布鲁顿酪氨酸激酶及其抑制剂研究进展
张鹏应 陈璐 陈海飞 李群益 施孝金

摘 要 布鲁顿酪氨酸激酶(Brutons tyrosine kinase, BTK)是B细胞抗原受体信号通路的关键调节因子,在多种B细胞恶性肿瘤的发生、发展中起着重要作用。BTK抑制剂是一类新型抗肿瘤药物,现已有3个BTK抑制剂获得美国FDA批准。多项大型临床试验证实,BTK抑制剂对慢性淋巴细胞白血病和套细胞淋巴瘤的疗效良好。BTK抑制剂联合其他化疗药物治疗多种实体瘤的临床研究亦已取得一定进展。本文概要介绍BTK的结构、功能及其抑制剂的临床研究进展。
关键词 布鲁顿酪氨酸激酶 依鲁替尼 B细胞恶性肿瘤
中图分类号:R979.19; R730.53 文献标志码:A 文章编号:1006-1533(2020)15-0008-05
Advances in Brutons tyrosine kinase and its inhibitors*
ZHANG Pengying1**, CHEN Lu1, CHEN Haifei1, LI Qunyi1, 2, SHI Xiaojin1, 2***(1. Department of Pharmacy, Northern Division of Huashan Hospital, Fudan University, Shanghai 201907, China; 2. Department of Pharmacy, Huashan Hospital, Fudan University, Shanghai 200040, China)
ABSTRACT Brutons tyrosine kinase (BTK) is a key molecule involved in multiple functions of B cells, and also plays an important role in the occurrence and development of various B cell malignancies. In the development of BTK inhibitors targeting BTK, there are currently three BTK inhibitors approved by the FDA for marketing. Several large-scale clinical trials have found that BTK inhibitors have excellent effects on chronic lymphocytic leukemia and mantle lymphoma. More progress has been also made in the trials around BTK inhibitor combined with other chemotherapeutic drugs to treat multiple solid tumors. The structure, functions and inhibitors of BTK are reviewed in this article.
KEY WORDS Brutons tyrosine kinase; ibrutinib; B cell malignancies
布魯顿酪氨酸激酶(Brutons tyrosine kinase, BTK)在肿瘤发生、发展中起着关键作用,对白血病等各种B细胞恶性肿瘤细胞的生存至关重要。1952年,美国儿科医生Ogdon Bruton首次发现,BTK在一种遗传性免疫缺陷疾病X连锁无丙种球蛋白血症患者中表达异常,与患者反复出现细菌感染有关[1-2]。BTK的小分子抑制剂在临床试验中显示有优异的抗肿瘤活性[3-4],引起人们极大的关注。BTK抑制剂依鲁替尼(ibrutinib)可口服给药,其通过与BTK活性位点的半胱氨酸残基形成共价键产生抑酶作用,2016年被美国FDA批准用于慢性淋巴细胞白血病(chronic lymphocytic leukemia, CLL)和小淋巴细胞淋巴瘤(small lymphocytic lymphoma, SLL)的一线治疗。2017年,第2个BTK抑制剂阿卡替尼(acalabrutinib)在美国获得批准。2019年,由中国药企百济神州医药公司研发的赞布替尼(zanubrutinib)获得美国FDA批准,成为第3个上市的BTK抑制剂。2020年,小野制药公司研发的BTK抑制剂tirabrutinib在日本获得批准,用于治疗原发性中枢神经系统淋巴瘤。目前,BTK抑制剂不仅已用于B细胞恶性肿瘤治疗,且也在进行治疗其他血液系统恶性肿瘤和实体瘤的临床研究。
1 BTK的结构及活化
BTK是Tec非受体酪氨酸激酶家族的5个成员之一[其余为肝细胞癌表达的酪氨酸激酶(tyrosine kinase expressed in hepatocellular carcinoma, TEC)、白细胞介素-2诱导性T细胞激酶(interleukin-2-inducible T-cell kinase, ITK)、静息淋巴细胞激酶(resting lymphocyte kinase, RLK)和骨髓激酶X(bone marrow tyrosine kinase on chromosome X, BMX)]。Tec非受体酪氨酸激酶家族高度保守[5]。BTK的结构与TEC和ITK相似,均包含5个不同的蛋白相互作用域。这些结构域包括氨基末端的PH域,富含脯氨酸的TEC域、SRC域、SH2域和SH3域,以及具有酶促活性的激酶域[5-6]。BTK通常存在于细胞质中,但在其PH域与磷脂酰肌醇-3激酶生成的3, 4, 5-三磷酸磷脂酰肌醇相互作用后会被短暂募集至细胞膜上。BTK的活化及被募集至细胞膜上分两步完成:首先,BTK激酶域Y551位点被Syk或Src激酶磷酸化[7],此磷酸化会提高BTK的催化活性;随后,BTK SH3域Y223位点发生自磷酸化,自磷酸化被认为可稳定BTK的活性构象并充分激活BTK[8]。
2 BTK参与多种信号通路的激活
2.1 参与B细胞抗原受体(B-cell antigen receptor, BCR)信号通路的激活
BCR信号通路是众多B细胞恶性肿瘤生长和播散的关键驱动者,而BTK是BCR信号通路激活不可或缺的参与者。BTK通过与BCR交联激活4种非受体酪氨酸激酶,包括磷脂酶Cγ、丝裂原活化蛋白激酶、核因子κB和蛋白激酶B。在缺乏BTK的情况下,B细胞凋亡显著增加,这与BCR介导的抗凋亡蛋白Bcl-xL的活性降低有关[9-10]。缺乏BTK的B细胞难以从细胞周期G1期转变至S期,此与无法诱导细胞周期蛋白D2的表达有关[11]。除与B细胞的存活和增殖有关之外,BCR还能通过BTK调控血管细胞黏附分子-1和纤连蛋白的整合素α4β1介导的B细胞的黏附作用[12]。
2.2 参与其他信号通路的激活
BTK对B细胞在各种淋巴组织间的定位非常重要。缺乏BTK的B细胞表现出体内迁移能力受损和细胞归巢特性缺失[13]。BTK与Toll样受体信号通路下游的4种不同蛋白相互作用,包括髓样分化因子88(myeloid differentiation factor 88, MyD88)、白细胞介素-1受体相关激酶-1、Toll/白细胞介素-1受体及其域接头蛋白[14]。 Toll样受体信号会诱导细胞内多种转录因子的激活,包括核因子кB、激活蛋白-1和干扰素调节因子-3,参与B细胞的活化、增殖以及促炎因子和抗体的分泌。BTK参与激活(含免疫受体酪氨酸激活基序)和抑制(含免疫受体酪氨酸抑制基序)免疫球蛋白可结晶片段C末端的受体的信号传导,平衡和调节多种髓细胞的激活、极化和吞噬过程[15-16]。
3 BTK与B细胞恶性肿瘤的关联
BTK的活性对B细胞白血病细胞的存活、增殖以及肿瘤微环境有着至关重要的影响。
3.1 与CLL的关联
CLL细胞表现出BCR信号通路相关激酶的持续性激活。对CLL患者细胞和小鼠模型的研究表明,BTK对CLL细胞的存活至关重要,可能与蛋白激酶B、胞外调节蛋白激酶(extracellular regulated protein kinases, ERK)和核因子кB等信号通路的激活有关[17-20]。BTK对由BCR和趋化因子调控的整合素α4β1介导的B细胞CLL细胞在肿瘤微环境中的存活和归巢至关重要[21]。
3.2 与套细胞淋巴瘤(mantle cell lymphoma, MCL)的关联
在原发性MCL细胞中,BTK高表达且其SH3域Y223位点被异常磷酸化,部分患者还伴有酪氨酸蛋白激酶Lyn、含SH2域的白细胞蛋白65 kD、Syk激酶和蛋白激酶Cβ的组成型磷酸化[22]。BTK的激活有利于MCL细胞在淋巴组织中的存活,因此可利用BTK抑制剂促使MCL细胞进入外周血,再使用抗肿瘤药物清除肿瘤细胞[23]。
3.3 与华氏巨球蛋白血症(Waldenstr?ms macroglobulinaemia, WM)的关联
大多数WM患者的MyD88 L265P位点存在替代性体细胞突变[24]。突变的MyD88 L265P可与磷酸化的BTK结合并进而激活核因子кB信号通路[25]。此外,约30%的WM患者存在趋化因子受体CXCR4 S338X体细胞突变,致使趋化因子受体配体CXCL12介导的蛋白激酶B和ERK活化增加[26]。研究证实,CXCR4和整合素α4β1的相互作用可调节WM细胞向骨髓的运输和黏附[27]。
4 BTK抑制剂的研究进展
4.1 依鲁替尼
依鲁替尼是一个可口服的不可逆BTK抑制剂,其能与BTK激酶域481位点的半胱氨酸残基共价结合,从而产生抑酶作用[28]。Honigberg等[29]首先在自身免疫性疾病小鼠模型和罹患自发性B细胞非霍奇金淋巴瘤的狗中证实了依鲁替尼的BTK抑制作用。Advani等[30]在对多种复发或难治性B细胞恶性肿瘤患者的临床研究中发现,使用依鲁替尼治疗安全、有效,尤其是对CLL和MCL患者。治疗有效患者表现为淋巴结病持续减少,同时绝对淋巴细胞计数短暂升高,这种现象被称为淋巴细胞增多。依鲁替尼治疗(采用连续给药方案)复发或难治性CLL患者的Ⅰb /Ⅱ期临床试验也发现,患者在开始接受治疗的最初几周出现淋巴细胞增多现象,但随着继续治疗,患者的淋巴细胞计数会恢复如初或降至基线以下[3]。依鲁替尼治疗复发或难治性CLL患者的独立于临床和基因组危险因素的总缓解率为71%(表1)。一项Ⅱ期临床试验亦显示,依鲁替尼治疗復发或难治性MCL患者的总缓解率为68%[31]。另有研究表明,对经治WM患者,依鲁替尼仍有很好的疗效[32]。依鲁替尼已于2013年11月起在美国先后获准治疗复发或难治性MCL、CLL、SLL和WM。
依鲁替尼治疗患者存在较大的出血风险,其中约3%的患者会发生严重出血事件[39],因此需同时使用抗凝药物和抗血小板药物。此外,多达16%的患者在接受依鲁替尼治疗后出现心房颤动合并高出血风险[40],这使得脑卒中预防成为治疗过程中必须要考虑的一个重要问题。依鲁替尼治疗的部分毒性和不良反应可通过其作用靶点的非特异性来解释:依鲁替尼不是BTK的特异性抑制剂,其还可能抑制同样在激酶域481位点含有半胱氨酸残基的其他激酶,包括其他Tec激酶家族成员(TEC、ITK、BMX和RLK)、Janus激酶-3,以及表皮生长因子受体(epidermal growth factor receptor, EGFR)[4, 29]。
为达到更好的治疗效果,目前人们也在进行依鲁替尼联合其他抗肿瘤药物治疗B细胞恶性肿瘤的各项研究,包括一些临床探索。
4.2 阿卡替尼
阿卡替尼于2017年獲得美国FDA批准,其属第二代不可逆BTK抑制剂,作用靶点具有高度选择性。阿卡替尼能与BTK激酶域481位点的半胱氨酸残基共价结合,但不会影响其他激酶(如ITK、RLK、Src激酶家族和Janus激酶-3)以及EGFR。一项在B细胞非霍奇金淋巴瘤犬模型中进行的临床前研究表明,与依鲁替尼治疗相比,阿卡替尼的抗肿瘤活性更强[41]。Ⅰ/Ⅱ期临床试验显示,阿卡替尼治疗复发或难治性CLL患者的总缓解率为95%[4]。迄今为止,尚无阿卡替尼治疗相关的剂量依赖性毒性、心房颤动和出血事件的报告。国外还在进行一项Ⅲ期临床试验,以直接比较依鲁替尼和阿卡替尼治疗复发或难治性CLL患者的作用。此外,一项Ⅱ期临床试验显示,阿卡替尼治疗复发或难治性MCL患者的总缓解率为81%,其中完全缓解率达40%[42]。阿卡替尼已于2017年10月起在美国先后获准治疗复发或难治性CLL、SLL和MCL。
4.3 赞布替尼
赞布替尼是又一个口服生物利用度和作用选择性均高于依鲁替尼的BTK抑制剂,其已被证实可抑制MCL和弥漫性大B细胞淋巴瘤(diffuse large B cell lymphoma, DLBCL)细胞株的增殖。Ⅰ/Ⅱ期临床试验显示,赞布替尼治疗的患者耐受性良好,其治疗45例CLL患者的总缓解率为90%,且在7.5个月的随访期内无患者出现疾病进展或Richter转化[38]。赞布替尼已于2019年11月在美国获准治疗经治MCL。
4.4 tirabrutinib
体外实验表明,tirabrutinib对DLBCL、滤泡型淋巴瘤、MCL和CLL细胞株均具有抗增殖作用[37]。tirabrutinib已于2020年在日本获准治疗原发性中枢神经系统淋巴瘤。
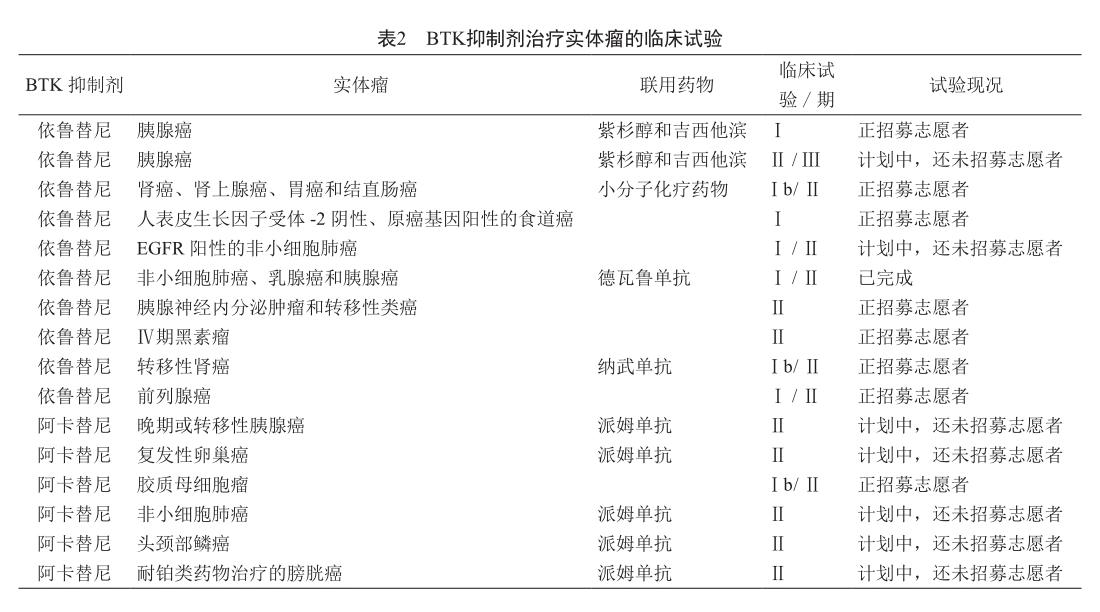
5 BTK抑制剂治疗实体瘤的临床试验
已在多种实体瘤中发现了BTK的异位表达,由此积累了BTK参与实体瘤发生、发展的证据[43-45]。这些发现也导致人们进行或计划进行数项Ⅰ/Ⅱ临床试验,以探索BTK抑制剂对晚期卵巢癌、结直肠癌、前列腺癌和脑癌等患者的治疗作用(表2)。
此外,在不表达BTK的BTK阴性实体瘤中,BTK抑制剂亦能通过调节肿瘤微环境中的多种类型细胞而产生一定的抗肿瘤作用。对胰腺癌、乳腺癌和BTK阴性结肠癌的动物模型研究表明,使用BTK抑制剂单药治疗仅可略微提高动物的生存率,但若联用化疗或免疫疗法药物,则动物的生存率大大提高[46-47]。因此,人们正在进行或计划进行依鲁替尼、阿卡替尼联用程序性细胞死亡受体-1/程序性细胞死亡受体配体-1抑制剂等治疗多种实体瘤的临床试验。
6 结语
临床试验证实,BTK抑制剂对CLL等多种B细胞恶性肿瘤具有良好的疗效。相关研究也已表明,在B细胞恶性肿瘤以及实体瘤治疗中,联用BTK抑制剂和其他多类抗肿瘤药物治疗能够提高疗效,减轻不良反应。未来需继续探索BTK抑制剂的联合治疗方案,以提高抗肿瘤疗效,同时避免BTK抑制剂的非靶点抑酶作用所带来的不良反应。
参考文献
[1] Vetrie D, Vo?echovsky I, Sideras P, et al. The gene involved in X-linked agammaglobulinaemia is a member of the Src family of protein-tyrosine kinases. 1993 [J]. J Immunol, 2012, 188(7): 2948-2955.
[2] Tsukada S, Saffran DC, Rawlings DJ, et al. Deficient expression of a B cell cytoplasmic tyrosine kinase in human X-linked agammaglobulinemia [J]. Cell, 1993, 72(2): 279-290.
[3] Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia [J]. N Engl J Med, 2013, 369(1): 32-42.
[4] Byrd JC, Harrington B, OBrien S, et al. Acalabrutinib (ACP-196) in relapsed chronic lymphocytic leukemia [J]. N Engl J Med, 2016, 374(4): 323-332.
[5] Bradshaw JM. The Src, Syk, and Tec family kinases: distinct types of molecular switches [J]. Cell Signal, 2010, 22(8): 1175-1184.
[6] Hyv?nen M, Saraste M. Structure of the PH domain and Btk motif from Brutons tyrosine kinase: molecular explanations for X-linked agammaglobulinaemia [J]. EMBO J, 1997, 16(12): 3396-3404.
[7] Rawlings DJ, Scharenberg AM, Park H, et al. Activation of BTK by a phosphorylation mechanism initiated by SRC family kinases [J]. Science, 1996, 271(5250): 822-825.
[8] Marcotte DJ, Liu YT, Arduini RM, et al. Structures of human Brutons tyrosine kinase in active and inactive conformations suggest a mechanism of activation for TEC family kinases [J]. Protein Sci, 2010, 19(3): 429-439.
[9] Anderson JS, Teutsch M, Dong Z, et al. An essential role for Brutons tyrosine kinase in the regulation of B-cell apoptosis[J]. Proc Natl Acad Sci U S A, 1996, 93(20): 10966-10971.
[10] Solvason N, Wu WW, Kabra N, et al. Transgene expression of Bcl-xL permits anti-immunoglobulin (Ig)-induced proliferation in xid B cells [J]. J Exp Med, 1998, 187(7): 1081-1091.
[11] Glassford J, Soeiro I, Skarell SM, et al. BCR targets cyclin D2 via Btk and the p85α subunit of PI3-K to induce cell cycle progression in primary mouse B cells [J]. Oncogene, 2003, 22(15): 2248-2259.
[12] Spaargaren M, Beuling EA, Rurup ML, et al. The B cell antigen receptor controls integrin activity through Btk and PLCγ2 [J]. J Exp Med, 2003, 198(10): 1539-1550.
[13] de Gorter DJ, Beuling EA, Kersseboom R, et al. Brutons tyrosine kinase and phospholipase Cγ2 mediate chemokinecontrolled B cell migration and homing [J]. Immunity, 2007, 26(1): 93-104.
[14] Jefferies CA, Doyle S, Brunner C, et al. Brutons tyrosine kinase is a Toll/interleukin-1 receptor domain-binding protein that participates in nuclear factor κB activation by Toll-like receptor 4 [J]. J Biol Chem, 2003, 278(28): 26258-26264.
[15] Nimmerjahn F, Ravetch JV. Fcγ receptors as regulators of immune responses [J]. Nat Rev Immunol, 2008, 8(1): 34-47.
[16] Bournazos S, Wang TT, Ravetch JV. The role and function of Fcγ receptors on myeloid cells [J/OL]. Microbiol Spectr, 2016, 4(6): 10.1128/microbiolspec.MCHD-0045-2016 [2020-03-04]. doi: 10.1128/microbiolspec.MCHD-0045-2016.
[17] Singh SP, Pillai SY, de Bruijn MJW, et al. Cell lines generated from a chronic lymphocytic leukemia mouse model exhibit constitutive Btk and Akt signaling [J/OL]. Oncotarget, 2017, 8(42): 71981-71995 [2020-03-04]. doi: 10.18632/ oncotarget.18234.
[18] Herman SE, Gordon AL, Hertlein E, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765 [J]. Blood, 2011, 117(23): 6287-6296.
[19] Ponader S, Chen SS, Buggy JJ, et al. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo[J]. Blood, 2012, 119(5): 1182-1189.
[20] Kil LP, de Bruijn MJ, van Hulst JA, et al. Brutons tyrosine kinase mediated signaling enhances leukemogenesis in a mouse model for chronic lymphocytic leukemia [J]. Am J Blood Res, 2013, 3(1): 71-83.
[21] de Rooij MF, Kuil A, Geest CR, et al. The clinically active BTK inhibitor PCI-32765 targets B-cell receptor- and chemokine-controlled adhesion and migration in chronic lymphocytic leukemia [J]. Blood, 2012, 119(11): 2590-2594.
[22] Pighi C, Gu TL, Dalai I, et al. Phospho-proteomic analysis of mantle cell lymphoma cells suggests a pro-survival role of B-cell receptor signaling [J]. Cell Oncol (Dordr), 2011, 34(2): 141-153.
[23] Chang BY, Francesco M, De Rooij MF, et al. Egress of CD19+CD5+ cells into peripheral blood following treatment with the Bruton tyrosine kinase inhibitor ibrutinib in mantle cell lymphoma patients [J]. Blood, 2013, 122(14): 2412-2424.
[24] Treon SP, Xu L, Yang G, et al. MYD88 L265P somatic mutation in Waldenstr?ms macroglobulinemia [J]. N Engl J Med, 2012, 367(9): 826-833.
[25] Yang G, Zhou Y, Liu X, et al. A mutation in MYD88 (L265P) supports the survival of lymphoplasmacytic cells by activation of Bruton tyrosine kinase in Waldenstr?m macroglobulinemia[J]. Blood, 2013, 122(7): 1222-1232.
[26] Hunter ZR, Xu L, Yang G, et al. The genomic landscape of Waldenstrom macroglobulinemia is characterized by highly recurring MYD88 and WHIM-like CXCR4 mutations, and small somatic deletions associated with B-cell lymphomagenesis [J]. Blood, 2014, 123(11): 1637-1646.
[27] Ngo HT, Leleu X, Lee J, et al. SDF-1/CXCR4 and VLA-4 interaction regulates homing in Waldenstrom macroglobulinemia [J]. Blood, 2008, 112(1): 150-158.
[28] Pan Z, Scheerens H, Li SJ, et al. Discovery of selective irreversible inhibitors for Brutons tyrosine kinase [J]. Chem Med Chem, 2007, 2(1): 58-61.
[29] Honigberg LA, Smith AM, Sirisawad M, et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy [J]. Proc Natl Acad Sci U S A, 2010, 107(29): 13075-13080.
[30] Advani RH, Buggy JJ, Sharman JP, et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies [J]. J Clin Oncol, 2013, 31(1): 88-94.
[31] Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma [J]. N Engl J Med, 2013, 369(6): 507-516.
[32] Treon SP, Tripsas CK, Meid K, et al. Ibrutinib in previously treated Waldenstr?ms macroglobulinemia [J]. N Engl J Med, 2015, 372(15): 1430-1440.
[33] Byrd JC, Brown JR, OBrien S, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia[J]. N Engl J Med, 2014, 371(3): 213-223.
[34] Coutré SE, Furman RR, Flinn IW, et al. Extended treatment with single-agent ibrutinib at the 420 mg dose leads to durable responses in chronic lymphocytic leukemia/small lymphocytic lymphoma [J]. Clin Cancer Res, 2017, 23(5): 1149-1155.
[35] Burger JA, Tedeschi A, Barr PM, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia [J]. N Engl J Med, 2015, 373(25): 2425-2437.
[36] Dreyling M, Jurczak W, Jerkeman M, et al. Ibrutinib versus temsirolimus in patients with relapsed or refractory mantlecell lymphoma: an international, randomised, open-label, phase 3 study [J]. Lancet, 2016, 387(10020): 770-778.
[37] Walter HS, Rule SA, Dyer MJ, et al. A phase 1 clinical trial of the selective BTK inhibitor ONO/GS-4059 in relapsed and refractory mature B-cell malignancies [J]. Blood, 2016, 127(4): 411-419.
[38] Thompson PA, Burger JA. Brutons tyrosine kinase inhibitors: first and second generation agents for patients with chronic lymphocytic leukemia (CLL) [J]. Expert Opin Investig Drugs, 2018, 27(1): 31-42.
[39] Jones JA, Hillmen P, Coutre S, et al. Use of anticoagulants and antiplatelet in patients with chronic lymphocytic leukaemia treated with single-agent ibrutinib [J]. Br J Haematol, 2017, 178(2): 286-291.
[40] Wiczer TE, Levine LB, Brumbaugh J, et al. Cumulative incidence, risk factors, and management of atrial fibrillation in patients receiving ibrutinib [J/OL]. Blood Adv, 2017, 1(20): 1739-1748 [2020-03-04]. doi: 10.1182/ bloodadvances.2017009720.
[41] Harrington BK, Gardner HL, Izumi R, et al. Preclinical evaluation of the novel BTK inhibitor acalabrutinib in canine models of B-cell non-Hodgkin lymphoma [J/OL]. PLoS One, 2016, 11(7): e0159607 [2020-03-04]. doi: 10.1371/journal. pone.0159607.
[42] Wang M, Rule S, Zinzani PL, et al. Acalabrutinib in relapsed or refractory mantle cell lymphoma (ACE-LY-004): a single-arm, multicentre, phase 2 trial [J]. Lancet, 2018, 391(10121): 659-667.
[43] Kokabee L, Wang X, Sevinsky CJ, et al. Brutons tyrosine kinase is a potential therapeutic target in prostate cancer [J]. Cancer Biol Ther, 2015, 16(11): 1604-1615.
[44] Grassilli E, Pisano F, Cialdella A, et al. A novel oncogenic BTK isoform is overexpressed in colon cancers and required for RAS-mediated transformation [J]. Oncogene, 2016, 35(33): 4368-4378.
[45] Zucha MA, Wu AT, Lee WH, et al. Brutons tyrosine kinase(Btk) inhibitor ibrutinib suppresses stem-like traits in ovarian cancer [J/OL]. Oncotarget, 2015, 6(15): 13255-13268 [2020-03-04]. doi: 10.18632/oncotarget.3658.
[46] Stiff A, Trikha P, Wesolowski R, et al. Myeloid-derived suppressor cells express Brutons tyrosine kinase and can be depleted in tumor-bearing hosts by ibrutinib treatment [J]. Cancer Res, 2016, 76(8): 2125-2136.
[47] Sagiv-Barfi I, Kohrt HE, Czerwinski DK, et al. Therapeutic antitumor immunity by checkpoint blockade is enhanced by ibrutinib, an inhibitor of both BTK and ITK [J]. Proc Natl Acad Sci U S A, 2015, 112(9): E966-E972.

