Air Temperature and Emersion Time Can Affect the Survival Rate and Ammonium Loading of Swimming Crab Portunus trituberculatus Exposed to Air
LU Yunliang, ZHU Boshan, ZHANG Dan, and LI Yuquan
Air Temperature and Emersion Time Can Affect the Survival Rate and Ammonium Loading of Swimming CrabExposed to Air
LU Yunliang1), *, ZHU Boshan2), 3), ZHANG Dan4), and LI Yuquan1)
1),,266109,2),,266003,3),,266235,4),,,300384,
Ammonium overloading is a common response of aquatic organisms to air exposure during transport. This study elucidated the relationship betweenammonium overloadingand mortality of crab.Additionally, we also explored the effects of emersion time and air temperature on ammonium loading and concomitant physiological change.To test the air temperature effect, the crab was exposed to 16, 23 and 30℃ in air for 3h, respectively, and then recovered in seawater at 23℃ for 12h. To test the emersion time effect, crab was exposed to 23℃ in air for 0.5 and 3h, respectively, and then recovered in seawater at 23℃ for 12h. In the control group, crab wasalways immersed at 23℃. At each time interval (0.5, 1.5 and 3h during air exposure and 0.5, 2, 4 and 12h during recovery), ammonium excretion rate, level of total ammonium, total free amino acids and urea concentration in hemolymph and thehepatopancreas enzyme activity involved in detoxifying ammonium were analysed. Results showed that crab mortality was positively related with emersion time and temperature while ammonium loading was lower at 16 and 30℃ than at 23℃. For crab experiencing thermal inconsistence of culture media (., 16 or 30℃), they were higher in ammonium excretion rate and activities of ammonium detoxification enzymes, which may be the reason that they had a lower ammonium loading. Prolonged emersion time (3.0h0.5h) increased the ammonium overloading and the activity of ammonium detoxification pathways in crab. Our results demonstrated that emersion-induced ammonium overloading may not bethe main reason leading todeath during air exposure and subsequent recovery. When the culture medium changed, thermal variation, compared with constant temperature, could reduce ammonium overloading in crab by elevating the activities of ammonium detoxification enzymes and ammonium excretion rate during recovery period.
; air temperature; emersion time; ammonium loading
1 Introduction
Transport of live animals is an important anthropogenic activity to obtain sea food with high quality. In this regard, a dry or semi-dry approach has been developed due to its unique advantages of lower transport cost and less dependency on water and essential equipment. This method, in some cases, is also adopted in transport of swimming crab, an important commercial crustacean in China. However,is a crustacean that is scarcely out of water. Therefore, this transport way, exposing crab to air, is suggested to have a greater influence on animal survival compared to the traditional water transport way. This viewpoint was confirmed by our pre-test results (Table 1) in which a lower survival rate was obtained during crab transport out of water. This phe- nomenon drives us to understand what is the cause ofdeath during air exposure or transport out of water. Undoubtedly, oxygen deprivationprobably is the major cause considering the change of crab living environment. At the same time, we also noticed a significant increase of hemolymph ammonium level inafter air exposure (Table 2). As ammonium is a toxic substance for most aquatic animals, we hypothesized that ammonium overloading may be partially responsible for crab death out of water. To test the hypothesis,was subject to air exposure at different temperatures in this experiment.
For crustaceans and fish species, ammonium excretion is mainly through gills. However, when these animals are exposed to air, gill filaments usually clump together due to the interruption of branchial water flow, reducing gill areas that exchange ions and gases with external environments and thus leading to the build-up of internal ammonium (Durand and Regnault, 1998; Bergmann, 2001). Though emersion condition has been well known to be a factor increasing the risks of morbidity and mortality of crustaceans (Ridgway, 2006; Lu., 2016b; Dong, 2019), whether this relates with emersion-induced ammonium overloading and how crustaceans respond in this aspect to air exposure are not fully understood.

Table 1 Survival rate of crabs during different transport way

Table 2 Ammonium and lactate concentrations during transport out of water at 23℃
Note: Lactate concentration was used to indicate the oxygen deprivation.
In the air, the impaired gill function can inhibit the am- monium excretion in aquatic animals, leading to adaptive physiological changes including detoxifying excess ammonium in tissues such as hepatopancreas and liver. For example, mudskippers can reduce the main origin of endogenous ammonium to slow down ammonium accumulation during air exposure (Lim, 2001). Durand(1999) reported thatcould detoxify ammo-nium by the synthesis of amino acids. Frick and Wright (2002) reported that the mangrove killifishcan elevate urea synthesis in response to air exposure. In contrast, African lungfish speciesanddepend more on decreasing the production of ammonium than increasing urea synthesis to detoxify ammonium (Loong, 2005). As important enzymes in ammonium detoxification system, glutaminate dehydrogenase (GDH), glutamine synthase (GS), glutaminase (Gln) and arginase (ARG) in the hepatopancreas have been widely reported in crustaceans (King, 1985; Weihrauch, 2004; Liu, 2012) and(Liu, 2014; Pan, 2018).
In our pre-test,survival decreased withincreasing emersion time while its internal ammonium le- vel increased drastically, indicating possible ammonium-related physiological changes in crab. Therefore, the following questions are raised: I) what relationship between ammonium overloading and crab survival is during dry or semi-dry transport; II) how ammonium-detoxifying path- ways (amino acids and urea synthesis) respond to air exposure; III) whether these changes relate with internal am- monium level (., ammonium detoxification); IV) what role emersion temperature plays in this process. To address these questions, we placedin air with- out water at 16, 23 and 30℃, respectively, and then analyzedtheir hemolymph and hepatopancreas parameters du- ring air exposure and following recovery period. Results of this study may be useful in finding out the reason of emersion-caused crab death and therefore provide a reference to optimizing the transport condition of.
2 Materials and Methods
2.1 Animal Collection and Maintenance
This experiment was conducted in Qingdao National Marine Science Research Center from July to August in 2013. Healthy subadults ofwere purchased from a local farm in Jiaonan district (Qingdao, Shan- dong, China), transported to laboratory and acclimated for two weeks in seawater at 23℃±1℃ and salinity 30 with a photoperiod of 14h:10h (light:dark). Each crab was placed in a separate chamber of tanks (50cm×40cm×30cm) for the prevention of cannibalism. Crab was fed once a day with fresh shellfish, and the un- consumed food and feces were removed after 3h of feeding. One third of the seawater each tank was exchanged daily with fully aerated seawater at 23℃. Each tank was continuously aerated with air stones. Asis not a protected species, no specific permits were required to conduct the experiment.
2.2 Experimental Procedure and Sample Collection
After acclimation, healthy crab with legal size (80–120g wet weight) at intermolt stage was randomly divided into three groups, 60 crab individuals each. Groups AT16, AT23 and AT30 expose to air at 16, 23 and 30℃, respectively. There was a medium thermal in consistence in AT16 and AT30 during experiment while temperature was maintained constant in AT30. The detailed information of three treatments was stated as following:
AT16 group: seawater (23℃)→air exposure (16℃)→recovery in seawater (23℃)
AT23 group: seawater (23℃)→air exposure (23℃)→recovery in seawater (23℃)
AT30 group: seawater (23℃)→air exposure (30℃)→recovery in seawater (23℃)
The thermal level and air exposure time were set according to our previous study (Lu., 2016a).
Crab in each group was deprived of food for 24h prior to experiment. Specimens were directly transferred from seawater (23℃) to air at different temperatures (., 16, 23 and 30℃). After 0.5, 1.5, 3h, crab in different groups was re-immersed into seawater (23℃), recovering for 0.5, 2, 4, 12h, respectively. The air exposure was performed in a light growth incubator where crab was placed into individual hollow plastic boxes (15cm×15cm×5cm) and air condition was controlled in accordance with the ambient environment of control group with humidity of 70%–80% and illumination of 50–100 lx. In the course of experiment, crab was sampled at 0.5, 1.5 and 3h during air exposure and at 0.5, 2, 4 and 12h during recovery.
Additionally, to determine the effect of emersion time on ammonium metabolism, we compared the difference between the parameters of other two groups,., AT23-0.5 and AT23-3.0 (exposure to air for 0.5 and 3.0h at 23℃,respectively) during recovery. Following re-immersion, each of the two groups was sampled at 0.5, 2, 4 and 12h. The detailed treatment information was stated as following:
AT23-0.5 group: seawater (23℃)→air exposure (23℃) for 0.5h→recovery in seawater (23℃)
AT23-3.0 group: seawater (23℃)→air exposure (23℃) for 3.0h→recovery in seawater (23℃)

Fig.1 Experimental design in this study. In experiment 1, all treatments are different only in air temperature. In experiment 2, all treatments are different only in air exposure time.
In this study, all treatments were triplicated (60 crab in- dividuals in each group) and crab cultured in seawater (23℃) without any treatment was used as control. At each sampling, 5 randomly chosen crab individuals were analyzed. Crab was dried with blotting paper, and then placed on an ice plate. Then 400μL of hemolymph was extracted from the ventral sinusthe arthrodial membrane at the base of the last pereiopod using 1mL chilled disposable syringes. Afterwards, the carapace was removed from crab and hepatopancreas was rapidly dissected out, placed in a 1.5mL microcentrifuge tube, flash-frozen in liquid nitrogen, and stored at −80℃ until further analysis.
2.3 Parameters Determination
2.3.1 Determination of ammonium excretion rate
Six crab individuals each group were used for the determination of ammonium excretion rate (AER) at 0 (., control), 0.5, 2, 4 and 12h during recovery. After AER was determined at 0h, crab was exposed to air for 0.5h (AT23-0.5 group) or 3h (AT16, AT30 and AT23/AT23-3.0 groups) and then recovered in seawater (23℃).
Prior to the experiment initiation, each crab was secured to an individual grid by tying the merus of each pereiopod to the corners of a grid in a suitable manner to minimize the injuries from different swimming activities. After 24h of starvation and immobilization, crab was carefully trans- ferred from acclimation tanks to individual respiratory chambers of 3.4L (29.7cm×15.2cm×11.8cm) filled with pre-aerated fresh seawater at 23℃while other two tanks without crab were used for each treatment as control. At each time, AER was determined according to the difference of ammonium concentration in water sample between pre- and post-experiment. The ammonium concentration was determined by indophenol blue photometric method (Lei, 2006).
2.3.2 Determination of hemolymph parameters
Hemolymph sample was mixed with three volumes of ice-cold 0.9molL−1perchloric acid (PCA) and centrifuged for 5min at 7000and 4℃ to remove precipitated protein. Then the supernatant was collected and neutralized to pH 6.5–7.0 with 3.75molL−1potassium carbonate. Precipitated potassium perchlorate was removed by a second centri- fugation at the same condition and the extracts were used for the determination of total ammonium (., NH3+NH4+), total free amino acids (TFAA) and urea concentration.
Ammonium level was analyzed with the electrode me- thod (Weihrauch., 1998; Martin., 2011).The electrode potentials (φ, mV) of (NH4)2SO4solutions (0–8.0μg total ammonium per mL) were measured with a gas sensitive NH3electrode which was connected to a PHSJ-3F pH meter (Shanghai Precision Scientific Instruments Co. Ltd., Shanghai, China). The constructed linear standard curve of log(C)–φ was used to calculate hemolymph ammonium concentration (μgmL−1).
The modified ninhydrin method (OD 570nm) (Moore and Stein, 1954) was employed to analyze TFAA level. Briefly, acetate buffer and ninhydrin reagent were added into hemolymph samples, mixed fully and boiled for 15min in dark. After the mixture was cooled down to room temperature, 3mL ethanol (60%) was added to stop the reaction. The absorbance of final solution was recorded at 570nm and used to calculate TFAA concentration (mmolL−1) against a standard curve prepared from glycine solution at concentrations from 0 to 0.3mmolL−1.
Twenty μL of sample was used to measure urea concen- tration with a urease method. The measurement was conducted according to the instruction of test kit (Nanjing Jiancheng Bioengineering Institute, China). The absorbance of final reaction solution was recorded at 640nm and used to calculate urea concentration (mmolL−1).
2.3.3 Determination of hepatopancreas parameters
Hepatopancreas tissue was powdered with mortar and pestle within liquid nitrogen. A certain amount of powder was homogenized in 9 volumes of ice-cold 0.9molL−1PCA. The homogenate was centrifuged for 5min at 4℃ and 7000to remove precipitated protein. Then the supernatant was collected and neutralized to pH 6.5–7.0 with 3.75molL−1potassium carbonate. Precipitated potassium perchlorate was removed by a second centrifugation at the same condition and supernatant was collected for the determination of ammonium concentration (μmolgww−1) in hepatopancreas.
Hepatopancreas powder was mixed with 9 volumes of 20 mmolL−1Tris-HCl containing 0.25molL−1sucrose, 0.15molL−1KCl, 1mmolL−1EDTA, 1mmolL−1dithiothreitol and 100μmolL−1phenylmethanesulfonyl fluoride; pH=7.6, homogenized at 4℃ and centrifuged at 4℃ and 9000. for 10min. Collected supernatant was used for the determination of activities of GDH (E.C. 1.4.1.3), GS (E.C. 6.3.1.2), Gln (E.C. 3.5.1.2), Urease (E.C. 3.5.1.5), and ARG (E.C.3.5.3.1). All preparation procedures were carried out at 4℃.
GDH, urease and ARG activities were determined by continuously monitoring the decrease in absorbance at 340nm using nicotinamide adenine dinucleotide (NAD)/ reduced form of nicotinamide-adenine dinucleotid (NADH) linked methods (Etienne, 2001; Richard, 2010). The molar extinction coefficient of NADH (6.22Lmmol−1cm−1) was used to calculate the enzyme activity. Gln activity was determined by the formation of ammonium in the reaction system (Dhale., 2011). The Nessler’s rea- gent method was applied for the measurement of ammonium concentration. The absorbance of final solution was read at 480nm and used to calculate enzyme activity against a standard curve. GS activity was determined by measuring the g-glutamyl monohydroxamate (GMH) formation (Maas, 2012). The absorbance of reaction system at 540nm was read and used for the calculation of GS activity against a standard curve prepared from GMH solution ranging from 0 to 1mgmL−1.
Specific activities of all enzymes were expressed as U(gprot)−1(unit per gram protein), where U was defined as the enzyme causing the conversion of 1μmol of substrate/min. Protein content was determined by Coomassie Brilliant Blue G250 dye binding method(Bradford, 1976), using bovine serum albumin (Sigma A7030) as standard. All ab- sorbance values at OD 595nm were read using the Syn- ergyTM2 automatic microplate reader.
3 Statistical Analysis
Data in this study were expressed as mean±standard deviation (mean±S.D.). Statistical analyses were perform- ed using SPSS 20.0 software. One-way ANOVA or Inde- pendent-Samplestest was employed to analyse the sta- tistical differences of different treatments.values lower than 0.05 were considered statistically significant.
4 Results
4.1 Effects of Air Temperature and Emersion Time on Crab Mortality
The crab survival during the entire experiment was shown in Table 3. During air exposure, the crab survival of AT16, AT23 and AT30 were 100%, 91.7% and 80%, respectively. Following recovery, the survival of AT16 was the same as that in air environment while survival of AT23 and AT30 decreased to 87.5% and 44.4%, respectively. At the same time, a lower survival of AT23-3.0 (87.5%) than AT23-0.5 (100%) was observed, indicating that prolonged air-emer- sion time decreased crab survival.
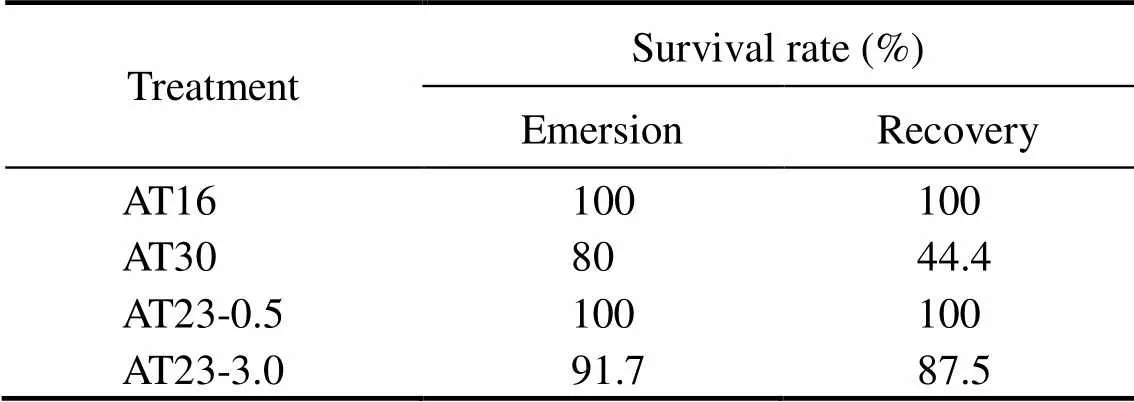
Table 3 Mortality rate of crab during the experiment
4.2 Effects of Air Temperature and Emersion Time on Ammonium Level in Hemolymph and Hepatopancreas and AER
In hemolymph (Fig.2A), an increase in the ammonium loading due to air exposure and a further increase following recovery was observed in all groups. For AT23, the ammonium level at each time interval was statistically different to that of control (<0.05). In hepatopancreas (Fig.2B), all groups showed a similar and significant vari- ation trend in the ammonium level: increasing during e- mersion and decreasing after re-immersion. During reco- very period, prolonged emersion time increased the am- monium level in hemolymph (Fig.2C) but not in hepa- topancreas (Fig.2D). Table 4 shows that after re-immer- sion, AERs of AT23-0.5 and AT23-3.0/AT23 were reduced by air exposure compared to control (<0.05), which was different to that of AT16 and AT30. According to Two-way ANOVA, the above three indicators were all signifi- cantly influenced by air temperature during recovery period (<0.05). After exposure of crab to air, both air tempe- rature and emersion time significantly influenced hemo- lymph ammonium level (<0.05) while only the latter had a significant effect on hepatopancreas ammonium level (<0.05).

Table 4 AER of crab during re-immersion (n=6)
Notes: Different super lowercase letter represents significant differences (<0.05) among time intervals in the same row (including control), and the symbol ‘*’ represents a significant difference (<0.05) compared with AT23-3.0/AT23 group at the same time interval.

Fig.2 Effects of air exposure temperature and time on ammonium levels during air exposure and subsequent recovery in seawater. (A, B): effects of air exposure temperature; (C, D): effects of air exposure time. The symbol ‘*’ represents a difference between each time interval and control (P<0.05), while ‘#’ represents a difference among treatments at the same time interval (P<0.05). The same as below.
4.3 Effects of Air Temperature on Hemolymph TFAA Level and Enzyme Activity Relating with Amino Acids Metabolism
As shown in Fig.3A, all groups had a similar and sig- nificant change in GDH activity (<0.05). However, fol- lowing re-immersion, AT23 group was higher in GDH activity than AT16 and AT30 groups. Compare to AT16 and AT30 groups, the AT23 group changed differently in the GS activity, Gln activity and TFAA level (Figs.3B–D). Two-way ANOVA showed that air temperature significantly influenced all these indicators during air exposure (<0.05) and following recovery period (<0.05). After air exposure, a significant effect of emersion time was only observed in the case of Gln and GDH activities (<0.05).
4.4 Effects of Emersion Time on Hemolymph TFAA Level and Enzyme Activity Related with Amino Acids Metabolism During Recovery Period
During recovery, AT23-0.5 and AT23-3.0 groups had a similar change trend in GDH (Fig.4A) and Gln (Fig.4C) activities while they were different in GS activity (Fig.4B) and TFAA level (Fig.4D). Two-way ANOVA showed that emersion time had a significant effect on GDH and GS activities and TFAA level during recovery period (<0.05).
4.5 Effects of Air Temperature on Hemolymph Urea Level and Enzymes Activities Relating with Urea Metabolism
A significant change (<0.05) was observed in ARG activity in all groups (Fig.5A). During experiment, ARG activities in AT16 and AT30 groups changed different to that in AT23 group. In the case of urease activity (Fig.5B), all groups changed significantly (<0.05). However, all groups showed a different response to air exposure: urease activity was higher in AT23 group. In Fig.5C, all groups changed similarly and significantly in hemolymph urea level (<0.05). Two-way ANOVA showed that there was a significant effect of air temperature on the three para- meters during air exposure (<0.05) and subsequent re- covery period (<0.05). Following exposure to air, emer- sion time significantly influenced ARG acitivity and urea level (<0.05).
4.6 Effects of Emersion Time on Hemolymph Urea Level and Enzymes Activities Relating with Urea Metabolism During Recovery Period
In Fig.6A, ARG activities in AT23-0.5 and AT23-3.0 groups changed oppositely, and a significant change was observed only in the latter group (=5,=3.900,=0.020). In terms of urease activity (Fig.6B), a significant change was only observed in AT23-3.0 group (=5,=7.876,=0.001). In Fig.6C, a similar and significant change in hemolymph urea level was recorded in AT23-0.5 and AT23-3.0 groups (<0.05). Two-way ANOVA showed that during recovery period, emersion time had a significant effect on ARG activity and urea level (<0.05) but not on urease activity (>0.05).

Fig.3 Effects of air exposure temperature on enzyme activities of GDH (A), GS (B) and Gln (C) in hepatopancreas and TFAA level (D) in hemolymph during air exposure and subsequent recovery in seawater.

Fig.4 Effects of air exposure time on activities of GDH (A), GS (B) and Gln (C) in hepatopancreas, and on TFAA level (D) in hemolymph during air exposure and subsequent recovery in seawater.
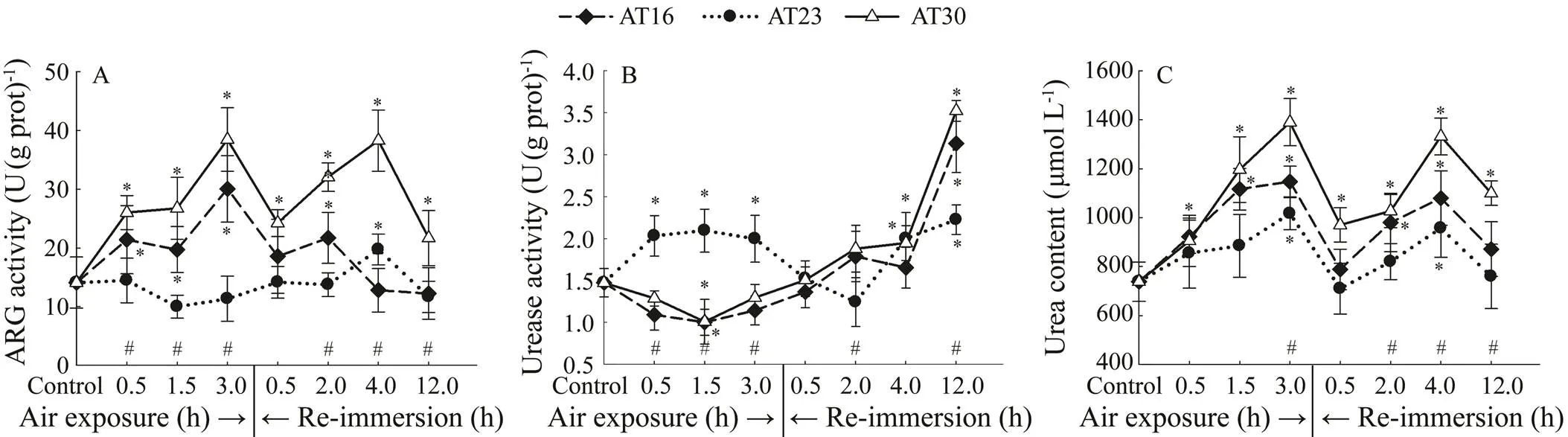
Fig.5 Effects of air exposure temperature on activities of ARG (A) and Urease (B) in hepatopancreas and urea level (C) in hemolymph.

Fig.6 Effects of air exposure time on activities of ARG (A) and Urease (B) in hepatopancreas and urea level (C) in hemolymph.
5 Discussion
Ammonium accumulation is a common phenomenon when aquatic crustaceans are captured, handled and trans- ported (Schmitt and Uglow, 1997; Lorenzon, 2008). In this study, we verified a significant effect of air tem- perature and emersion time on ammonium metabolism ofout of water.
李老黑倒背起双手,翻起白眼往天上看去。他这个动作我很熟悉。李老黑仰脸看天,并不代表天上有什么好奇的东西吸引着他,这是他思考问题时的习惯性动作。李老黑这个动作充满个性,也有气势,在沙河村独一无二。这表明,只有宽阔的天空才能容得下他深刻的思考。这时候我要是跟着往天上翻白眼,或者左顾右盼什么的,那就是极不明智的,所以我就专心致志地盯着李老黑的白眼。果然,李老黑的眼皮毫无征兆地耷拉下来了,结束了他的深刻思考。
5.1 The Relationship Between Emersion-Induced Ammonium Build-Up and Crab Survival
Ridgway(2006) and Barrento(2011) report- ed that increasing temperature can lower crab survival when crab was transported out of water, which was further confirmed by our results. It was noticed that the increase in air temperature even resulted in a further reduction of crab survival when animal was recovered in seawater. Accord- ing to Chen and Chen (1998), the lower accumulation of ammonium and lactate was considered as a guarantee on higher survival ofat lower temperatures (6 and 12℃). In the present study, the prolonged e- mersion time decreased crab survival and concomitantly increased internal ammonium loading. This information seemed to indicate a possible role of ammonium elevation in affectingsurvival. However, when air temperature increased from 23 to 30℃, ammonium accumulation was astonishingly lowered in crabs. This was in conflict with the fact that crab survival was reduced du- ring air exposure and subsequent recovery. Thus, we spe- culated that the emersion-induced ammonium buildup might not be one main factor responsible forsurvival following exposure to air,., during dry or semi-dry transport.
5.2 The Relationship Between Ammonium Overloading and AER During Recovery
According to the present study, the buildup of internal ammonium loading inwas stimulated by air exposure. This phenomenon usually disappears when animals return to aquatic environment, such as(Durand and Regnault, 1998). However, the species-specific resilience to air exposure retards some animals in internal ammonium returning to normal level,., the am- monium concentration ofwas still higher than the control level even after a recovery of 24h (Durand and Regnault, 1998). Forin this study, the ammonium buildup was not eliminated following recovery in seawater; however, it was exacerbated. This su- ggested a weaker resiliency to air exposure for. Durand and Regnault (1998) reported that pro- duced ammonium can be temporally reserved in tissues when crustaceans are exposed to air, which can also be observed from the significantly elevated ammonium level in hepatopancreas in the present study. Following re-im- mersion, the rapid reduction in hepatopancreas ammonium level (Fig.3) may be indicative of ammonium release into hemolymph. This, together with an accelerated ureolysis (Fig.5), might jointly contribute to the further elevation of internal ammonium loading in crabs following re-immer- sion. As shown in Table 4, air exposure inhibited crab AERif temperature kept stable throughout the experiment (AT23-0.5 and AT23-3.0 groups) and if not, a contrary result was obtained (AT16 and AT30 groups). It can be concluded that the media thermal inconsistence can stimulate AERs of crabs, which may be one reason of lower ammonium loading in AT16 and AT30 groups than in AT23 group du- ring re-immersion.
5.3 The Relationship Between Ammonium Over-loading and Adaptive Physiological Activities
Ammonium excretion and conversion into less toxic substances are two important pathways for aquatic animals to buffer the stress-induced ammonium change (Chen and Chen, 2000; Weihrauch and Allen, 2018). It has been re- ported that high environmental ammonium can inhibit am- monium excretion of immersed crustaceans, and the levels of substances such as glutamine, glutamate and urea were concomitantly elevated, indicating that the activation ofthese substances synthesis may be employed by crusta- ceans as ammonium toxicity buffering pathways in response to internal ammonium buildup (Hong, 2007; Martin, 2011; Liu, 2014). For air-exposed crus- taceans and fishes, ammonium conversion into less toxic substances is important in detoxifying ammonium since the ammonium excretion through gills is disrupted. Our re- sults showed that this mechanism inis influenced by air temperature.
Glutamate and glutamine are two major amino acids in organisms and their metabolism is important in regulating internal ammonium level (Weihrauch, 2004). GS, GDH and Gln are three key enzymes in the pathway of glutamate-glutamine, while GS and GDH function in ami- no acids synthesis and Glnplays a role in glutamine de- composition (King, 1985). In response to high environmental ammonium,can increase the amino acids synthesis as indicated by elevated GS and GDH activities (Liu, 2014). Emersion condition has also been shown to be a factor activating this mechanism in aquatic animals (Durand, 1999; Jow, 1999). In the present study, the induced ammonium buildup elevated hemolymph TFAA level, in particular when emersion time was prolonged or temperature was inconsistent between air and water medium. Through comparing GS and GDH activities, we found that the former was more related with the change of TFAA level during air exposure, while the latter was surprisingly suppressed by media thermal inconsistence and even by prolonged emersion time during recovery period (Fig.4). Thus the emersedmay have a more reliance on glutamine synthesis system to fight against ammonium toxicity in terms of amino acids synthesis. We also noticed that following re-immersion, TFAA level was lower in AT16 and AT30 groups than in AT23 group, which was in coincident with the performance of Gln activity rather than GS activity. In organisms, glutamine is known as one of the ‘carriers’ of tissue ammonium, and in hepatopancreas am- monium was released from this ‘carrier’ by Gln to participate in further reactions (Wang, 2002). In our study, though Gln activity was elevated in all groups during exposure of crabs to air, thermal inconsistence between culture media leads Gln activity to drop by 61%–81% com-pared to AT23 group during recovery period. The utiliza- tion of ammonium was thus restrained in, leading to fewer raw materials used for amino acids synthesis. This mightcause the decrease of TFAA level in AT16 and AT30 groups during re-immersion.
Urea cycle has been reported to be another important ammonium-detoxifying mechanism (Peng, 2017; Wil-kie, 2017). Our results showed that emersion-in- duced ammonium overloading promoted the elevation of urea level in, which was in accord with that in emersed(Bernardi, 2015). It thus comes to the conclusion that the activity of urea cycle can be stimulated by air exposure to a higher level in order to trap more ammonium to decrease its toxicity. However, some other aquatic animals likecould not employ this strategy to detoxify ammonium (Jow, 1999), which may be due to the species-specific lifestyle. As a key enzyme in urea cycle, ARG activity ofcan be significantly elevated by ammonium stress, accelerating ammonium conversion into less toxic urea (Liu, 2014). In the present study, thermal inconsistence between culture media, rather than constant temperature, could stimulate urea cycle and depress urealysis in emersed. These synergistically contribute to the elevation of urea level during air exposure. This phenomenon gra- dually went off during recovery, which might be due to the restoration of ARG activity and enhancement of urease activity which is involved in urealysis. It should be noted that following re-immersion, the urea level was reduced by prolonged emersion time, which could not be well ex- plained by the enhancement of urea synthesis and reduction of urealysis. Therefore, we speculated that the reduction of urea level may be due to the enhancement of urea excretion rate by prolonged emersion time which was not measured in this study.
6 Conclusions
High air temperature had a negative effect onsurvival during air exposure and subsequent recovery. Our study demonstrated that ammonium buildup might be not one of the major factors influencing emersed crab survival during dry or semi-dry transport and subsequent recovery. The elevated ammonium loading could be alleviated by thermal inconsistence between environment media, because of elevated activities of ammonium-de- toxifying pathways and reduced decomposition of amino acids. Additionally, the elevated ammonium excretion and urea synthesis also contributed to lower ammonium loading during re-immersion. For the amino acids synthesis pathway, crabs relied more on glutamine synthesis systemto detoxify ammonium.
Acknowledgements
This work was supported by the National Natural Science Foundationof China (No. 31972784),the Shandong Province Natural Science Fund Project (No. ZR2018MC028), the High-levelTalents Research Fund of Qingdao Agricultural University(No. 663/1119032), the Scientific Research Project of Tianjin Education Commission (No. 2017KJ186), the Shrimp & Crab Innovation Team of Shan- dong Agriculture Research System (No. SDAIT-15-011), the ‘First Class Fishery Discipline’ programme in Shan- dong Province, China, and Marine and Fishery Science andTechnology Innovation Project of Shandong Province (No. 2017YY20).
Bergmann, M., Taylor, A. C., and Geoffrey Moore, P., 2001. Physiological stress in decapod crustaceans (and) discarded in the Clyde Nephrops fishery., 259: 215-229.
Bernardi, C., Baggiani, L., Tirloni, E., Stella, S., Colombo, F., Moretti, V. M., and Cattaneo, P., 2015. Hemolymph parame- ters as physiological biomarkers in American lobster () for monitoring the effects of two commercial maintenance methods., 161: 280-284.
Bradford, M. M., 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding., 72: 248-254.
Chen, J. C., and Chen, J. S., 1998. Acid–base balance, ammonia and lactate levels in the haemolymph ofduring aerial exposure., 121: 257-262.
Chen, J. M., and Chen, J. C., 2000. Study on the free amino acid levels in the hemolymph, gill, hepatopancreas and muscle ofexposed to elevated ambient ammonia., 50: 27-37.
Dhale, M. A., Puttananjaiah, M. K., Sukumaran, U. K., and Go- vindaswamy, V., 2011. Production ofpig- ments; influenced by amidase and acid protease activity., 35: 1231-1241.
Dong, Z., Mao, S., Chen, Y., Ge, H., Li, X., Wu, X., Liu, D., Zhang, K., Bai, C., and Zhang, Q., 2019. Effects of air-ex- posure stress on the survival rate and physiology of the swim- ming crab., 500: 429-434.
Durand, F., and Regnault, M., 1998. Nitrogen metabolism of two portunid crabs,and, during prolonged air exposure and subsequent recovery: A compa- rative study., 201: 2515-2528.
Durand, F., Chausson, F., and Regnault, M., 1999. Increases in tissue free amino acid levels in response to prolonged emer- sion in marine crabs: An ammonia-detoxifying process effi- cient in the intertidalbut not in the subtidal., 202: 2191-2202.
Etienne, R., Fortunat, K., and Pierce, V., 2001. Mechanisms of urea tolerance in urea-adapted populations of., 204: 2699-2707.
Frick, N.T., and Wright, P. A., 2002. Nitrogen metabolism and excretion in the mangrove killifishII. Sig- nificant ammonia volatilization in a teleost during air-exposure., 205: 91-100.
Hong, M., Chen, L., Sun, X., Gu, S., Zhang, L., and Chen, Y., 2007. Metabolic and immune responses in Chinese mitten-handed crab () juveniles exposed to elevated ambient ammonia., 145: 363-369.
Jow, L., Chew, S., Lim, C., Anderson, P., and Ip, Y., 1999. The mar-ble gobyactivates hepatic glutamine synthetase and detoxifies ammonia to glutamine during air ex- posure., 202: 237-245.
King, F. D., Cucci, T. L., and Bidigare, R. R., 1985. A pathway of nitrogen metabolism in marine decapod crabs., 80: 401-403.
Lei, Y. Z., 2006.. China Agriculture Press, Beijing, 45pp.
Lim, C. B., Chew, S. F., Anderson, P. M., and Ip, Y.K., 2001. Re-duction in the rates of protein and amino acid catabolism to slow down the accumulation of endogenous ammonia: A strate-gy potentially adopted by mudskippers (snd) during aerial expo- sure in constant darkness., 204: 1605-1614.
Liu, H., Sun, W., Tan, B., Chi, S., Dong, X., and Yang, Q., 2012. Molecular cloning and expression of hepatopancreas gluta- mine synthetase in the Pacific white shrimp,, induced by acute hypo-osmotic stress., 362: 80-87.
Liu, S., Pan, L., Liu, M., and Yang, L., 2014. Effects of ammonia exposure on nitrogen metabolism in gills and hemolymph of the swimming crab., 432: 351-359.
Loong, A. M., Hiong, K. C., Lee, S. M. L., Wong, W. P., Chew, S. F., and Ip, Y. K., 2005. Ornithine-urea cycle and urea synthesis in African lungfishes,and, exposed to terrestrial conditions for six days., 303(5): 354-365.
Lorenzon, S., Giulianini, P. G., Libralato, S., Martinis, M., and Ferrero, E. A., 2008. Stress effect of two different transport systems on the physiological profiles of the crab., 278: 156-163.
Lu, Y., Wang, F., Li, L., and Dong, S., 2016a. Responses of me- tabolism and haemolymph ions of swimming crabto thermal stresses: A comparative study between air and water., 47(9): 2989-3000.
Lu, Y., Zhang, D., Wang, F., and Dong, S., 2016b. Hypothermal effects on survival, energy homeostasis and expression of ener- gy-related genes of swimming crabsduring air exposure., 60: 33-40.
Maas, A., Seibel, B. A., and Walsh, P. J., 2012. Effects of ele- vated ammonia concentrations on survival, metabolic rates, and glutamine synthetase activity in the Antarctic pteropod mollusk., 35: 1123-1128.
Martin, M., Fehsenfeld, S., Sourial, M. M., and Weihrauch, D., 2011. Effects of high environmental ammonia on branchial ammonia excretion rates and tissue Rh-protein mRNA expre- ssion levels in seawater acclimated Dungeness crab., 160: 267-277.
Moore, S., and Stein, W. H., 1954. A modified ninhydrin reagent for the photometric determination of amino acids and related compounds., 211: 907-913.
Pan, L., Si, L., Liu, S., Liu, M., and Wang, G., 2018. Levels of metabolic enzymes and nitrogenous compounds in the swim- ming crabexposed to elevated am- bient ammonia-N., 17(4): 957-966.
Peng, R. B., Le, K. X., Wang, P. S., Wang, Y., Han, Q. X., and Jiang, X. M., 2017. Detoxification pathways in response to environmental ammonia exposure of the cuttlefish,: Glutamine and urea formation., 48(2): 342-352.
Richard, L., Vachot, C., Brèque, J., Blanc, P. P., Rigolet, V., Kau- shik, S., and Geurden, I., 2010. The effect of protein and me- thionine intake on glutamate dehydrogenase and alanine amino- transferase activities in juvenile black tiger shrimp., 391: 153-160.
Ridgway, I. D., Taylor, A. C., Atkinson, R. J. A., Stentiford, G. D., Chang, E. S., Chang, S. A., and Neil, D. M., 2006. Morbidity and mortality in Norway lobsters,: Physi-ological, immunological and pathological effects of aerial ex- posure., 328: 251-264.
Schmitt, A. S. C., and Uglow, R. F., 1997. Haemolymph consti- tuent levels and ammonia efflux rates ofduring emersion., 127: 403-410.
Wang, J. Y., Zhu, S. G., and Xu, C. F., 2002.. Higher Education Press, Beijing, 310pp.
Weihrauch, D., and Allen, G. J., 2018. Ammonia excretion in aqua- tic invertebrates: New insights and questions., 221(2): jeb169219.
Weihrauch, D., Becker, W., Postel, U., Riestenpatt, S., and Sie- bers, D., 1998. Active excretion of ammonia across the gills of the shore craband its relation to osmore- gulatory ion uptake., 168: 364-376.
Weihrauch, D., Morris, S., and Towle, D. W., 2004. Ammonia ex-cretion in aquatic and terrestrial crabs., 207: 4491-4504.
Wilkie, M. P., Clifford, A. M., Edwards, S. L., and Goss, G. G., 2017. Wide scope for ammonia and urea excretion in foraging Pacific hagfish., 164(6): 126.
. E-mail: yun.2004@163.com
February 26, 2019;
June 3, 2019;
November 13, 2019
(Edited by Qiu Yantao)
 Journal of Ocean University of China2020年3期
Journal of Ocean University of China2020年3期
- Journal of Ocean University of China的其它文章
- Simulation of the Power Take-off System for a Heaving Buoy Wave Energy Converter
- Biochemical Factors Affecting the Quality of Products and the Technology of Processing Deep-Sea Fish,the Giant Grenadier Albatrossia pectoralis
- Study on Wave Added Resistance of a Deep-V Hybrid Monohull Based on Panel Method
- Preparation of Clay/Biochar Composite Adsorption Particle and Performance for Ammonia Nitrogen Removal from Aqueous Solution
- Estimation of the Reflection of Internal Tides on a Slope
- Propulsion Performance of Spanwise Flexible Wing Using Unsteady Panel Method
