activation of the signal translator and activator of transcription 1 increased in the intestines of patients with IBD[72]. Daidzein and genistein were shown to inhibit signal translator and activator of transcription 1 and then to reduce the expression of inducible nitric oxide synthase (iNOS)[73]. The overproduction of NO caused by the induction of iNOS could lead to massive prostaglandin E2 (PGE2)production through cyclooxygenase-2 S-nitrosylation[74]. PGE2 has a proinflammatory effect[75]and massive PGE2 could damage the intestinal integrity[76].
Isoflavones were shown to inhibit the tumor necrosis factor α (TNF-α) induced production of chemokine IL-8 by Caco-2 cells[77], reduce the inflammatory cell infiltration in the intestines, decrease the level of myeloperoxidase, inhibit the nuclear factor-κB (NF-κB) pathway and the expression of inflammatory factors (such as IL-6,IL-1 β, and TNF-α), and reduce the levels of NO and PGE2 in colonic tissues[65].
NF-κB is a family of transcription factors, including RelA (p65), c-Rel, RelB,p105/p50, and p100/p52[78], and almost all cells in the human body can express NFκB[79]. The activity of NF-κB is controlled by the inhibitory κB protein and the inhibitory κB protein kinase.There are two ways to activate NF-κB. One is the classical way: Bacterial products and proinflammatory factors, such as TNF-α and IL-1, can phosphorylate and degrade IκB α and activate p65/p50 through the IκB kinase (IKK)complex. Another is the nonclassical pathway: The TNF cytokine family, additional to TNF-α, can phosphorylate p100 and activate p52/RelB through the IKK complex[79,80].The NF-κB signaling pathway plays a dual role in the gut[81]. On the one hand, the activation of NF-κB induces the expression of chemokines, cytokines, and adhesion factors to promote the development of inflammation[78]. On the other hand, NF-κB is important in maintaining the integrity of the intestinal mucosal barrier and intestinal mucosal homeostasis[82].
Daidzein reduced the secretion of pro-inflammatory factors by inhibiting the NF-κB signaling pathway[65]. Kimet al[83]found that soybean isoflavones inhibited the activation of macrophages by inhibiting the phosphorylation of the IκB protein and the degradation of IKK in a DSS-induced colitis mouse model. M1 macrophages damaged the intestinal epithelial cell barrier through TNF-α[84]; however, genistein promoted the transformation of M1 macrophages into M2, reduced cytokines, and reduced T cells in the lamina propria of the colon[85]. It was reported that soybean isoflavones may reduce DSS-induced intestinal inflammation by inhibiting the TLR4/MyD88 signal[86]. Stimuli in the intestinal tract could activate NF-κB through TLR4/MyD88[78].
In addition, isoflavones can inhibit the NOD-, LRR-, and pyrin domain-containing protein 3 (NLRP3) inflammasome. Abnormal activation of the NLRP3 inflammasome may be an important mechanism of IBD occurrence and development. The NF-κB pathway could induce the expression of the NLRP3 inflammasome[87]. Baueret al[88]used DSS to induce colitis in mice and found that the NLRP3 inflammasome played an important role in the development of intestinal inflammation. DSS activated caspase-1 through NLRP3 inflammasome and transformed IL-1β and IL-18 into their active forms. IL-1β and IL-18 are important pro-inflammatory cytokines[89], and IL-1β can increase intestinal tight junction permeability through the classical NF-κB pathway[90,91].
However, the effect of IL-18 on the intestinal mucosal barrier is controversial[92,93].Both a protective effect and a destructive effect have been reported. Genistein inhibited the NLRP3 inflammasome in macrophages through the G-protein-coupled bile acid receptor 1, also known as TGR5[94]. However, Hirotaet al[95]found that, in mice that had lost the NLRP3 inflammasome, experimental colitis was likely to appear. A lack of the NLRP3 inflammasome can damage the intestinal barrier function and cause inflammation, which may be related to promoting epithelial cell apoptosis and reducing epithelial cell proliferation[96].
IL-17 also plays an important role in IBD. IL-17A and IL-17C can regulate occludin and maintain the integrity of the barrier[97,98]. Inhibiting IL-17 weakens the barrier function of intestinal epithelial cells. Therefore, anti-IL-17A treatment will aggravate IBD[99,100]. Isoflavones increased the gene expression ofIL-17Ain immune cells[101].Biochanin A activatedIL-17transcription, which is dependent on retinoic acid receptor-related orphan receptor γ[102]. However, IL-17 by itself could gather inflammatory cells and promote the development of inflammation in coordination with other cytokines[103], and IL-17 increased in the serum and intestinal mucosa of IBD patients[104]. As a result, the up-regulation of IL-17 by isoflavones may play a role in promoting the development of IBD.
The estrogen-like effect of isoflavones also has an effect on IBD. Estrogen was found to alleviate endoplasmic reticulum stress, reduce the production of proinflammatory factors through epithelial cells, and improve the barrier function of intestinal epithelial cells (by up-regulating the tight junction proteins)[105]. The colon estrogen β receptor signaling pathway up-regulated the expression of occludin and the junctional adhesion molecule A in epithelial cells and reduced the intestinal permeability[106]. Therefore, the estrogen-like effect of soybean isoflavones could improve the gut barrier function[107].
Soybean isoflavones can also regulate the intestinal flora. Genistein reduced the growth rate ofLactococcus lactis subsp. lactis,Slackia equolifaciens, andBacteroides fragilis,while genistein and equol increased the growth rate ofF. prausnitziiandLactobacillus rhamnosus[108].Lactobacillus rhamnosusGG had an anti-inflammatory effect bothin vivoandin vitro[109], andLactobacillus rhamnosusCNCM I-3690 had a protective effect on the intestinal barrier[110].F. prausnitziiis a flora in the human intestinal tract, which was shown to produce butyrate and play an anti-inflammatory role by regulating the balance of Th17/Treg in the intestinal tract. Butyrate is a kind of SCFA, which is very important for intestinal health. Isoflavone could increase butyrate and acetate[63,111]. In addition, isoflavones promoted the production of SCFAin vitro[112]. Therefore,isoflavones can increase intestinal SCFA, and SCFA can further promote the growth of flora that can produce butyrate, have an anti-inflammatory effect, and protect the intestinal barrier function. This may form a virtuous cycle and finally promote the recovery of IBD.
However, different opinions have been expressed in certain studies. An increased intake of isoflavones increased the risk of UC, especially in the female population[113].Moreover, the early adoption of an isoflavone-rich diet did not avoid the occurrence and development of IBD in a rat model[114]. This may be related to the estrogen effect of isoflavones. The effect of estrogen on IBD has been reported. One study found that postmenopausal women who take estrogen or progesterone supplements had an increased risk of UC[115], and another study also found that patients who take oral contraceptives had an increased risk of IBD[116]. The effects of isoflavones on IBD are shown in Figure 2.
CONCLUSION
The incidence of IBD is increasing worldwide. The quality of life of IBD patients is seriously impacted and IBD cannot be cured at present. How to control IBD effectively for a long time is, therefore, of particular importance. Isoflavones have certain therapeutic effects on IBD through inhibiting inflammation, as well as regulating intestinal flora and its metabolites. Therefore, isoflavones may have a certain potential in the treatment of IBD. However, the two-way effect of the NLRP3 inflammasome and IL-17 would lead to a dual effect of isoflavones on IBD. The effects of isoflavones may be related to the dosage, frequency of use, intestinal flora, and the type and severity of IBD. Therefore, we suggest that future research should focus on the dosage and frequency of use of isoflavones in relation to their positive effects and compare the disease characteristics and intestinal flora characteristics of people with different effects, so as to determine how to take advantage of the beneficial role of isoflavones for IBD.

Figure 2 The effects of isoflavones on inflammatory bowel disease. SCFA: Short-chain fatty acids; STAT1: Signal transducer and activator of transcription 1;COX2: NO: Nitric oxide; iNOS: Inducible nitric oxide synthase; PGE2: Prostaglandin E2; NF-κB: Nuclear factor-κB; NLRP3: NOD-, LRR-, and pyrin domain-containing protein 3; TNF-α: Tumor necrosis factor α; IL-8: Interleukin-8.
 World Journal of Clinical Cases2020年11期
World Journal of Clinical Cases2020年11期
- World Journal of Clinical Cases的其它文章
- Tumor circulome in the liquid biopsies for digestive tract cancer diagnosis and prognosis
- Cytapheresis for pyoderma gangrenosum associated with inflammatory bowel disease: A review of current status
- Altered physiology of mesenchymal stem cells in the pathogenesis of adolescent idiopathic scoliosis
- Association between liver targeted antiviral therapy in colorectal cancer and survival benefits: An appraisal
- Peroral endoscopic myotomy for management of gastrointestinal motility disorder
- Clinical prediction of complicated appendicitis: A case-control study utilizing logistic regression
