Oral encapsulated transforming growth factor β1 reduces endogenous levels: Effect on inflammatory bowel disease
Laura Hammer, Stacia Furtado, Edith Mathiowitz, Dominick L Auci
Abstract
Key Words: Transforming growth factor beta; All trans retinoic acid; Ulcerative colitis;Crohn’s disease; Inflammatory bowel disease; Regulatory T cells
INTRODUCTION
TreXTAM®is a proprietary micro-encapsulated drug product in development as an oral treatment for inflammatory bowel disease (IBD).It is the combination of the key regulatory cytokine transforming growth factor beta (TGFβ) encapsulated in into polylactic acid (PLA) particles; along with a signaling form of vitamin A, all trans retinoic acid (ATRA), encapsulated in poly D,L-lactide-co-glycolide (PLGA) particles[1].Simultaneous ATRA and TGFβ signals synergize in promoting the differentiation and stabilization of regulatory T cells[2].This is a completely novel strategy for the treatment of IBD, as no similar products exist.However, unlike ATRA, TGFβ is a protein macromolecule that must be protected against hydrolysis in the stomach to be effectiveviathe oral route[3].
Encapsulation remains one of the most promising methods to protect drug substances and to achieve local, sustained release.Efforts have generally focused on siRNA[4], small molecules[5], peptides[6]and cytokines[7]and involve polymer encapsulation accomplishedviacombinations of phase separation or precipitation,emulsion/solvent evaporation[8-15]and/or spraying methods[16-20].However, loss of bioactivity during manufacturing, poorly controlled release rates, and difficulties with large-scale production of accurately sized particles are some of the formidable challenges preventing commercialization.
To address these challenges, we pioneered the development of phase inversion nano-encapsulation (PIN®) technology that utilizes a non-mechanical approach to preserve the structural integrity of macromolecules during the drug product manufacturing process.PIN encapsulated cytokines have demonstrated stability,bioactivity and efficacy in various preclinical models[21-26].Particles with an average diameter of 0.1-5 microns[6], are ideally suited to oral delivery as particles smaller than 5 microns in diameter readily traverse the gastrointestinal barrier[27-29].Indeed, we had previously shown that orally administered insulin encapsulated in PIN particles resulted in localization of drug product to the gut, and efficient uptake at the intestinal border[6,7].
More recently we applied PIN technology to the development of TreXTAM and showed that oral administration effectively ameliorated disease in two different rodent IBD models[1].Broadly, treatment of mice with established disease using the optimized dose/frequency regimen, achieved a dramatic 2 to 9-fold reduction in multiple markers of disease compared to control groups within 2 wk, in some cases approaching normal values.Importantly, treatment enhanced long-term survival over eight weeks with no detectable toxicity.Activity was associated with enhanced Foxp3 expression in the colonic lamina propria CD4+ CD25+ T-cells, and required both TGFβ and ATRA for maximal efficacy.We have recently reviewed potential cellular and molecular mechanisms driving synergy, including cross-talk between ATRA and TGFβ signal transduction pathways[2].
During TreXTAM development, we studied TGFβ pharmacokinetics after oral administration of TreXTAM, or after the encapsulated cytokine (TPX6001) was given alone, without ATRA.We made the surprising discovery that oral administration of TreXTAM dramatically reduced TGFβ levels in colon and in blood, to below baseline levels.When encapsulated TGFβ (TPX6001) was given alone, three times a week for 25 d, we likewise observed serum TGFβ decreases below baseline (untreated) levels.Oral treatment with TPX6001 alone transiently ameliorated weight loss in the murine adoptive cell transfer (ACT) model of IBD.These observations suggest a negative feedback mechanism in the gut whereby local delivery of TGFβ results in reduced local and systemic levels of the active form of TGFβ.This finding suggests potential clinical implications for use of encapsulated TGFβ in the context of IBD and/or pathological TGFβ signaling.
MATERIALS AND METHODS
Preparation and characterization of TGFβ and ATRA loaded formulations
Microsphere preparation: For tissue studies, TGFβ (Peprotech, Rocky Hill, NJ, United States) was encapsulated into bench-top scale, poly-lactic acid (PLA) particles (0.285 mg TGFβ per gram of final drug product; for simplicity and clarity the abbreviation TGFβ refers specifically to TGFβ1, unless otherwise noted) using PIN as described previously[30].ATRA (Sigma) was encapsulated into poly-lactic-co-glycolic acid(PLGA) particles (1 mg of ATRA per gram of particles) using a modification of the solvent evaporation technique as in previous studies[31].
For PK studies, TGFβ and ATRA loaded microspheres (denoted TPX6001 and TPX7001, respectively) were synthesized at Lonza-Bend, Bend Oregon using a proprietary two-step spray dry process to manufacture larger, scaled up quantities.Briefly, in Step 1, lyophilized protein is mixed with excipients and dispersed.In Step 2,micronized protein + excipients are encapsulated, precipitated and collected.To reduce dose mass, TGFβ and ATRA spray dry drug products were loaded at 1 mg/g and 2 mg/g w/v.The release kinetics, bioactivity, morphology, long-term (1 year)stability, as well as the physicochemical properties of glass transition temperature and crystallinity were essentially identical in the bench lots and spray dried particles (data not shown).
TGFβ and ATRA loaded PLA and PLGA particles were mixed cage-side in the indicated proportions to create TreXTAM, a proprietary combinatorial product designed to provide both TGFβ and ATRA signals thought to drive the development of regulatory T cells[32-35].
In vitro drug substance release:Formulations were release-tested using anin vitrorelease assay described previously[24].Briefly, for TGFβ, 0.2 mL of a 10 mg/mL particle suspension was transferred to the wells of a 96-well plate in triplicate.The plate was incubated at 37oC in 5% CO2, the supernatants were sampled at the indicated time points and stored at -20oC until use.ATRA was extracted and measured by HPLC as in our previous studies[36].The immune-reactive, active form of TGFβ was measured by assaying non-acidified samples in an ELISA (R&D Systems Quantikine ELISA kit Catalog# MB100B).This assay does not have significant cross-reactivity or interference with TGFβ2 or TGFβ3, and does not detect the latent form of TGFβ1 without acid treatment.ATRA extraction and analysis was performed as follows: 10 ± 0.1 mg ATRA containing microspheres were weighed into 15 mL Falcon tubes for each terminal time-point.1 mL of 1 × PBS was added and placed on end-over-end rotator at 37 ºC.At predetermined time-points, the tubes were centrifuged and supernatant discarded.The remaining microspheres were flash frozen and lyophilized for 24 h.Microsphere samples were then extracted by adding 5 mL of pH 7 mobile phase (68:24:8 ratio of acetonitrile: 1% glacial acetic acid:ethanol) and bath sonicating for 45 min.Extracted samples were then run on a HPLC using a Waters Symmetry C18 Column (5.0 µm, 3.9 mm × 150 mm) at a flow rate of 1 mL/min using pH 7 mobile phase.Absorbance was measured at 356 nm.
Pharmacokinetic studies
Animals: The in-life phase of these studies was performed at Comparative Biosciences,Sunnyvale, California.7 to 9-wk old Sprague-Dawley rats (males and females) were kept under standard laboratory conditions with free access to food and water.They were allowed to adapt one week before starting the study.The care and use of laboratory animals was in accordance with relevant IACUC-approved animal use protocols.
Administration of encapsulated drug products:A 0.5-mL aliquot of TreXTAM (or TPX6001 alone) in aqueous suspension was prepared by reconstitution of drug products (TPX7001 and/or TPX6001) with distilled water and mixed in appropriate w/v proportions to achieve the targeted dosing.Animals were dosed by oral gavage.Blood samples were collected at fixed times after dosing.
Tissues analysis:7 to 9-wk old male Sprague-Dawley ratsn= 3 per group) were untreated, or treated (oral gavage) with TreXTAM three times per week for four weeks.Four hours after the final dose, gut tissues were taken, frozen at -20oC and stored until used.Tissues were then thawed and homogenized using a glass tube with the pestle insert, in the presence of EDTA-free SIGMAFAST™ Protease Inhibitor Cocktail Tablets (Sigma-Aldrich) used as per manufacturer’s instructions.Levels of TGFβ1 and ATRA in lysates were measured as described above.
Serum analysis:Serum levels of TGFβ1 were measured using an ELISA kit (R&D Systems, Minneapolis, MN; see above) with a slight modification from manufacturer’s instructions.Samples were not acid- activated, minimizing detection of endogenous latent cytokine.For ATRA, a high-performance liquid chromatograph combined with a triple quadrupole mass spectrometer was used as in our previous studies[36].
SCID mouse CD4+CD25- T cell transfer colitis model
The model was chosen because it recapitulates a regulatory T cell immunological basis of colitis, and was performed as in our previous studies[1].Briefly:
Animals:Six to 8-wk old BALB/c and CB-17 SCID mice (males and females; Jackson Laboratories, Bar Harbor, MA, United States) were kept under standard laboratory conditions with free access to food and water and allowed to adapt one week before starting the study.The care and use of laboratory animals was in accordance with a University at Buffalo IACUC-approved animal use protocol.
Isolation of CD4+CD25- T cells:CD4+ CD25- T-cells were purified from the spleens of naïve BALB/c mice by magnetic bead separation using MACS®column and separator according to manufacturer’s instructions (Miltenyi Biotech, San Diego, CA,United States).Purity and viability (> 95%) were assessed by flow cytometry(FACScan, Becton Dickinson, San Jose, CA, United States).
Induction of colitis:Purified CD4+CD25- T-cells were adoptively-transferred to SCID recipients (4 × 105cells per mouse, i.p.).Mice were randomized into groups when 10%of mice show 5% or greater weight loss and/or soft or bloody stools and treatment (3 ×per weekviaoral gavage) started.Daily disease score was recorded for each animal as in our previous studies[1]and summarized for each group as cumulative disease score during treatment.Last recorded values of animals that died during treatment were brought forward.At the end of the treatment period, all mice were sacrificed, and colons scored grossly for pathology on a 0 (normal) to 5 (diseased; elongated,inflamed, lacking definable stools) scale.Histology was also performed as in our previous studies[1].Six to eight H&E sections of colon representing ascending,transverse and descending colon per mouse were evaluated independently, in blinded fashion, by a board-certified pathologist (Pacific Tox Path, LLC, Ellensburg, WA,United States).A composite inflammation score was calculated based on (0-3) severity and extent of cellular infiltration, amount of mucus and degree of proliferation(maximum score of 12).
Statistical analysis
Significance (P≤ 0.05) between experimental and control groups was determined using Student’st-test analysis.In experiments with multiple groups, homogeneity of inter-group variance was analyzed by ANOVA.
RESULTS
In vitro release patterns of TGFβ and ATRA and typical appearance of PLA and PLGA microsphere particles
For tissue studies, and for ACT studies, TGFβ and ATRA were encapsulated using PIN or solvent evaporation techniques bench top-processes (respectively) at 0.285 mg/g and 1 mg/g w/w, respectively.At 24 h, both TGFβ and ATRA drug products released bioactive (confirmed using TGFβ sensitive mouse lymphoblast cell line HT-2 or ATRA sensitive murine melanoma B16-F1cells; data not shown) TGFβ or ATRA (Figure 1A and B respectively) as expected, indicating that both drug substances could potentially be delivered in active forms, simultaneously,in vivoafter oral administration.
TGFβ and ATRA in small and large intestine and MLN after oral Administration of TreXTAM to male rats
To assess delivery of ATRA and TGFβ to gut, male Sprague-Dawley rats were fed with either blank particles or with TreXTAM (60 mg/kg and 30 mg/kg of TGFβ and ATRA loaded particles; denoted TPX6001 and TPX7001, respectively, and loaded at 0.286 mg/g and 1 mg/g, approximately 17 and 30 μg/kg respectively) three times per week for four weeks.Four hours after the final treatment, small intestine, large intestine and MLN were collected from each animal and frozen at -20oC.ATRA and TGFβ levels in small and large intestine, as well as MLN, were determined by HPLC or ELISA,respectively (limit of detection 0.75 ng/mL and 0.4 pg/TGFβ/100 μg of protein,respectively).Levels of ATRA in small intestine and MLN of treated and untreated animals were at the limit of detection.Levels of ATRA in colon were virtually the same in treated and untreated animals (data not shown).TGFβ was also negligible in small intestine and MLN of treated and untreated animals.However, TGFβ levels in colon of treated animals were decreased over 50% compared to untreated animals (Figure 2).This difference was significant (P= 0.025) suggesting a treatment associated attenuation of endogenous active TGFβ in colon tissue.Since those initial studies, we scaled up production of PLA encapsulated TGFβ (TPX6001) using the proprietary twostep stray dried manufacturing process described in the methods section.Production of PLGA encapsulated ATRA (TPX7001) has also been scaled up using spray drying methods.Release rates and physiochemical properties of the spray dried and bench top materials were virtually identical (data not shown).All pharmacokinetic work to follow was performed using spray-dried TGFβ PLA and ATRA PLGA (loaded at 0.1 and 0.2%, respectively) material.
Pharmacokinetics following oral administration of TreXTAM
We could not directly demonstrate simultaneous delivery of TGFβ or ATRA to gut tissue by oral TreXTAM (although we could see biological effects[1]).To further investigate this issuein vivo, and as part of our development efforts, we tested oral TreXTAM in a 28-d GLP rat toxicology study.The relevant pharmacokinetic for ATRA after TreXTAM administration has been published previously[36].Those studies reported that after a single oral TreXAM administration, serum ATRA levels peaked with a Tmaxof 60 min and t ½ of 143 min.
We report here that after oral administration of TreXTAM (30 mg/kg spray-dried encapsulated TGFβ and 30 mg/kg PLGA encapsulated ATRA) three times a week for 25 d, serum TGFβ levels were significantly reduced compared to those observed in the same animals on day 0, prior to any TreXTAM dosing (Figure 3; NB: The level of ELISA detection is approximately 150 pg/mL).This finding was reminiscent of our observations of reduced TGFβ in colon after dosing (Figure 2).We also note that in the pre- dose, naïve animalsn= 24 per sex) females had higher endogenous levels of TGFβ compared to males (492 ± 107 pg/mLvs324 ± 14 pg/mL).This difference was highly significant (P< 0.0001).Similar observations were reported previously by Knabbeet al[37].
Oral treatments with PLA encapsulated TGFβ reduce serum levels of TGFβ
We also tested spray-dried PLA encapsulated TGFβ (TPX6001; loaded at 1 mg/g w/w)given alone in a similar 28-d GLP rat toxicology study (Figure 4).Once again, when similar analyses were performed on naive animals and on the same animals that had been dosed three times per week for 25 d, a dramatic and highly significant (P< 0.01)treatment-related reduction in serum TGFβ levels was evident for all dose groups(Figure 4).Indeed, the reduction in baseline serum TGFβ was dose dependent, in that the difference between the low and high dose group was also significant (P< 0.03).It was also interesting to once again note that in naïve pre-dose animalsn= 24 per sex),females had significantly (P= 0.001) higher levels of TGFβ than males, (492 ± 107 pg/mLvs324 ± 14 pg/mL) indicating the same gender bias.

Figure 1 Release profiles of transforming growth factor -loaded poly-lactic acid microspheres and all trans retinoic acid-loaded polylactic-co-glycolic acid microspheres.Transforming growth factor (TGF) was encapsulated in poly-lactic acid (PLA) microspheres (285 μg of TGF per gram of particles) using Phase Inversion Nano-encapsulation (PIN).All trans retinoic acid (ATRA) was encapsulated into poly-lactic-co-glycolic acid (PLGA) microspheres (1 mg of ATRA per gram of particles) using a modification of the solvent evaporation technique (see methods section).A: TGF-loaded microspheres were release-tested using the in vitro release assay as described in the methods section; B: ATRA-loaded microspheres were release-tested using an in vitro extraction assay as described in the methods section.Data are expressed as pg/mL or as μg/mL ± SE.TGFβ: Transforming growth factor ; ATRA: All trans retinoic acid.

Figure 2 Effect of oral treatment with TreXTAM on levels of transforming growth factor in colon.Treated rats (n =3) received TreXTAM [60 mg/kg encapsulated transforming growth factor (TGF) and 30 mg/kg encapsulated all trans retinoic acid (ATRA)] three times a week for four weeks.Colons of these animals were taken 4 h after the final dose, along with tissues from age and sex matched untreated animals.All tissues were frozen at -20 oC and stored until used.Tissues were then thawed and homogenized using a glass tube with the pestle insert, in the presence of EDTA-free SIGMAFAST™ Protease Inhibitor Cocktail Tablets (used as per manufacturer’s instructions).Levels of TGF in lysates were determined by ELISA according to manufacturer’s instructions but without acid activation.(Quantikine, R&D Systems, Minneapolis, MN, United States).Total protein concentration was determined by BCA Protein Assay- Pierce (Thermo Fisher Cat# 23227) Data are expressed as pg/100 μg protein ± standard deviation.TGFβ: Transforming growth factor .
Effect of TPX6001 on disease in the SCID mouse CD4+CD25- ACT model of IBD
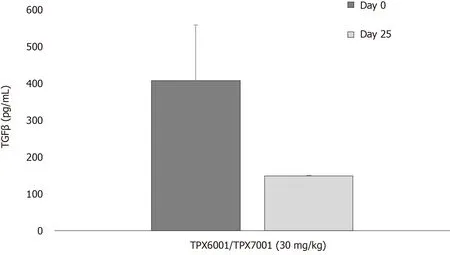
Figure 3 Baseline serum levels of transforming growth factor in naïve and TreXTAM treated animals.Blood was taken from naïve male and female Sprague-Dawley rats (males and females, 3 per sex) before and after treatment with TreXTAM [30 mg/kg encapsulated transforming growth factor (TGF) and 30 mg/kg encapsulated all trans retinoic acid (ATRA)].Animals were dosed by gavage 3 × per week for 25 d.Serum levels of TGFβ were determined by ELISA without acid activation (R&D Systems Quantikine ELISA Catalog# MB100B).TGFβ: Transforming growth factor .

Figure 4 Baseline serum levels of transforming growth factor in naïve rats and in poly-lactic acid encapsulated transforming growth factor treated rats.Blood was taken from naïve Sprague-Dawley rats (males and females, 6 per sex) before and after treatment with poly-lactic acid encapsulated transforming growth factor (TGF).Animals were treated by gavage at doses of 5, 15 or 30 mg/kg, 3 × per week for 25 d.Serum levels of TGFβ were determined by ELISA without acid activation (R&D Systems Quantikine ELISA Catalog# MB100B).Statistical significance (P ≤ 0.02) vs day 25 at 5 mg/kg vs day 25 at 30 mg/kg.All differences between day 0 pre-dose and day 25 pre-dose were significant (P ≤ 0.01).TGFβ: Transforming growth factor .
We next tested the IBD therapeutic potential of TPX6001 oral treatments when given alone, without ATRA, in the SCID mouse ACT model of IBD.This single, preliminary study used a highly challenging therapeutic iteration of the model.Treatments began at disease onset.There were no significant differences between groups in terms of body weight or disease score at the start of treatment.We found that TPX6001 treatment resulted in significant attenuation of weight loss (Figure 5).The differences between the 5 mg and 40 mg doses (days 3 to 12) were significant (P= 0.01) compared to animals treated with blank microspheres.The difference between the 10 mg and blank groups during that same period achieved only a trend (P= 0.12), possibly because of two deaths in the 10 mg group.It is also interesting to note that the high dose group showed the most benefit for the first 7 d of treatment, but then deteriorated rapidly.At the end of the study, for each group, we calculated cumulative disease score during treatment (blank fed group = 52; 5, 10 and 40 mg treatment groups = 49.5,52.6, and 47, respectively); colon weight to length ratios (blank fed group = 55.3 ± 14.3;healthy age and sex matched controls = 27.9 ± 4.4; 5, 10 and 40 mg treatment groups =52.8 ± 9.1, 56.5.± 15.3, and 57.4 ± 9.9, respectively), gross pathology (blank fed group =2.9 ± 0.9; 5, 10 and 40 mg treatment groups = 3.7 ± 1.4, 2.4.± 1.4, and 3.8 ± 1.1,respectively) and histology composite inflammation scores (blank fed group = 8.75,and 5, 10 and 40 mg treatment groups = 9.4, 7.8, and 8.1, respectively).We found no significant differences, except at the 10 mg dose (P= 0.003), which again, may have been biased by the deaths of 2 animals in that group.We also note a trend in favor of treatment with respect to cumulative disease score, in the high dose group (P= 0.08).Therefore, we conclude only slight, transient benefit of TPX6001 treatment in this iteration of the ACT model.
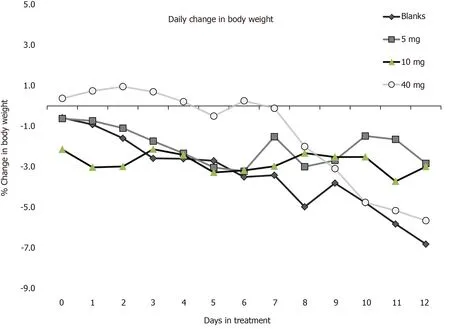
Figure 5 Therapeutic activity of transforming growth factor β loaded particles (TPX6001) in the SCID mouse adoptive CD4+ CD25- T-cell transfer model of inflammatory bowel disease.Mice (n = 6-9 per group) with established disease were weighed (day 0) and fed transforming growth factor β1 microspheres (5, 10, or 40 mg/mouse), or blank microspheres (40 mg/mouse) in 0.2 ml water 3 times per week for 2 wk.Mice were monitored for overall disease score and weighed 3 times per week for two weeks.Mice were sacrificed 2 d after the last dose, serum taken, colons weighed and measured; and colons samples prepared for histological analysis (five randomly selected sections from each mouse).Data are expressed as % change in body weight relative to day of first treatment.5 and 40 mg/mouse TPX6001-treated groups were significantly different (P = 0.01 on days 3-12) from animals treated with blank microspheres.
Multiple (28 d) oral treatments (thrice weekly) with either TreXTAM or encapsulated TGFβ were safe and well tolerated at the highest doses tested
For both TreXTAM and PLA encapsulated TGFβ GLP pharmacokinetic studies, full industry standard toxicology analyses, including clinical observations clinical pathology, necropsy, histopathology and ophthalmology, were also performed on both male and female animals.There were no statistically significant differences in body weights or weekly food intake among groups, and no significant organ weight changes.There were no test article-related histopathological or other findings and no fibrosis was observed with even the highest doses at the end of treatment (Day 28) or at the end of a 56-d recovery period (data not shown).Encapsulated TGFβ, when given alone was as safe and as well tolerated as the TreXTAM combination.
DISCUSSION
We report here that oral TreXTAM produced a surprising and dramatic decrease in serum and colonic TGFβ levels.While we could not directly demonstrate simultaneous delivery of both drug substances to gut tissues, ourin vitroand pharmacodynamics observations suggest that it was achieved.In animals given either TreXTAM or PLA encapsulated TGFβ (TPX6001) alone 3 times per week for 25 d, we observed dramatically lower serum TGFβ compared to the same animals before dosing.We also found evidence for a transient benefit of oral TPX6001, at least in terms of weight loss attenuation, in the murine adoptive cell transfer (ACT) model of IBD.
TreXTAM is being developed as treatment for Crohn’s disease (CD) and ulcerative colitis (UC).CD and UC are chronic disorders of the GI tract causing significant morbidity for over 1.4 million Americans[38].An appreciation of common inflammatory pathways led to the joint designation “IBD”.Symptoms include diarrhea, nausea,abdominal pain, weight loss, increased risk for colorectal cancer[39]and can be fatal[40].Although etiologies are incompletely understood, genetic, immunologic and environmental factors all make significant contributions[38,41].Human and animal studies implicate abnormal responses to commensal microflora and perturbed local immune homeostasis[38,39,41].‘Biologics’, macromolecules that target inflammatory lymphocytes or the cytokines they produce[42]have emerged as a new class of highly effective treatments.However, an estimated 30% of patients will not respond and of those who initially respond, 50% relapse within a year.A more recent review indicates only modest impact on surgical intervention rates[43].The need for novel, targeted therapies remains acute.TreXTAM aims to address that need by taking advantage of the synergistic effects of ATRA and TGFβ on the differentiation and stabilization of regulatory T cells[2].
TGFβ is a pleiotropic cytokine with multiple effects on many cell types.It is a key regulator of T-cell biology, impacting thymocyte development, differentiation and effector function[44].On the one hand, complete loss of TGFβ signaling leads to lymphoproliferative autoimmunity[45-47], on the other hand, systemic administration in microgram doses protects in several autoimmune disease models[48-51].Unfortunately,TGFβ is also associated with serious side effects, including pulmonary fibrosis[52-55],scleroderma[56], chronic GVHD[57]and glomerulonephropathies[58].To circumvent these toxicities, local deliveryviagene therapy has been proposed, but is inconvenient,transitory, imprecise and immunogenic[48,50,59].There is no means to control signal transcription or translation, dose schedule, release rates or unwanted immune responses.TreXTAM, aims to circumvent this problems by local delivery and reduces systemic exposure of drug substances with the hope of reducing effective doses and toxicities.
Because of the known fibrotic effects of TGFβ, exacerbation of fibrosis in the context of IBD was a serious concern of oral TreXTAM treatment.The results reported here suggest the opposite might be true, especially in colon, where TreXTAM reduced endogenous TGFβ levels.28-d TreXTAM repeat dosing studies in rats, like the one reported here for encapsulated TGFβ alone, showed no TreXTAM induced fibrosis in any organ including small intestines and colon (data not shown).Further, we tested TreXTAM, both in healthy mice and in SCID animals with CD4+ CD25- induced colitis, for up to 8 wk, and likewise, found no increases in fibrosis in any organ (Auciet al, unpublished observations).Considering the results reported here, oral treatment with TreXTAM, or even treatment with encapsulated TGFβ alone, may be useful to stimulate autocrine negative feedback and reduce TGFβ levels, to prevent IBD associated fibrosis.
Our inability to detect increased TGFβ in small intestine and MLN after TreXTAM treatment may be due to insignificant amounts of TGFβ delivered despite effective particle uptake in the Peyer’s patches and MLN(7).This may relate to the failure of the particles to reach the colon or rapid degradation and/or deactivation of the released TGFβ.Uptake by other tissues, binding to cell surface proteins or other factors, as well as the potential conversion of TGFβ1 to TGFβ2, 3 or its latent form, would have prevented an increase from being detected.Perhaps most surprisingly, we observed a highly significant TreXTAM-associated decrease (approximately 50%) of active TGFβ in the colon.While this may relate to effects of the particles themselves, a more intriguing possibility involves ATRA amelioration of TGFβ expression and signaling[60].Several studies report ATRA decreases TGFβ levels and/or signaling in various tissues[61-64].ATRA modification of TGFβ signaling may also help explain the lack of treatment associated fibrosis observed in our previous studies[1].Reduction of endogenous TGFβ in colon and its simultaneous delivery to immune structures such as Peyer’s patches and MLN may contribute to the TreXTAM-associated benefits in models of IBD.Like observations in colon, decreases in systemic TGFβ were observed when the encapsulated cytokine was delivered with ATRA in the form of TreXTAM,but also when given alone.Therefore, at least the systemic attenuation of TGFβ levels do not require ATRA and can be achieved with just the encapsulated cytokine.The role of TreXTAM in IBD, including its prophylactic and/or therapeutic usefulness for Crohn’s disease and/or colitis, awaits further studies in various models aimed at determining the contribution each component plays in the efficacy observed.
Our finding of higher levels of TGFβ in femalevsmale rats is reminiscent of other studies in humans[65]and in non-human primates, where TGFβ levels were found to be higher in young females as compared to males.Interestingly, TGFβ levels decreased with age in females, and increased with age in males, suggesting effect of sex hormones[66].The wide literature describing activities of TGFβ in the context of autoimmunity and infection has already been extensively reviewed[67], and its consideration is beyond the scope of this work.Suffice to say that an important intersection for the cross talk between TGFβ signaling pathways and sex hormones may lie at the generation and stabilization of regulatory T cells.
Reminiscent of our observations with TreXTAM in tissue, we found that oral TPX6001 when given alone, without ATRA, also reduced serum levels of the endogenous cytokine.The mechanism(s) by which oral treatment with encapsulated TGFβ could lead to reduction in systemic and tissue levels remain unknown.They may relate to synthesis or release of mediators by cells, increased uptake and/or deactivation by other tissues[68]and/or effects on pathways specific to immune structures of the gut.TGFβ is synthesized as an inactive precursor, a complex consisting of a TGFβ dimer, the latency-associated protein, and latent TGFβ binding protein[69].Before TGFβ can exert its biological effects, both must be dissociated.Therefore, our findings may also relate to specific activation/deactivation pathways,which may be controlled by the gut.It is also possible that our findings relate to switching between immunologically (ELISA) distinct isoforms of TGFβ (1, 2 or 3)[70].The potential biological significance of such switching is unclear.
To our knowledge, we were the first to administer PLA encapsulated TGFβviathe oral route[1].Our preliminary observations in the ACT model of IBD suggest only a transient benefit of oral TPX6001 treatment.However, several studies report activities of oral TGFβ, when given as an intact protein.Shiouet al[71]reported that oral administration of TGFβ (30 ng/mL) suppressed pro inflammatory cytokine production (including IL-6 and IL-8) in the gut of rat pups.The suppression was associated with suppressed NF-κB signaling.Systemic TGFβ levels were not measured.An earlier publication by Andoet al[72]reported increased serum TGFβ in mice after oral administration of the intact protein.Those studies also reported enhancement of oral tolerance.Additional studies in the ACT model, as well as other models of acute and chronic IBD, will be necessary to fairly evaluate the therapeutic potential of oral TPX6001 when given alone in IBD and perhaps also in other specific clinical situations where increasing TGFβ levels are pathogenic, for example against certain challenging forms of breast cancer[73].Such studies are subjects of forthcoming work from our laboratories.
CONCLUSION
These observations suggest a negative feedback mechanism in the gut whereby local delivery of TGFβ results in reduced local and systemic levels of the active form of TGFβ.Our findings suggest potential clinical implications for use of encapsulated TGFβ, perhaps in the context of IBD and/or other instances of fibrosis and/or pathological TGFβ signaling.
ARTICLE HIGHLIGHTS
Research background
TreXTAM®is a combination of transforming growth factor beta (TGFβ) and all trans retinoic acid (ATRA) microencapsulated for oral delivery to immune structures of the gut.It is in development as a novel treatment for inflammatory bowel disease (IBD).
Research motivation
When given together, ATRA and TGFβ signals synergize in promoting the differentiation and stabilization of regulatory T cells.
Research objectives
This is a completely novel strategy for the treatment of IBD, as no similar products currently exist.TreXTAM would represent an entirely novel IBD treatment modality.
Research methods
During TreXTAM development, we studied TGFβ pharmacokinetics after oral administration of TreXTAM, or after the encapsulated cytokine (TPX6001) was given alone, without ATRA.This is required for combinatorial products.
Research results
We made the surprising discovery that oral administration of TreXTAM dramatically reduced TGFβ levels in colon and in blood, to below baseline levels.When encapsulated TGFβ (TPX6001) was given alone, three times a week for 25 d, we likewise observed serum TGFβ decreases below baseline (untreated) levels.Oral treatment with TPX6001 alone transiently ameliorated weight loss in the murine adoptive cell transfer model of IBD, chosen because it recapitulates regulatory T cell immunology associated with disease.
Research conclusions
These observations suggest a negative feedback mechanism in the gut whereby local delivery of TGFβ results in reduced local and systemic levels of the active form of TGFβ.This finding suggests potential clinical implications for use of encapsulated TGFβ in the context of IBD and/or pathological TGFβ signaling.
Research perspectives
Additional studies in the ACT model, as well as other models of acute and chronic IBD, will be necessary to fairly evaluate the therapeutic potential of oral TreXTAM, as well as TPX6001 when given alone in IBD, autoimmune diseases, and perhaps also in other specific clinical situations where increasing TGFβ levels are pathogenic, for example against certain challenging forms of breast cancer.Such studies are subjects of forthcoming work from our laboratories.
 World Journal of Gastrointestinal Pharmacology and Therapeutics2020年5期
World Journal of Gastrointestinal Pharmacology and Therapeutics2020年5期
- World Journal of Gastrointestinal Pharmacology and Therapeutics的其它文章
- Bowel adhesion and therapy with the stable gastric pentadecapeptide BPC 157, L-NAME and L-arginine in rats
- Do liver metastases from gastric cancer contraindicate aggressive surgical resection? A 14-year single-center experience
