Water and nutrient recovery from urine: A lead up trail using nano-structured In2S3 photo electrodes*
R Jayakrishnan, T R Sreerev, and Adith Varma
1Department of Physics,University of Kerala,Thiruvananthapuram,Kerala 695581,India
2Photovoltaic Research Laboratory,Christian College Chenagnnur 689122,India
Keywords: semiconductors,water quality,thin film structure and morphology,electrolysis
1. Introduction
One of the most demanding and challenging problems today on the face of earth and on any space mission is on“urine recycling”. The costs of transporting water to the International Space Station and beyond are reported to be so cost-prohibitive that it seeks new inventions. The world is on the lookout for new technologies to solve problems associated with energy requirements,life support and recycling.[1]Recycling of urine will be a key deterrent in cost escalation of space missions.[2]Mature technologies for urine recycling will play a vital role in policy framing and planning for public utilities.[3,4]Urine is also one of the largest sources of waste water on earth.[5]If urine is applied in its inherent form directly over arable lands,it will lead to problems of increased soil salinity,pH,and volatilization of intrinsic ammonia, which in turn result in poor crop growth, productivity or even crop failures.[6]
The composition of human urine has been studied and 158 different chemical constituents are summarized in the NASA Bioastronautics Data Book.[7]Out of these 42 compounds constitute 98% of the total solute concentration in urine. These compounds can be classified into inorganic salts,urea, organic compounds and organic ammonium salts. It is difficult to treat urine by conventional treatment method for the purpose of recycling, especially because of the strong odor emitted from ammonia. Water makes up more than 95%of urine by weight. The largest solid constituent of urine is urea.[8]Bacteria produce urease enzymes which hydrolyze urea to bicarbonate and ammonia resulting in strong odor.This makes the practical storage and recycling of the urine very difficult. Successful removal of ammonia from water has been reported to use indirect anodic ammonia oxidation with the existence of chlorides.[9]Ammonia can be removed by the conventional method of air-stripping, biological treatment, or breakpoint chlorination.[10–12]
Practically recycling of human urine has been explored using techniques such as alkaline urine dehydration, struvite precipitation, evaporation, reverse osmosis, nitrification,precipitation,ammonia stripping,electro-dialysis,nanofiltration and advanced oxidation.[13–15]Distillation is the oldest method and least energy intensive technique for urine recycling. However distillation cannot remove constituents with boiling points lower than 100°C,which may become concentrated in the product water. Another disadvantage of distillation is cost. Distilled water can also be very acidic. It lacks oxygen and minerals and has a flat taste, which is why it is mostly used in industrial processes. An electrolysis process can be a viable technology for urine recycling as it involves two simultaneous processes: (1) the oxidation of water and urine, (2) the reduction of proton and water. Recent studies demonstrate that electrolysis can be used to treat water and waste water along with simultaneous generation of molecular hydrogen efficiently and cost effectively.[2,4]
The aim of our work is to explore the feasibility of designing a system which utilizes two processes, viz, solar distillation and solar powered electrolysis sequentially utilizing the abundant sunlight as the source for input energy and demonstrate the removal/recovery efficiency of nutrients and water from urine.
2. Materials and methods
A solar still was fabricated using steel. A trapezoidal collector with back-end height of 30 cm and front-end height of 15 cm was fabricated. The schematic representation is given in Fig.1. A mirror was placed vertically along the back walls of the collector. A tray with the dimension of 28 cm×22 cm×2 cm(L×W×H)could be inserted into the collector from the front end. The tray served as the basin for the feedstock urine. The upper surface of the machine is covered with transparent glass with an inclination of about 70°from the horizontal plane. The apparatus is made air tight by silicon synthetic rubber, and teflon tape. This apparatus is placed on a stand whose height can be adjusted. A parabolic reflector is placed on another stand which is used to concentrate sunlight on to the set up. The arrangement is such that light rays focused by the reflector fall on the mirror, which in turn reflects the rays onto the feed stock as shown in Fig.2.
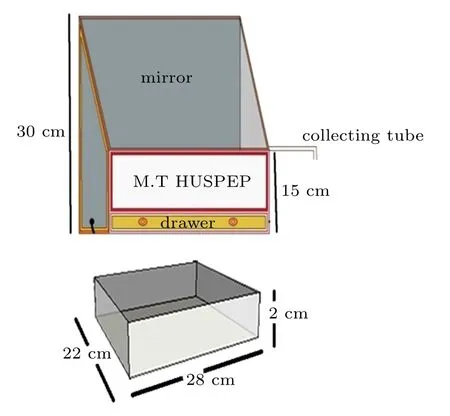
Fig.1. Schematic of the solar still.

Fig.2. Picture of the solar still and reflector constructed by us.
Undiluted urine-100 ml,was fed into the solar still.When exposed to sunlight the temperature of the urine increased by means of convection and the greenhouse effect. The urine was vaporized as a result of the heating. The glass cover, which was at a relatively lower temperature because of cooling by the wind,facilitated the vapor inside the still to condense and form small droplets at the underside of the glass cover. Under the influence of gravity, these droplets rolled down the glass cover into a drainage pipe, and were extracted from the unit.The volume of generated condensate was determined using a measuring cylinder. Total nitrogen and pH of the condensate were also measured.The solar still was set up in an open space in order to allow for maximum exposure to direct sunlight.
The water collected from the solar still was fed into an electrolytic reactor designed and fabricated in the lab. The reactor was powered by a 12 V,0.5 A c:Si photovoltaic module.The electrolysis reactor was cylindrical in shape with height of 15 cm and diameter of 7.5 cm. The reactor contained a copper cathode (99.9% pure) and an In2S3anode grown on the ITO substrate using chemical spray pyrolysis technique.We have reported on the In2S3film deposition process using chemical spray pyrolysis technique before.[16]In this work we have used a nebulizer to spray a mist of the precursor solutions onto the ITO substrate maintained at 573 K.The areas of the cathode and anode were identical ~5 cm × 2 cm and were separated by a distance of 1 cm using ceramic discs. A gas outlet was provided at the top to extract gaseous electrolysis byproducts. Schematic of the reactor is shown in Fig.3. The electrolysis was conducted for a period of eight hours. At the end of eight hours a dense brownish coagulated sedimentation was obtained in the electrolysis cell.Using a syringe the colorless liquid was extracted from the cell and subjected to further testing.The volume of the collected liquid was measured.The coagulation was also extracted and its weight was measured.The coagulation was dried in the solar still and the mass was sent for chemical analysis.

Fig.3. Picture of the electrolysis unit assembled.
3. Results and discussion
The structural analysis of the anode using x-ray diffraction analysis showed that the deposited film was the β-In2S3phase with preferential orientation along the(400)plane. The cubic β-In2S3was identified as per JCPDS card No. 32-456.Figure 4 shows the x-ray diffraction pattern obtained for the anode material. The presence of indium hydroxide impurity was also identified as per JCPDS card No.85-1338 in the x-ray diffraction pattern. The broad nature of the diffraction pattern indicated that the film contained particles in the nano-regime.

Fig.4. X-ray diffraction spectrum for the In2S3 thin film used as electrode.
The crystallite size was calculated using the Scherrer formula

where K =0.9, d represents crystallite size, λ is the wavelength of Cu-Kα radiation, and β is the half width of the diffraction peak. The crystallite size along the different β-In2S3planes was calculated and it was found to be ~2.89 nm along the (400) plane and to be ~3.76 nm along the (109)plane.
The solar still distillation process was conducted for six hours. After a treatment period of six hours, the liquid fraction would evaporate completely and the sediment solids were recovered and weighed.The evaporation of 100 ml of the feedstock urine resulted in ~3 mg residue. Table 1 represents the chemical composition of the urine derived nutrients obtained from the residue on the photo-reactor plate after the complete evaporation of the liquid phase. The residue was found to be a good source of agrarian nutrients composed of nitrogen,phosphorous, potassium, sodium and chlorine, which are generously used by farmers.

Table 1. Comparison of nutrient content in residual mass after solar still evaporation and electrolysis.
Table 1 shows the comparison of the results of biochemistry analysis conducted for the urine sample and the condensate. From the results it is found that there is considerable decrease in the amount of constituents of urine in the solar still distilled output collected as compared to the original constituents of the input urine sample. The amount of chlorine in the input and output samples showed the minimum decrease in concentration. The pH of the input feedstock was 9.3 and that of the output condensate was slightly higher at 9.9.
Table 2 contains the results of chemical analysis conducted on the residual mass collected after electrolysis. All other nutrients observed earlier were also obtained at the end of this process. Table 1 summarizes the biochemistry of the electrolyzed recycled liquid output showing the effect of electrolysis on the condensate. Electrolysis reduced the biological chemicals to a large extent.

Table 2. Comparison between bio-chemistry of input feedstock,output water from the solar still and electrolyzed recycled water. Here mg/dl means milligram per deciliter,mEq/L means milli-equivalent per liter.
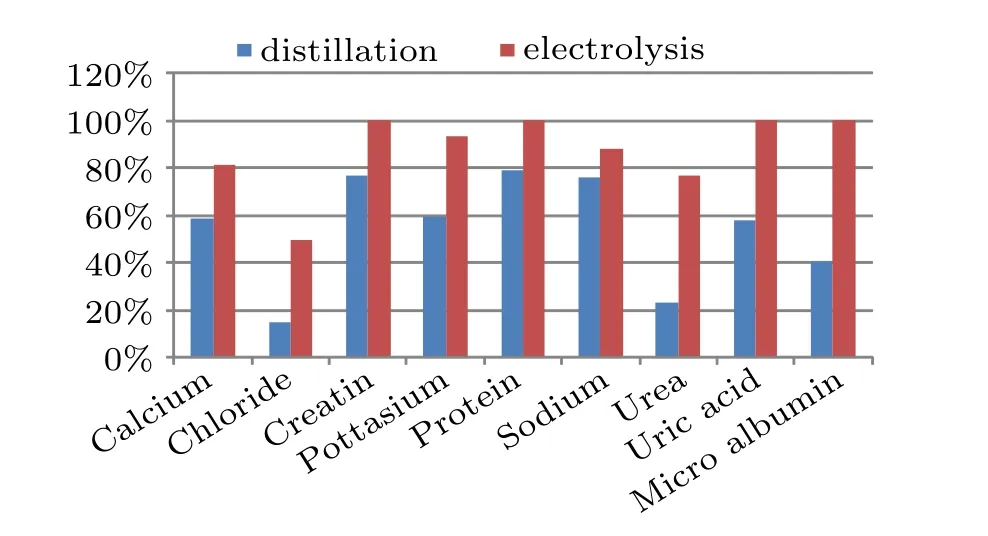
Fig. 5. Extraction efficiency of the process sequence on individual chemical compounds.
Figure 5 shows a histogram showing the relative efficiency of the two processes in removing the organic constituents from urine. It is evident that our process yields 100% removal of micro albumin, creatine, proteins and uric acid from the recycled water. Thus complete removal of organic materials could be achieved. Among the constituents the lowest removal of 49% was achieved for chlorides. It is known that by passing sufficient electricity through human urine, most of the dissolved organic compounds can be converted to hydrogen, oxygen, nitrogen, and carbon dioxide,which are out gassed, leaving behind semi-purified urine that contains primarily inorganic salts.Table 3 summarizes the water quality analysis conducted on the liquid samples collected at the end of the electrolyte free electrolysis process envisaged by us. The results are encouraging as the COD and TOC have been reduced to safe limits.
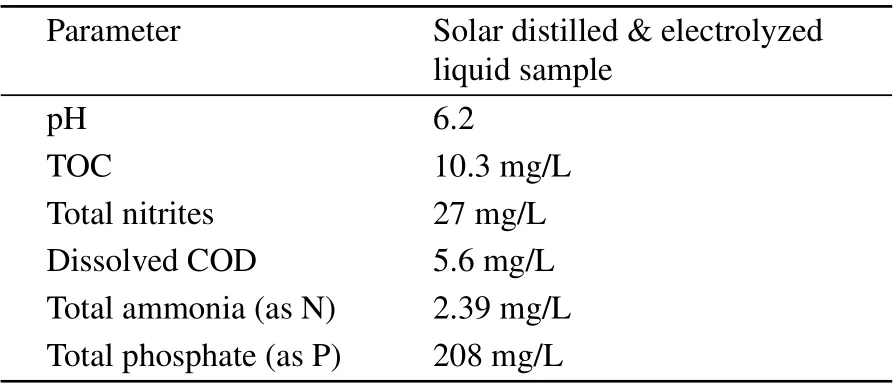
Table 3. Water quality parameters tested for the electrolyzed recycled water.
During electrolysis the liberation of gas was observed in the form of air bubbles. We propose that the following reactions may have occurred in our solar powered electrolytic cell:anode reaction
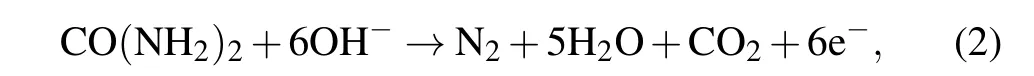
cathode reaction
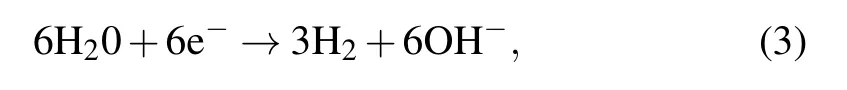
overall reaction
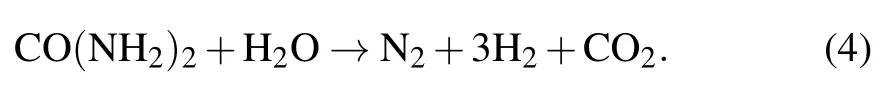
Figure 6 shows the AFM image of the ITO/In2S3working anode before and after the electrolysis. A distinct change in surface morphology was observed for the working anode.Figure 6(a) shows the surface topology for the as-prepared electrode. Figure 6(b) shows the topology after it functioned for eight hours. Figure 6(c)shows the topology after it functioned for 40 hours. The rms roughness Rqas defined in the following equation quantifies the surface roughness to a single value which expresses the variation of the surface height function h(r,t)over a two-dimensional surface with linear size L,

where r refers to the point at which the height function was measured.[17]The rms roughness was found to be 18.34 nm and 54.62 nm for the ITO/In2S3electrode before and after electrolysis. The electrode was found to have no damage on physical verification with the naked eye after eight hours of operation.
To quantify the damage threshold of the In2S3electrode,the process cycle was repeated and after each cycle an optical microscope was used to visualize its surface damage.

Fig. 6. AFM surface topologies for (a) as-prepared ITO/In2S3, (b)ITO/In2S3 after eight hours of electrolysis,(c)ITO/In2S3 after 40 hours of electrolysis.
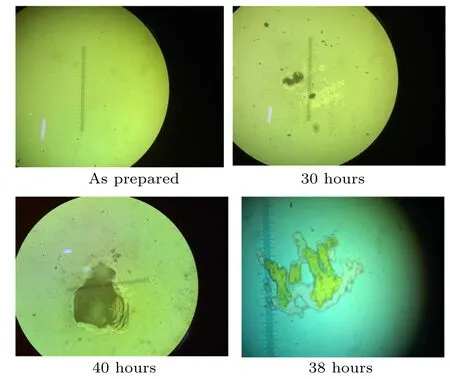
Fig.7. Optical microscopic views of the surface of the In2S3 electrode.
Figure 7 shows the images recorded over the microscope focused over a specific region of damage for different hours of the operation progress. It was observed that the process efficiency was lost after 30 hours of operation. Figure 6(c)shows the AFM topology of the electrode after 40 hours of operation.The rms roughness was found to be 183 nm for this sample.
Off the 100 ml urine fed to the solar still only 60 ml could be recovered for passing to the second stage. During electrolysis the formation of coagulated sediments restricted 100% recovery of recycled water. Out of the 60 ml fed to the electrolytic reactor 40 ml only could be extracted with the designed model. The total residual mass recovered from the process chain was approximately 13 mg.
4. Conclusion
We have deposited nano-structured In2S3thin films by spray pyrolysis method. It is demonstrated that solar distillation and solar powered electrolysis are lucrative processes chains for recycling urine. We have developed a laboratory scale proto-type for recycling human urine using direct sunlight driven processes sequentially utilizing equipment designed and developed in-house. Nano-structured In2S3thin films served as the anodes for the electrolysis process. Since the processes utilize direct sunlight they are not energy intensive processes. A random urine sample of 100 ml could be distilled in 6 hours’time using the solar still facility. Nutrients such as nitrogen, phosphorus and potassium could be recovered from the solar still. The loss of 40%of the liquid fraction at the solar still stage was probably due to poor insulation of the proto type,and could easily be improved for future experiments. The distilled liquid sample when electrolyzed for 8 hours resulted in a coagulated solid mass precipitation. Our analysis on the recycled water proves that it may be used for domestic purposes. The residual mass sediment obtained after solar distillation and electrolysis contain rich plant nutrients. The lowered COD and TOC in the recycled water warrant merit and scope for further improvement. The conduct of electrolysis without an electrolyte in the present work opens up several opportunities in study of the efficacy of nutrient and water recovery using targeted electrolytes and chemicals using In2S3and the anode material.
- Chinese Physics B的其它文章
- Corrosion behavior of high-level waste container materials Ti and Ti–Pd alloy under long-term gamma irradiation in Beishan groundwater*
- Degradation of β-Ga2O3 Schottky barrier diode under swift heavy ion irradiation*
- Influence of temperature and alloying elements on the threshold displacement energies in concentrated Ni–Fe–Cr alloys*
- Cathodic shift of onset potential on TiO2 nanorod arrays with significantly enhanced visible light photoactivity via nitrogen/cobalt co-implantation*
- Review on ionization and quenching mechanisms of Trichel pulse*
- Thermally induced band hybridization in bilayer-bilayer MoS2/WS2 heterostructure∗

