锂金属电池中的复合负极
赵雨萌,任凌霄,王澳轩,罗加严
天津大学化工学院,天津 300072
1 Introduction
With the rapid development of portable electronic devices and electric vehicles, the demand for high-energy-density storage systems is increasing dramatically1,2. Since the 19th century, the commercial application of lead-acid battery, nickel chromium battery, nickel hydrogen battery and lithium-ion battery has changed our life and production profoundly with incomparable power3,4. Nowadays, lithium-ion batteries have occupied more than 60% of the market share4. However, lithium intercalated anodes, represented by graphite, have been approaching the limitation of their theoretical energy density5,6, which tremendously hindered the development of new energy industry.
Lithium metal anode is regarded as an especially attractive candidate for the next generation energy storage devices because of its high theoretical specific capacity of 3860 mAh·g−1and low electrochemical potential of −3.04 V (vsStandard Hydrogen Electrode)7-9. Despite its advantages, the commercialization of lithium metal batteries (LMBs) is hampered by several challenges, such as the formation of dendrites2,10,11, unstable solid electrolyte interface (SEI)12,13, and infinite anode volume change14,15. In current literatures, most of the studies have used excess lithium metal, electrolyte and applied low current density,which profit uniform deposition/dissolution behavior16.Nevertheless, in practical applications, there is no doubt that fast charge of portable electronic products and electric vehicles is necessary. Generally speaking, high current density tends to worsen the deposition/dissolution behavior of lithium metal17.Inhomogeneous distribution of charges usually leads to aggregation and deposition of Li+at the “hot spot”. The nonuniform deposition of Li+usually presents a shape of branch,known as dendrites18. Furthermore, the structure and stability of SEI formed spontaneously are poor, and it will be further deteriorated by dendrites, causing the formation of “dead Li” and infinite volume fluctuation19. These ultimately lead to the declination of Coulombic efficiency, irreversible capacity loss,short circuit, thermal runaway and even explosion.
The key to solve the above-mentioned problems is inhibiting the formation of lithium dendrites. A lot of strategies have been put forward, including electrolyte additives9,20, artificial SEI21,22,composite anodes16,23-26, promoted solid-state electrolytes(SSEs)27,28, and modifying separators29,30. As we all know,LMB is composed of cathode, lithium anode, electrolyte and separator31, which are inseparable from each other and impact the battery performance synergistically. In addition, the operating conditions and working environment are also important. However, most of the methods mentioned above only optimize the performance of LMB from a single aspect, which have great limitations.
In recent years, great progress has been made in the research of composite lithium metal anodes through comprehensive consideration and greatly optimized the performance of LMBs.
2 Mechanisms of lithium dendrite formation and growth
In previous studies, researchers have proposed several models of lithium dendrite formation and growth, which laid a solid theoretical foundation for the suppression of lithium dendrites(Table 1). Chazalviel32put forward space charged model in1990, which is the most widely accepted classical model to analyze the nucleation and growth of lithium dendrites from the perspective of kinetics. The model points out that ion concentration distribution is relatively uniform at low current density, so that the trend of dendrite formation is relatively small.However, when the current density exceeds the limit current density, rapid depletion of Li+leads to a sudden decrease of ion concentration in the vicinity of the anode in dilute solution, and thus creating a large space charge region. Strong electric field can accelerate the deposition of a large number of Li+in a short time, resulting in an uneven lithium metal deposition morphology. It also points out that the time needed for nucleus formation is named “Sand’s time” (τ), which can be calculated from equation (1) and (2).
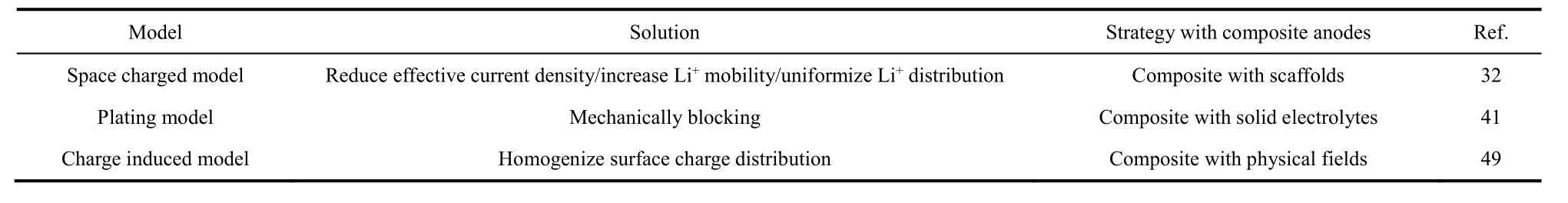
Table 1 Theoretical models of lithium dendrite formation and corresponding strategies using composite anodes for LMBs.

where,Dis the ambipolar diffusion constant,eis elementary charge,C0is the initial concentration of the electrolyte,Jis the effective current density,taandtLi+ are the transference number of anions and Li+, respectively,μaandμLi+are the mobility of anions and Li+, respectively.
The largerτ, the more difficult for lithium dendrite formation.Based on the model, whereτis proportion toJ−2and positively correlated toμLi+, decreasingJand increasingμLi+ can be effective methods to postpone lithium dendrite formation.
Meanwhile, the model proposed by Monroe and Newman33indicates that the growth rate of lithium dendrite is also positively correlated with current density in the liquid electrolytes.

where,vtipis the growth rate of lithium dendrite,Jnis the current density,Vis the molar volume of lithium, andFis the Faraday’s constant. Furthermore, from the perspective of kinetics, Li+is deposited by obtaining electrons. Thus, the distribution of electrons and ions is equally important. Therefore, suitable materials, such as electron-conductive (EC) materials24,34-38,ion-conductive (IC) materials39,40,et al. can be used to composite with lithium metal forming composite anodes,reducing effective current density, increasing the mobility of Li+and uniformizing the distribution of Li+. Thus, finally, the formation of lithium dendrites can be postponed.
Yamakiet al.41demonstrated that the formation of lithium dendrite is due to the release of mechanical stress. Based on the plating model, pressure difference (ΔP) is related to surface tension (γ) and orthogonal radius of curvature (R1andR2).

When ΔPis greater than the creep strength of lithium metal,in other words, whenγis greater than 0.2 N·m−1, the formation of lithium dendrites can be restrained. That is to say, lithium dendrites can be blocked by mechanical strength of a “hard film”2. Compared with the liquid electrolyte, SSEs and quasisolid-state electrolytes have higher mechanical strength, which can inhibit the formation of lithium dendrites mechanically and make battery safer. However, poor interface contact performance has been seriously criticized42,43. Recently, some researchers composited SSEs and quasi-solid-state electrolytes with lithium metal anodes, effectively reduced the interface resistance and increased the ionic conductivity, thus greatly improved the performance of LMBs44-48.
Charge induced model indicates that, the morphology of lithium metal deposition is related to the surface charge distribution of the substrate49. A uniform electric field can induce homogeneous Li+distribution, so that uneven lithium deposition can be suppressed. It can be confirmed from theory and experiments that physical fields, such as electric field and magnetic field, can induce uniform distribution of Li+in the vicinity of the substrate through the effect of physical force on Li+(equations (5) and (6))11. Li+can be evenly distributed and nucleated, so as to obtain uniform lithium metal deposition morphology.

where,FEandFBare the forces generated by electric field and magnetic field, respectively,zis the amount of charge carried by an ion,e0is the elementary charge,qis the charge number,vis the velocity of the ion,EandBare the electric field intensity and magnetic field intensity, respectively.
From the above-mentioned theoretical models, it can be concluded that the formation of lithium dendrites is affected by complex factors. Smaller current density, greater Li+transference number, higher mechanical strength of electrolyte,and more homogeneous distribution of Li+on the substrate, are beneficial for uniform morphology of lithium metal deposition.
Briefly, from the perspective of integral structure of LMB,composite anodes can be summarized as follows:
(i) The internal composite of lithium metal anode: composite lithium metal with suitable materials to reduce the effective current density and homogenize the distribution of Li+in the vicinity of the anode.
(ii) The internal composite of LMB: composite lithium metal anode with SSE and quasi-solid-state electrolyte to enhance the interface contact performance and block the lithium dendrites mechanically.
(iii) The overall composite of internal environment and external operating conditions of LMB: composite LMB with physical fields to uniform Li+distribution in the vicinity of the anode and accelerate the migration rate of Li+in the electrolyte.
3 Internal composite of lithium metal anode
From the perspective of internal structure of lithium metal anode, the first strategy to enhance the performance of LMBs is compositing lithium metal with host scaffolds to obtain composite lithium metal anodes. In recent years, more and more attentions have been paid to the design of host scaffolds in order to suppress lithium dendrites and inhibit volume expansion15,35,50,51. Generally speaking, suitable host scaffolds should have the following characteristics: stable chemical properties and certain mechanical strength to maintain the morphology and physicochemical properties of the scaffolds during the repeated deposition/dissolution process, large surface area and porous to serve as the host for deposited lithium metal.The host scaffolds can be roughly divided into four categories according to their conductivity: non-conductive (NC) scaffolds,EC scaffolds, IC scaffolds and mixed ion and electronconductive (MIEC) scaffolds.
3.1 Compositing with NC scaffolds
Using 3D scaffolds is an effective strategy to suppress lithium dendrites and weaken volume fluctuations. The fundamental reason for lithium dendrites lies in the uneven distribution of Li+on the anode surface. Therefore, the solution to lithium dendrites is to homogenize the distribution of Li+on the anode. Several NC materials with great quantities of polar functional groups are layered on the top of the current collector as scaffolds40,52,53. The interaction between these polar functional groups and Li+can weaken the impact of tip effect, thereby preventing the accumulation of Li+, making them uniformly distribute on the surface of the lithium metal anode, finally inhibiting dendrites(Fig. 1a). Moreover, because of the strong affinity of polar functional groups, lithium metal will grow along these NC scaffolds, although they do not participate in the deposition process of lithium metal actually52.
In this context, Cuiet al.40proposed a lithium-coated polymeric matrix to inhibit lithium dendrites and volume expansion. In order to increase the lithiophilicity of polyimide(PI), a layer of ZnO was attached to PI fibers through atomic layer deposition, which the melting lithium could react with. The most worth mentioning is that the anode prepared by this method did not require an additional current collector, and the diffused lithium metal itself served as the only conductive material, so that the process of gaining and lossing electrons of the composite anode were separated from the matrix. Most Li+or lithium metal only got or lost electrons from the bottom lithium metal that has been deposited. Lithium metal could only grow from bottom to top, thus avoiding the problem that Li+were directly deposited on the top of the current collector causing the formation of dendrites, while the vacancies inside were not filled (Fig. 1b).Based on the above advantages, this composite anode can circulate smoothly for more than 100 cycles in carbonate and ether electrolyte even at the current density of 5 mA·cm−2.
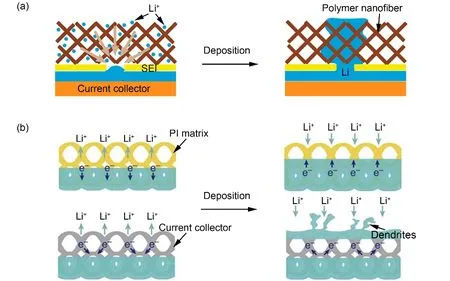
Fig. 1 Compositing with NC scaffolds.
3.2 Compositing with EC scaffolds
EC scaffolds, usually called current collectors, have excellent electron-conductivity. Current collector is an important part of the electrode, which has an important influence on the nucleation and electrodeposition behavior of lithium metal. Copper (Cu)foil is the most commonly used collector, but there are defects in its micro morphology, such as terraces, kinks, and steps, where electrons gather easily and lithium tends to deposit and finally lead to the formation of dendrite23. Porous metal-based and carbon-based materials with large surface are popular in the study of 3D collectors, which can significantly reduce the local current density and thus inhibiting the generation of dendrites according to space charged model.
3.2.1 Compositing with metal-based scaffolds
Cu and nickel (Ni) are often used as collectors for their high mechanical strength and structural stability. Luet al.51used 3D porous Cu as collector to provide a “cage” for lithium, thus effectively alleviating the volume expansion and dendrite formation. Zhang and co-workers36proposed a thermal diffusion method to diffuse molten lithium metal into Ni foam(Fig. 2a). Because of the high melting point and low surface energy of Ni, molten lithium metal infiltrated into its porous structure easily. Furthermore, Ni foam not only acted as a host scaffold to encapsulate lithium metal, but also accommodated the surface energy between the composite anode and the electrolyte, so that no dendrite was generated during the cycle.LMBs with such anode can circulate more than 100 cycles with an overpotential of less than 200 mV in carbonate-based electrolyte at a high current density of 5 mA·cm−2.
However, the application of melting and thermal diffusion to prepare composite lithium metal anode is greatly limited, for the reason that it requires lithiophilic sites on the scaffold surface and high temperature during operation. Compared with the thermal diffusion method, electrochemical deposition is the most widely used method to prepare composite lithium metal anode16. Guo and co-workers38prepared a 3D current collector with a submicron skeleton which was composed of Cu fibers,with micron-level diameters and nano-level secondary protrusions on the surface. For normal Cu foil, once lithium metal nucleated, it further led to the deposition of lithium metal to form dendrites. As for the 3D current collector with submicron skeleton fibers which can serve as charge centers and nucleation centers, it was conducive to forming a uniform electric field distribution (Fig. 2b). At the same time, high surface area of the 3D current collector provided larger electroactive surface area for Li+to deposit densely in the pores. As a consequence, by electrochemically depositing lithium on such 3D collector to serve as anode, LMB can run for 120 cycles at 0.2 mA·cm−2and 50 cycles at 0.5 mA·cm−2with Coulombic efficiency of 97%.However, the curved Cu fiber that formed by chemical methods has certain irregularities, and the tortuosity of the electrode grievous hindered the mass transfer process.
Similarly, Dasguptaet al.37also proposed a kind of 3D current collector composed of Cu pillars (Fig. 2c). However, the Cu pillars were highly uniform vertically aligned, which greatly reduced the transmission resistance of Li+. Inspired by the fact that the nucleation overpotential on the surface of Au, Ag, Zn is basically zero54, a layer of ZnO was deposited on the surface of the pillars by atomic layer deposition to tune interfacial chemistry and further accelerate the nucleation process of lithium metal. Because of the coupling effect of lithium affinity through interface optimization and the uniform electric field distribution of the 3D current collector, homogeneous lithium metal deposition morphology without dendrites and extremely high Coulombic efficiency (99.5% at the current density of 0.5 mA·cm−2) can be obtained.
In addition to the above-mentioned 3D protruding fiber array,the recessed array structure of current collector can also be used to suppress dendrites. 3D Cu scaffold with vertically aligned microchannels (Fig. 2d), developed by Guo and co-workers55,could induce Li+to deposit on the inner wall of the channels,because the current density in the microchannels were significantly greater than that on the upper surface of the porous Cu. By adjusting radii, depth and distribution of the microchannels, LMB can achieve stable cycle performance with higher average Coulombic efficiency within 200 cycles.
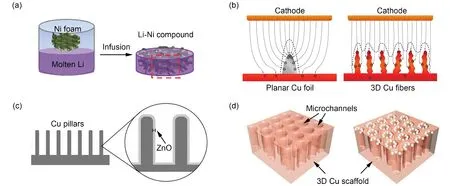
Fig. 2 Compositing with metal-based EC scaffolds.
Apart from these, there have been many composite anode designed with lithium alloys56-58. Generally speaking, lithium alloys have good wettability for lithium, which is beneficial to the nucleation of lithium metal. However, the alloyed lithium anode generally faces the problems of reduced capacity of LMBs and increased reduction potential of the lithium anode, which is unsatisfactory.
Although metal-based scaffolds have made certain progress in uniform deposition without dendrites and volume expansion for lithium metal anode, the introduction of bulky metal scaffold will seriously offset the advantage of high specific capacity of lithium anode. Besides, the metal-based scaffolds, represented by Cu, ususlly have poor wettability to lithium metal, which also severely limits the further improvement of the performance.
3.2.2 Compositing with carbon-based scaffolds
Carbon-based materials are more and more widely used in the scaffolds of composite lithium metal anodes, due to their numorous advantages. High surface area and electronic conductivity are beneficial to reduce the effective current density, thereby inhibiting tip effect, so that lithium metal is deposited uniformly. The porous structure can encapsulate lithium metal and suppress volume change. Meanwhile, the mass capacity loss of lithium metal can be highly avoided besause of its lightweight. Due to the above characteristics, the use of carbon-based materials as lithium metal anode scaffolds has received more and more attention, such as graphene24,59, carbon fiber60, carbon nanotubes61, reduced graphere oxide13and so on.
In this context, Cuiet al.35used electrospun carbon fiber net as the skeleton and modified the 3D porous carbon matrix with Si through chemical vapor deposition, which could form binary alloy with lithium to increase the lithium affinity of the porous carbon material62, so that molten lithium metal could quickly diffuse into the pores (Fig. 3a). Due to the stable structure,special lithiophilic interface and unblocked electron conductive pathway of Li/C composite anode, the capacity can reach up to 2000 mAh·g−1, and LMB can cycle for more than 80 cycles at a high current density of 3 mA·cm−2without dendrites and visible volume expansion.
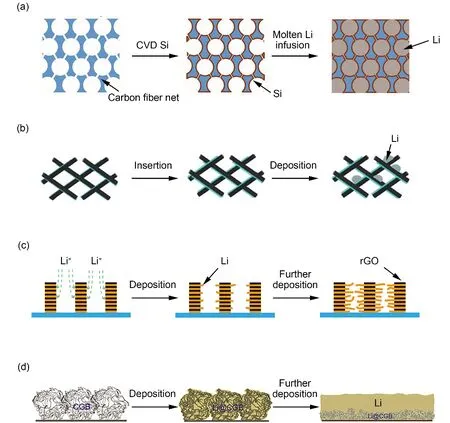
Fig. 3 Compositing with carbon-based EC scaffolds.
To further increase the capacity of LMB, Guo and co-workers34designed graphitized carbon fibers (GCF) as 3D current collector, of which the most prominent advantage was combining interaction and lithium metal deposition as lithium storage process (Fig. 3b). In the process of discharge, the first step was the intercalation of Li+. When the potential dropped below 0 V, the deposition process of lithium metal began.Therefore, the capacity of LMB using this composite anode was as high as 8 mA·h·cm−2without obvious dendrite formation.Besides, GCF with high graphitization degree had surperior conductivity and large electroactive surface, providing fast electronic transmission channel and reduced local current density, resulting in high Coulombic efficiency of the GCF composite anode. Except for 1D carbon materials, 2D carbon materials can also be used as EC scaffolds of composite anode.Luo and co-workers23creatively developed a patterned reduced graphere oxide composite anode (p-rGO/Li). The composite anode presented a layer by layer morphology of p-rGO and lithium metalviaa method of template flash etching GO film followed by Li infusion. Li+tended to deposit and grow horizontally at the edges of the empty areas, where electric field lines were denser, and finally filled up the vacant parts horizontally with small volume fluctuations and negligible dendrites growth (Fig. 3c). What's more, direct deposition on the layer of lithium metal that had been deposited horizontally already did not require extra nucleation energy barrier, thus reducing the interface resistance. As a result, symmetrical battery using the horizontal centripetally grown lithium metal anode can circulate smoothly for more than 2000 h, and it can still maintain a stable deposition/dissolution voltage distribution even at a current density of 10 mA·cm−2.
In addition to using 2D carbon materials as a layered structure,Luoet al.24assembled rGO balls into a stretchable continuous graphite solid as scaffords. The crumpled paper ball-like graphene particals were lithiophilic, ensuring the rapid transmission of Li+and effectively reducing the nucleation overpotential of Li+without any coating of the scaffolds.Furthermore, lithium metal could deposite inside the folds of the rGO balls and between the graphene particles of them, so that volume fluctuations were minimized while the capacity was increased (Fig. 3d). As a result, it was possible to deposit lithium metal with a height of 100 μm with 12 mAh·cm−2without any dendtries.
Although EC materials increase the electrochemically active area of the anodes and significantly reduce the current density,inhibiting the generation of dendrites and volume fluctuation, it also increases the contact area between lithium anode and electrolyte, leading to intensified side reactions. Moreover,owing to its electrical conductivity property, lithium metal is more likely to deposit on the upper surface of the EC scaffolds instead of inside of it, especially at high current densities,resulting in the formation of dendrites and serious safety accidents.
3.3 Compositing with IC scaffolds
From the kinetics point of view, the two elements of lithium metal deposition are Li+and electrons, both of which are indispensable63. Therefore, in addition to EC scaffolds, it is also possible to composite IC scaffolds with lithium metal as anodes.Accelerated ion transmission rate and uniform Li+flux are also conducive to obtaining a lithium anode with high capacity and no dendrites. Cuiet al.39prepared a LixSi-LiO2 matrix with embedded Li domains by reacting SiO with a pverstoichiometric amount of Li, forming a special structure that directly encapsulated lithium metal inside the IC scaffolds. Compared with the open-framework 3D Lithium metal anode, the special composite anode eliminated the direct contact between lithium metal and the electrolyte, thereby avoiding side reactions and the generation of excess SEI (Fig. 4a). Moreover, the lithium metal encapsulated inside the matrix did not lose electrochemical reaction activity because of the advanced ion conductivity of the scaffold.
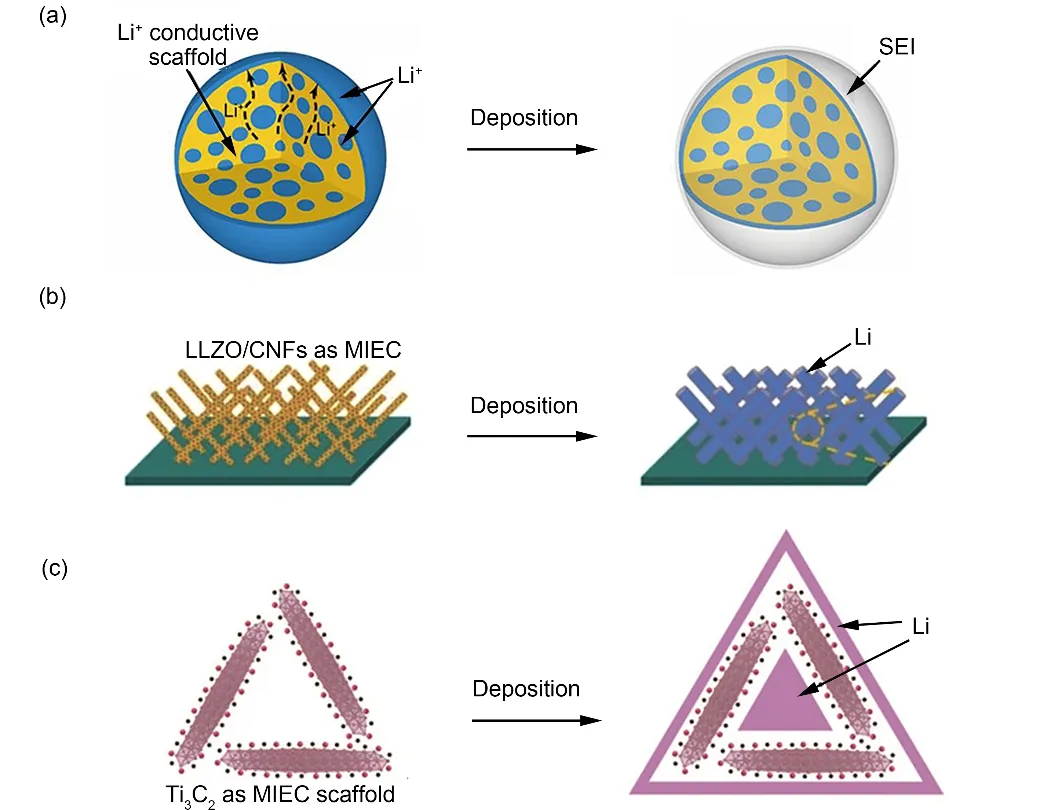
Fig. 4 Compositing with IC scaffolds and MIEC scaffolds.
3.4 Compositing with MIEC scaffolds
EC, IC and NC scaffolds all show their own advantages in composite lithium metal anodes, but they also have potential shortcomings. As mentioned earlier, both electron transport and ion transport are of importance in the process of lithium metal deposition, especially under high rate. IC scaffolds may hinder the electron transport process, and pure EC scaffolds may be restricted due to slow ion diffusion25, let alone NC scaffolds.Therefore, compared with NC, EC and IC scaffolds, MIEC scaffolds have received more and more attention25,26,64-66.
Recently, Luoet al.64incorporated Li6.4La3Zr2Al0.2O12(LLZO) nanoparticles into 3D carbon nanofibers scaffold through an electrospinning process followed by heat treatment.On the one hand, CNFs with excellent electronic conductivity effectively reduced the current density. On the other hand, LLZO accelerated the transport of ions, uniformed the distribution of Li+and improved the surface roughness of CNFs which could reduce the interface energy (Fig. 4b). Therefore, the area capacity of the composite anode can reach up to 16 mAh·cm−2,and the volume capacity is as high as 1600 mAh·cm−3. Besides,it can still operate smoothly for more than 1000 h even at a high current density of 5 mA·cm−2.
For the first time, Luo and co-workers26explored the potential of MXene aerogel as lithium metal anode scaffolds, because both theoretical and experimental studies have proved that Ti3C2, a kind of MXene, has excellent electronic conductivity and extremely low Li+diffusion barrier (Fig. 4c). For one thing, the abundant oxygen-containing functional groups on MXene aerogel induced uniform deposition of lithium metal, and the interconnected MXene network provided continuous electronic and ion pathways. For another, the aerosol voids served as a host for lithium metal, thereby inbihiting volume expansion during the repeated deposition and dissolution of lithium metal.Therefore, LMBs with this anode can operate stably at a current density of 10 mA·cm−2with low overpotential.
4 Internal composite of the LMB
As we all know, the most fundamental problem that causes the safety hazards of LMBs lies in the active properties of lithium metal and the inherent properties of organic liquids, which could cause serious catastrophe. In recent years, the academic community has mainly focused on SSEs and quasi-solid-state electrolytes to improve the safety performance of LMBs, ranging from inorganic electrolytes to polyer elecrtolyte14,67. However,no matter which kind of electrolytes, all face critical challenges.For SSEs, including solid polymer electrolytes (SPEs), inorganic solid electrolytes and polymer-inorganic composite electrolytes,the most prominent problem is poor physical contact between solid lithium metal anode and SSE48,68. As for quasi-solid-state electrolyte, represented by gel polymer electrolyte, it combines the advantages of liquid electrolyte and SSE while retaining shortcomings of the both at the same time, and the conductivity,tenacity, interfacial stability and compatibility need to be considered during the preparation process. Therefore, from the perspective of internal structure of LMBs which contain electrode, electrolyte and separator, the second strategy to enhance the performance of LMBs is compositing lithium metal anode with electrolyte.
4.1 Compositing with SSEs
Compared with liquid electrolytes, SSEs can mechanically block the formation of dendrites, owing to their high shear moduli69. On the other hand, SSEs are not flammable and have relatively high thermodynamic and electrochemical stability44.However, SSEs generally have almost no fluidity, and poor interface contact with the lithium anode at room temperature.Therefore, it is difficult for electrolyte to form continuous ionic and electronic contact with anode, resulting in large interface impedance, which seriously weakens the electrochemical performance. Moreover, the volume change of lithium metal during continuous deposition and dissolution process will further lead to interface fluctuations, especially in the case of large current density and high capacity, which further limits the performance of solid-state batteries. Some treatments have been applied to improve the interface compatibility between SSEs and lithium anode, such as lithiophilic coating70,71, surface chemical treating72,73,etc., which can only regulate the compatibility of lithium anode and SSEs from the perspective of contact surface.Nevertheless, the bulk anode has a greater impact on the compatibility of SSEs and lithium anode during cycles.Therefore, SSEs and lithium metal anode are composited to form an integrated design of electrolyte system and electrode structure, so that continuous contact between lithium anode and SSEs can be successfully achieved.
4.1.1 Compositing with inorganic solid electrolyte
In the past few years, several types of inorganic solid electrolytes have been explored, such as perovskite type electrolytes74, garnet based electrolytes75and sulfurized based electrolytes76. However, among these electrolytes, garnet electrolyte is the most favored because of its high ionic conductivity, wide electrochemical window, thermodynamic and electrochemical stability.
In this context, Huet al.47proposed a 3D lithium metal anode compositing with garnet solid electrolyte Li7La3Zr2O12(LLZO).They fabricated an asymmetric 3D LLZO based on a porousdense bilayer structure garnet electrolyte through co-sintering.In order to increase the lithium affinity of the porous layer, ZnO was attached to the inner surface of porous layer, which not only acted as a 3D scaffold for the lithium anode, but also provided continuous ion channels and greatly overcame the problem of volume expansion (Fig. 5a). The dense layer acted as a seperator,mechanically suppressing the dendrites. Furthermore, the other side of it was highly flat, which was beneficial to the assembly of cathode. Benefitting from this, 3D lithium metal anode based bilayer framework greatly improves cycle stability, produces extremely low overpotential and prevents short circuit.
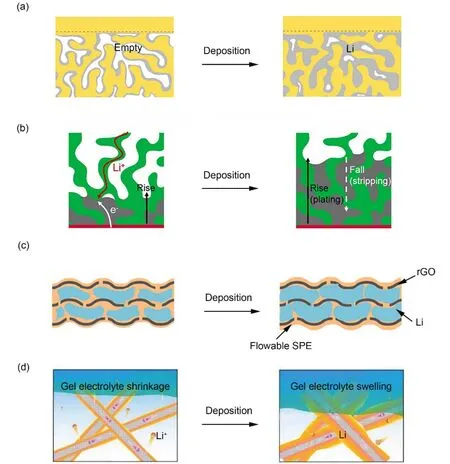
Fig. 5 Compositing with electrolytes.
However, when using the bilayer garnet framework, lithium metal may be deposited on the outside of the framework, because SSE was in direct contact with the stainless steel battery case.Therefore, a trilayer garnet structure electrolyte with a porousdense-porous structure and a thin layer of Cu on the bottom was introduced44. The Cu current collector at the bottom was deposited on the surface of the anode by electron beam evaporation, greatly reducing the interface impedance of the battery. Consequencely, both the deposition and dissolution processes of lithium metal only started from the surface of the deposited lithium metal, far away from the intermediate dense layer, so the penetration of the intermediate layer was thoroughly avoided (Fig. 5b). As a result, this composite anode is extremely stable and can cycle for 300 h with a low overpotential at a current density of 0.5 mA·cm−2without generating dendrites.
4.1.2 Compositing with SPEs
Compared with inorganic solid electrolytes, SPEs, composed of polymer chains and lithium salts, are flexible, processable and stretchable with good film-forming properties27,28. However,how to maintain continuous ionic/electronic contact in the case of unstable interface morphology produced by the deposition and dissolution of lithium metal, especially under high rate, is still a great challenge.
Cuiet al.48composited lithium metal anode with a fluidized SPE by pouring liquid SPE on the Li/rGO composite material which have been obtained before by the capillary force. After flowable SPE penetrated, it completely occupied the nanopores of Li/rGO, and a composite anode with the polymer electrolyte was finally obtained. On the one hand, the lithium metal was encapsulated in rGO layer by layer, thereby suppressing the volume change. On the other hand, SPE with certain fluidity had good adaptability to volume changes. When lithium metal was stripped from rGO layer, SPE filled in the pores, thereby maintaining the stability of the composite metal anode. When lithium metal was redeposited, SPE could be easily extruded owing to the part of the lithium metal remaining in the rGO which acted as nucleation sites (Fig. 5c). Therefore, continuous contact process between the electrode and the electrolyte was maintained during the repeated disolution and disposition, thus reducing the interface resistance. As a result, the assembled solid-state full-cell, paired with high loading LiFePO4, has a specific capacity of 110 mAh·g−1, and at a current density of up to 3 mA·cm−2, it still has a capacity retention rate of 93.6% after 300 cycles.
4.2 Compositing with gel polymer electrolyte
Compared with SSEs and SPEs, gel polymer electrolyte has stronger fluidity under room temperature, which is beneficial to improve the interface contact performance between electrolyte and electrode. However, it is a enormous challenge to maintain good interface contact and long-term cycle stability under the condition of constant volume change of lithium metal anode during the cycle. With good liquid storage performance, gel electrolyte is a suitable material to composite with 3D lithium metal anode to form an integrated system which is an effective strategy to meet the above challenges.
In particular, Guo and co-workers45reported an integrated system, compositing 3D carbon fiber (Li/CF) anode with anin situautopolymerized gel electrolyte, successfully encapulating liquid electrolyte within 3D Li/CF anode and improving the interfacial contact through interfacial polymerization (Fig. 5d).Thein situautopolymerized gel electrolyte acted as a reservoir that regulated the supply of liquid electrolyte, which absorbed and released liquid electrolyte during the volume change of lithium anode. In addition, the formed gel polymer electrolyte was highly ion-conductive and flexible, which was conducive to the formation ofin situcoating on irregular 3D Li/CF, thereby realizing continuous supply of Li+along the interface of the 3D lithium metal anode. The integral structure of the 3D lithium anode andin situpolymer gel electrolyte had successfully solved the problems of lithium dendrite formation, volume change and discontinuous interface contact in solid-state LMBs. Owing to these, the capacity of full-cell Li/LiFePO4 reaches 129 mAh·g−1,and the capacity retention rate after 120 cycles is as high as 88%.
5 Overall composite of internal environment and external operating conditions of the LMB
It is widely accepted that lithium dendrites are caused by diffusion limitation. That is to say, when the electrochemical reaction rate is greater than the diffusion rate of Li+, a large concentration gradient will be formed in the vicinity of anode surface leading to uneven electric field distribution, and finally dendrites are formed77,78. In terms of dynamics, as a positively charged particle, the diffusion direction and velocity of Li+can be impacted by the force of magnetic field, electric field11, even the temperature field. Therefore, from the overall perspective of LMB, the external physical fields can be applied to the battery to adjust the distribution of Li+in the vicinity of the anode, so that dendrite-free deposition morphology can be attained.
5.1 Compositing with external magnetic field
It is well known that when the trajectory of a charged particle cuts magnetic line, the charged particle will be forced by Lorentz force, which will deflect its origin movement, and Li+is no exception. There is an inherent electric field between the anode and cathode inside the battery. That is to say, when an external magnetic field is applied outside the battery, the charged particles in the electrolyte will be forced by both electric field and magnetic field thus producing vortices, which is called magnetohydrodynamic (MHD) effect79,80. Luoet al.81composited external magnetic field with LMB and used MHD effect to solve the tip effect of Li+, causing the solution to vortex on the protrusion of lithium metal anode, thereby accelerating the diffusion of Li+on the electrode surface (Fig. 6a). Instead of accumulating Li+somewhere, it increased the deposition area of lithium metal, thereby effectively inhibiting the growth of dendrites. In ether electrolyte, the deposition morphology of lithium metal presented a flattened petal-like morphology.Simultaneously, Luet al.82also adopted the coincidence effect of the applied magnetic field, which accelerated the migration speed of Li+and changed the movement direction of Li+on the anode surface, so that Li+were uniformly distributed in the vicinity of the anode surface, and a uniform deposition morphology of lithium metal was obtained.
5.2 Compositing with external electric field
Similar to the external magnetic field, the uniform distribution of Li+on the surface of lithium anode can also be achieved by the external electric field. Hanet al.83explored the influence of alternating current field on the distribution of Li+in the vicinity of the lithium anode. By placing a sinusoidal alternating current field (AC) vertically on the lithium anode, the distribution of Li+near the lithium anode was significantly more uniform, avoiding the accumulation of Li+at the tip. Besides, considering that lithium dendrites was affected by the diffusion rate of Li+in the electrolyte, an external direct current field (DCF) was installed(Fig. 6b). Owing to the applied DCF, the concentration gradient was significantly reduced, minimizing the driving force for the dendrite formation. Finally, increased lateral migration rate of Li+on the surface of the anode and accalated rate of Li+moving towards the anode, owing to the applied ACF and DCF respectively, make it possible for 5 times the cycle life extension at high current density.
5.3 Compositing with external temperature field
The generally accepted theory is that as the current density increases, the formation of lithium dendrites will be accelated.But Koratkar and coworkers84believed that when the current density was large enough, dendrites that have been generated could be self-healed due to the self-heating phenomenon of batteries, which indicated the important influence of temperature(Fig. 6c). It was reported that when the current density was 15 mA·cm−2, the predicted self-heating temperature was about 40-60 °C, which was far less than the temperature at which lithium metal could be dissolved. Hence, the reason for dendrite selfhealing may be that the temperature was sufficient enough to allow Li+to diffuse on the surface of the dendrites and fill the gaps between the dendrites, finally forming a uniform deposition morphology.
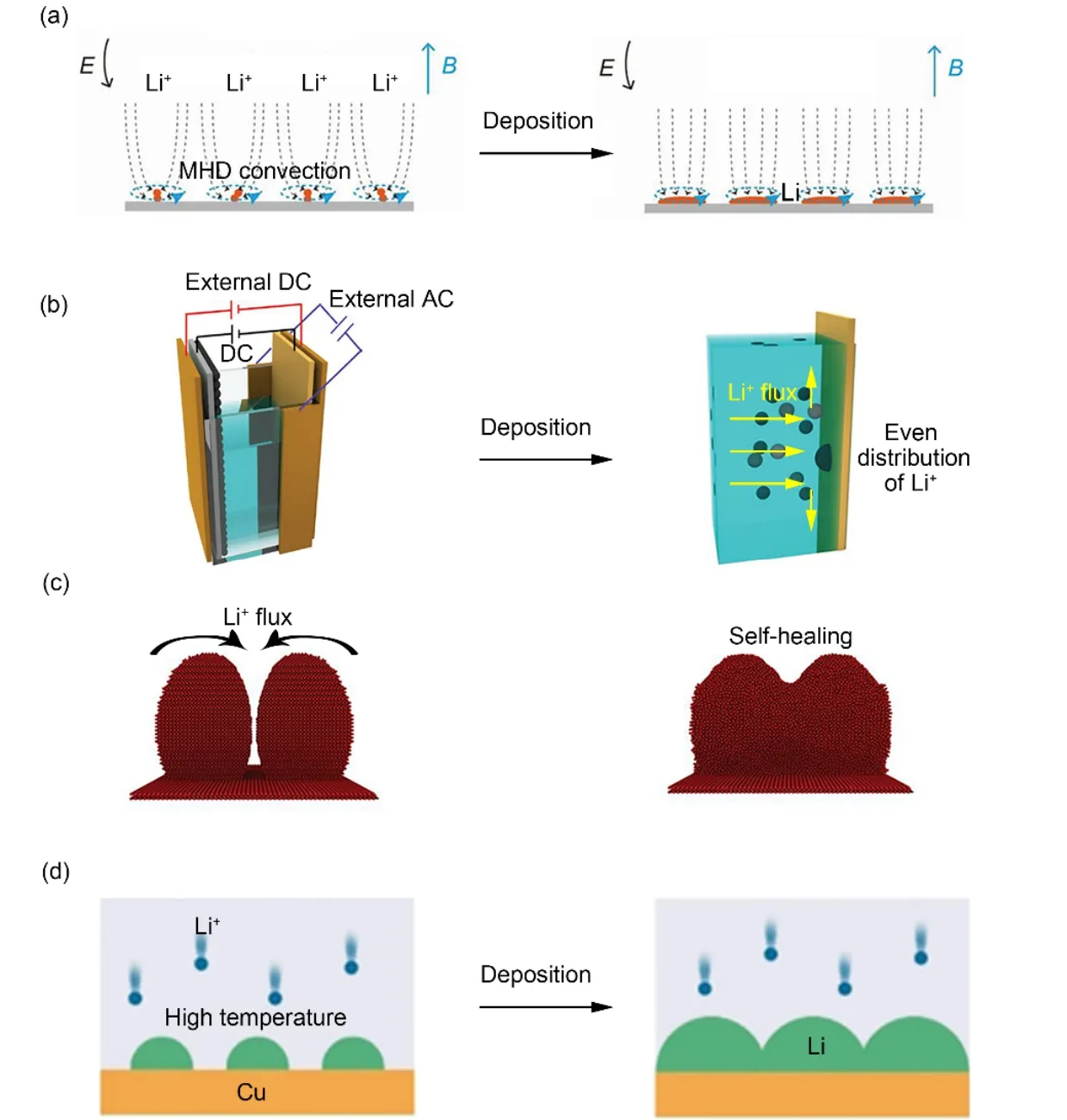
Fig. 6 Compositing with external physical fields.
Inspired from this, Wanget al.85combined temperature field with lithium anode and achieved uniform deposition of lithium metal. The nucleation characteristics of lithium metals were affected by temperature from the beginning. High temperature was beneficial to large and sparse Li nuclei formation, while low temperature corresponded to the formation of small and dense Li nuclei (Fig. 6d). Meanwhile, the rapid ion mobility caused by high temperature led to the change of Li+nucleation position. As the electroplating continues, large and sparse Li nuclei gradually grew until they fused with their neighbors, finally forming a uniform deposition morphology without dendrites. Furthermore,due to the increased temperature, the rate of Li+passing through SEI was increased owing to the accelarated desolvation rate of Li+. Finally, whether in half-cell or full-cell, LMB can be dendrite-free with high Coulombic efficiency and long cycle life at 60 °C.
6 Conclusions
Numerous factors from internal to external constitute a unified whole of LMB, such as the internal structure (composed of anode, cathode, separator and electrolyte), the distribution of internal physical field, the external operating conditions, and so on. All these factors are interrelated and have a significant impact on the performance of LMB. In recent years, great efforts have been made to improve the performance of LMBs through composite anodes. From the overall point of view, composite anodes for LMBs can be divided into the following categories from internal to external:
(i) The internal composite of lithium metal anode. Composited with NC scaffolds, tip effect can be weakened through the effect of NC material on Li+, so as to uniform the distribution of Li+in the vicinity of anode. The composite of lithium metal and EC scaffolds can effectively reduce the local current density, while IC scaffolds can increase ion flux and induce uniform deposition of Li+from bottom to top. MIEC scaffolds provide fast ion/electron channels for the deposition and dissolution of lithium metal simultaneously, so that lithium metal can be uniformly and densely deposited without dendrite and visible volume fluctuation.
(ii) The internal composite of LMB. Compared with liquid electrolytes, SSEs and quasi-solid-state electrolytes are much safer. Nevertheless, their interfacial contact with lithium metal anodes has been criticized. From the internal point of view of LMB, lithium metal anode can be composited with SSE or quasisolid-state electrolyte to inhibit the volume change during continuous deposition and dissolution of lithium metal anode,optimize the interface contact performance and reduce the interface resistance, so as to realize the reversible deposition and dissolution of lithium metal under high capacity.
(iii) The overall composite of internal environment and external operating conditions. The internal electric field distribution of LMB is usually uneven due to the defects of anodes, especially in the vicinity of anodes. Composited with the external physical fields, such as electric field and magnetic field,the distribution of Li+can be homogeneous. In addition, the initial nucleation morphology of lithium metal can be changed by the application of temperature field, and the mobility of Li+on the surface of lithium anode can be accelerated.Consequently, the formation of lithium metal dendrites can be inhibited.
These composite strategies based on lithium metal anode have greatly optimized the performance of LMBs. However, these composite anodes are still only involved in one or two aspects of the LMB structure, which are still far from practical application.In the future, a deeper understanding of composite anode is expected. More researches based on internal or external physical, chemical field distribution and multi-level composite for LMBs are supposed to get explored furtherly, for instance,integrated the design of electrolyte, separator and even cathode with the design of lithium metal anode. It is believed that they will play a significant role in promoting the development of LMBs.

