STAT3、E-cadherin、Vimentin在食管鳞癌上皮间质转化中的研究
徐夏 江海 陈豫民 王琰
【摘要】 目的:探究STAT3、E-cadherin、Vimentin在食管鱗癌上皮间质转化过程中产生的影响。方法:采用RT-qPCR方法检测50例食管鳞癌组织及癌旁正常组织中STAT3、E-cadherin、Vimentin的mRNA水平表达,分析癌组织、癌旁正常组织相对表达量与临床病理特征间的关系;随机抽取4例食管鳞癌患者组织标本,采用Western blot方法检测STAT3、E-cadherin、Vimentin蛋白水平表达。采用RT-qPCR及Western blot方法检测三种食管鳞癌细胞株中STAT3、E-cadherin、Vimentin表达,筛选出1株高表达STAT3的菌株,用STAT3的小干扰RNA转染。对照组转染双链无义RNA,转染成功后进行细胞划痕实验及Transwell细胞侵袭实验。结果:50例食管鳞癌组织中STAT3、E-cadherin、Vimentin相对表达量分别为(1.81±0.62)、(0.68±0.23)、(1.48±0.56);癌旁正常组织为(0.54±0.23)、(2.03±0.64)、(0.61±0.21)。食管鳞癌中STAT3、Vimentin在mRNA水平高表达及E-cadherin低表达,在浸润深度、淋巴结转移方面比较,差异均有统计学意义(P<0.05)。检测随机4例食管鳞癌患者组织标本蛋白水平表达,癌组织中STAT3、Vimentin均比癌旁正常组织高(P<0.05),而癌组织中E-cadherin比癌旁正常组织低(P<0.05)。STAT3小干扰RNA转染高表达STAT3的细胞株EC109后,STAT3表达在mRNA水平较对照组下调,E-cadherin表达较对照组相对上调;Vimentin表达较对照组相对下调,差异均有统计学意义(P<0.05);在蛋白水平,转染STAT3小干扰RNA组较对照双链无义RNA组成功抑制STAT3蛋白表达(P<0.05),E-cadherin蛋白表达相对上调(P<0.05),Vimentin蛋白表达相对下调(P<0.05)。细胞划痕结果显示,转染STAT3小干扰RNA组EC109的36、72 h迁移率均低于对照双链无义RNA组,差异均有统计学意义(P<0.05);侵袭实验结果显示,转染STAT3小干扰RNA组的EC109穿入小室膜底部的细胞总数较对照双链无义RNA组减少,差异有统计学意义(P<0.05),下调STAT3表达后食管鳞癌细胞株EC109迁移、侵袭能力减弱。结论:STAT3可能通过调控E-cadherin、Vimentin影响食管鳞癌上皮间质转化,从而影响肿瘤迁移与侵袭。
【关键词】 食管鳞癌 STAT3 E-cadherin Vimentin 上皮间质转化
[Abstract] Objective: To research the effect of STAT3, E-cadherin, Vimentin in the transformation of epithelial mesenchymal transition in esophageal squamous cell carcinoma. Method: RT-qPCR was used to detect the mRNA expression of STAT3, E-cadherin and Vimentin in 50 cases of esophageal squamous cell carcinoma and corresponding normal tissues, and the relationship between the relative expression of STAT3, E-cadherin and Vimentin in esophageal squamous cell carcinoma and corresponding normal tissues was analyzed. A simple randomized method was used to chose 4 patients with esophageal squamous carcinoma and the expression of STAT3, E-cadherin and Vimentin were detected with Western blot method. The expression of STAT3, E-cadherin, and Vimentin in three esophageal squamous cancer cell lines were detected by RT-qPCR and Western blot method. A strain expressing STAT3 was screened and transfected with STAT3 small interfering RNA. The control group was transfected with double stranded nonsense RNA. The cell migration and invasion test were detected in both group through the cell scratches experiment and the Transwell cell invasion experiment. Result: The relative expression levels of STAT3, E-cadherin and Vimentin in 50 cases of esophageal squamous cell carcinoma were (1.81±0.62), (0.68±0.23), (1.48±0.56) respectively; those in adjacent normal tissues were (0.54±0.23), (2.03±0.64), (0.61±0.21). At mRNA level, the expression of STAT3 and Vimentin were higher and that of E-cadherin was lower in esophageal squamous cell carcinoma (P<0.05). During 4 cases were randomly selected, the expression of STAT3 and Vimentin in esophageal squamous cell carcinoma tissues were higher than that in adjacent normal tissues (P<0.05), while E-cadherin in esophageal squamous cell carcinoma tissues was lower than that in adjacent normal tissues (P<0.05). After transfection with STAT3 siRNA, the expression of STAT3 mRNA was down regulated, E-cadherin expression was up-regulated, Vimentin expression was down regulated, the difference was statistically significant (P<0.05). At the protein level, the expression of STAT3 protein was successfully inhibited in the STAT3 siRNA transfection group compared with the control double stranded nonsense RNA group (P<0.05), and E-cadherin expression was down regulated, the expression of herin protein was up-regulated (P<0.05), while the expression of Vimentin protein was down regulated (P<0.05). The results of cell scratch showed that 36, 72 h cells of EC109 transfected with STAT3 siRNA were significantly higher than those of the control double-stranded meaningless RNA group, the differences were statistically significant (P<0.05). The results of invasion test showed that the total number of EC109 cells penetrating into the bottom of ependyma in the STAT3 siRNA group was significantly lower than that in the control double stranded nonsense RNA group (P<0.05), and the migration and invasion ability of esophageal squamous cell carcinoma cell line EC109 decreased after down regulating STAT3 expression. Conclusion: In esophageal squamous carcinoma, STAT3 may affect epithelial interstitial transformation phenotypes and thus affect tumor migration and invasion by mediating E-cadherin and Vimentin expression.
[Key words] Esophageal squamous cell carcinoma STAT3 E-cadherin Vimentin Epithelial-mesenchymal transition
First-authors address: Shiyan Renmin Hospital, Shiyan 442000, China
doi:10.3969/j.issn.1674-4985.2021.11.002
上皮間质转化(epithelial-mesenchymal transition,EMT)是胚胎发育、组织重建和伤口修复中的基础过程,同时也是肿瘤侵袭、转移的重要机制之一[1]。STAT3作为STAT家族的重要成员之一,激活其可引起细胞异常增殖,与肿瘤的发展密切相关[2]。在高表达的肿瘤细胞中,STAT3与肿瘤恶性程度密切相关[3];并参与调控肿瘤细胞的生长、凋亡等多方面的过程[4]。本实验旨在探讨食管鳞癌细胞中EMT分子标志物E-cadherin和Vimentin在调控STAT3表达后的变化,来探讨STAT3在食管鳞癌细胞EMT中的作用。
1 材料与方法
1.1 材料 食管鳞癌细胞株KYSE70、KYSE140、EC109由十堰市人民医院临床医学研究所提供。收集了十堰市人民医院心胸外科手术治疗的50例食管鳞癌患者的食管鳞癌和癌旁正常组织标本,储存在-80 ℃冰箱备用。所有病例符合以下条件:(1)常规病理诊断均为食管鳞状细胞癌;(2)每例均包括癌组织及相应的癌旁正常黏膜组织;(3)临床资料完整;(4)术前未接受任何放疗、化疗及免疫抑制治疗。STAT3小干扰RNA及其对照双链无义RNA、PCR上下游引物,实时荧光定量聚合酶链反应试剂盒,STAT3、E-cadherin抗体、Vimentin抗体,Transwell实验试剂盒,Matrigel基质蛋白胶。
1.2 方法
1.2.1 RNA或蛋白质提取及质检 新鲜冰冻样品采用液氮研磨法提取总RNA或总蛋白;用紫外分光光度计检测RNA样品的纯度和浓度,BCA法测量总蛋白浓度。
1.2.2 实时荧光定量聚合酶链反应(RT-qPCR) 严格按照试剂盒说明操作,检测组织或细胞中STAT3、E-cadherin、Vimentin表达水平,以2-ΔΔCt表示在mRNA水平相对表达量。以甘油醛-三磷酸脱氢酶(GAPDH)作为内参。STAT3引物序列,上游:F5-CAAGCAGTTTCTTCAGAGCA-3,下游:R5-CGTCACCACGGCTGCTGT-3;E-cadherin引物序列上游:5-GAACGCATTGCCACATACAC-3;下游5-GAATTCGGGCTGTTGTCAT-3;Vimentin引物序列:上游引物5-CGGGAGAAATTGCAGGAGGA-3,下游引5-AAGGTCAAGACGTGCCAGAG-3。
1.2.3 Western blot检测 提取标本总蛋白,利用BCA法测定总蛋白浓度,定量取30 μg总蛋白,严格按照说明书行电泳、电转、抗体孵育及ECL发光法显影。利用微管蛋白(Tubulin)作为蛋白质标准化内参,利用Imagine J软件进行蛋白质灰度分析。
1.2.4 干扰抑制STAT3表达细胞模型构建 将实验用的食管鳞癌细胞株接种于6孔板中,采用Lipo2000脂质体转染法将STAT3小干扰RNA片段及其对照双链无义RNA片段转染入不同孔细胞,培养24~48 h后行后续实验。
1.2.5 细胞划痕实验 将细胞株接种于6孔板中,脂质体法转染24 h后使铺板密度达80%~90%,用10 μL枪头垂直于6孔板底部直线划痕,先后于0、36、72 h显微镜下拍照记录细胞划痕愈合情况,以划痕愈合百分比表示细胞迁移情况。
1.2.6 细胞侵袭实验 采用Transwell小室及Matrigel人工基质胶法行细胞侵袭实验。每组设3个平行复孔,培养24 h后取出Transwell小室,棉签擦拭小室内面基质胶,4%中性甲醛固定并0.1%结晶紫染色,显微镜下观察照相。随机选取5个视野进行细胞计数,算出平均值。
1.3 统计学处理 应用SPSS 13.0统计软件分析,计量资料用(x±s)表示,比较采用t检验;计数资料以率(%)表示,比较采用字2检验。以P<0.05为差异有统计学意义。用GraphPad Prism制作统计图。
2 结果
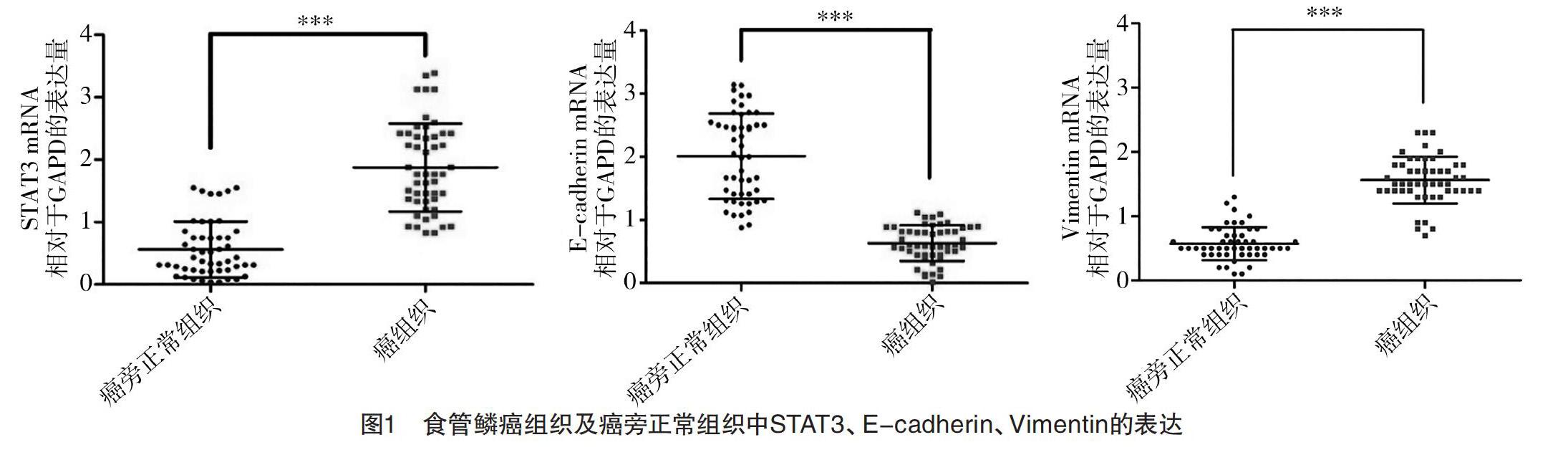
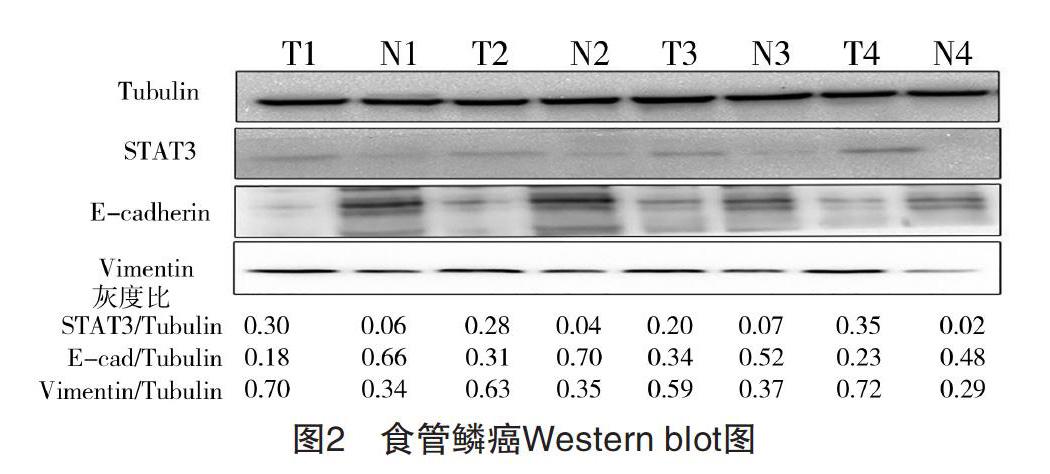
2.1 食管鳞癌组织及癌旁正常组织中STAT3、E-cadherin、Vimentin的表达 RT-qPCR检测提取的50例食管鳞癌患者标本总RNA,结果显示,在癌组织及癌旁正常组织中,STAT3分别表达为(1.81±0.62)、(0.54±0.23),E-cadherin分别表达为(0.68±0.23)、(2.03±0.64),Vimentin分别表达为(1.48±0.56)、(0.61±0.21),差异均有统计学意义(P<0.05),见图1。Western blot检测随机抽取的4例食管患者癌组织(T,tumor)及癌旁正常组织(N,normal),结果显示,癌组织中STAT3、Vimentin表达分别为(0.27±0.05)、(0.64±0.05),均高于癌旁正常组织(0.04±0.02)、(0.34±0.03)(P<0.05),而E-cadherin表达在癌组织为(0.26±0.06),明显低于癌旁正常组织(0.57±0.08)(P<0.05)。见图2。
图2 食管鳞癌Western blot图
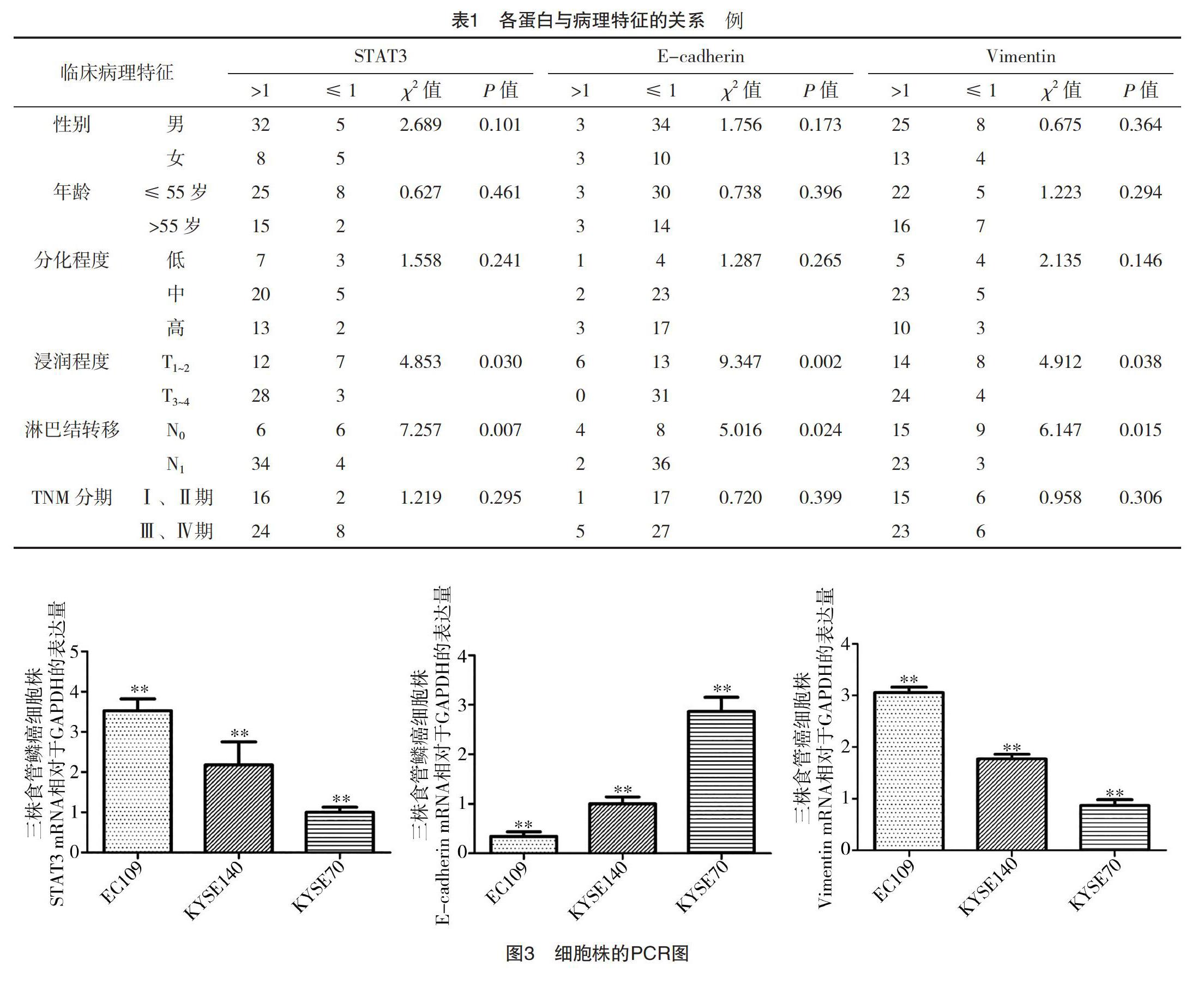
2.2 食管鳞癌中STAT3、E-cadherin、Vimentin的表达与其临床病理特征的关系 STAT3中癌组织和癌旁正常组织相对表达量比率>1和≤1例数分别为40例和10例,E-cadherin分别为6例和44例,Vimentin分别为38例和12例。食管鳞癌中STAT3、Vimentin在mRNA水平高表达及E-cadherin低表达,在浸润深度、淋巴结转移中比较,差异均有统计学意义(P<0.05),见表1。
2.3 食管鳞癌中STAT3对E-cadherin、Vimentin表达的影响 三种食管鳞癌细胞株中,相对于GAPDH在mRNA水平表达,STAT3、Vimentin由高到低依次为EC109、KYSE140、KYSE70,分别为(3.48±0.28)、(2.12±0.57)、(0.98±0.34)和(3.02±0.32)、(1.84±0.55)、(0.91±0.26),差异均有统计学意义(P<0.05);E-cadherin由高到低依次为KYSE70、KYSE140、EC109,分别为(2.86±0.13)、(1.12±0.03)、(0.46±0.04),差异有统计学意义(P<0.05),见图3。STAT3、Vimentin、E-cadherin各蛋白相对Tubulin在蛋白水平表达趋势一致,STAT3、Vimentin由高到低依次为EC109、KYSE140、KYSE70,分别为(0.38±0.10)、(0.27±0.11)、(0.17±0.05)和(0.67±0.11)、(0.28±0.06)、(0.16±0.05),差异均有统计学意义(P<0.05);E-cadherin由高到低依次为KYSE70、KYSE140、EC109,分别为(0.21±0.11)、(0.15±0.06)、(0.09±0.03),差异有统计学意义(P<0.05),见图4。
2.4 转染STAT3小干扰组RNA对STAT3、E-cadherin、Vimentin表達的影响 选取EC109作为siRNA-STAT3实验用细胞株,PCR结果显示,转染STAT3小干扰RNA组的表达(0.84±0.23),低于对照双链无义RNA组(3.00±0.19)(P<0.05);而同时E-cadherin上调,转染STAT3小干扰RNA组为(2.72±0.26),高于对照双链无义RNA组(0.32±0.17)(P<0.05);Vimentin下调,转染STAT3小干扰RNA组为(0.91±0.15),低于对照双链无义RNA组的(2.81±0.27)(P<0.05),见图5。Western blot结果显示,转染STAT3小干扰RNA组的表达(0.09±0.05),低于对照双链无义RNA组(0.32±0.17)(P<0.05);而同时E-cadherin上调,转染STAT3小干扰RNA组为(0.75±0.10),高于对照双链无义RNA组的(0.15±0.04)(P<0.05);Vimentin下调,转染STAT3小干扰RNA组为(0.18±0.05),低于对照双链无义RNA组的(0.87±0.13)(P<0.05)。见图6。
2.5 下调STAT3对食管鳞癌细胞株迁移、侵袭的影响 STAT3表达下调后,细胞划痕实验显示,转染STAT3小干扰RNA组EC109的36、72 h迁移率分别为(16.6±4.3)%、(42.3±4.3)%,均低于对照双链无义RNA组的(55.3±4.2)%、(73.5±3.7)%,差异均有统计学意义(P<0.05),见图7;细胞Transwell侵袭实验结果显示,转染STAT3小干扰RNA组的EC109穿入小室膜底部的细胞总数为(14.7±3.1)个,少于对照双链无义RNA组的(61.7±6.5)个(P<0.05),差异有统计学意义(P<0.05),见图8。
3 讨论
生物胚胎发生过程中的EMT在许多恶性肿瘤的发生、进展中发挥重要作用[5],E-cadherin和Vimentin是作为EMT中重要的分子标志物,可特异性反应EMT的发生[6]。E-cadherin与Vimentin在上皮细胞间结合形成复合体可防止肿瘤细胞转移和侵袭,而在恶性肿瘤细胞中EMT发生,允许细胞相互分离,从而提高了细胞的迁移能力[7-8];此外,肿瘤细胞经历EMT可能获得肿瘤干细胞特征[9],已经被认为与许多恶性肿瘤的萌生和发展有关,包括肿瘤的转移、侵袭[10-11]。
STAT家族蛋白质SH2区域的酪氨酸残基被JAK蛋白磷酸化后,STAT聚集形成同源或异源二聚体并发挥功能[12],提高细胞抗感染能力[2]。目前研究显示,STAT3在调控食管鳞癌发展过程中起一定作用[13]。本研究通过RT-qPCR方法检测食管鳞癌患者组织标本中,STAT3、E-cadherin与Vimentin的表达在癌组织浸润深度、淋巴结转移方面比较,差异均有统计学意义(P<0.05);在患者性别、年龄、分化程度和TNM分期方面比较,差异均无统计学意义(P>0.05)。提示STAT3、E-cadherin与Vimentin表达可能与食管鳞癌发生、发展有关。再次证实STAT3在食管鳞癌中异常高表达,其相对表达水平与食管鳞癌浸润深度和淋巴结转移相关。这些结果提示了STAT3可能成为发现高危食管癌和预防复发的分子生物学标志之一。
肿瘤细胞增殖及侵袭与其发展、浸润、复发、转移等生物学行为及预后密切相关[14-15]。文献[16-17]研究表明,包括STAT3在内的分子标志物是反映食管鳞癌细胞增殖水平的指标,STAT3信号通路的异常激活与肿瘤EMT及侵袭转移密切相关。文献[18-19]在肝癌细胞EMT过程中发现STAT3过表达可降低E-cadherin表达而增加Vimentin表达,相反,抑制STAT3表达可显著增加E-cadherin表达而降低Vimentin表达[18-19]。本研究通过对食管鳞癌细胞株的研究结果表明,STAT3对食管鳞癌中Vimentin表达正性影响,E-cadherin表达负性影响,EC109细胞通过转染法建立STAT3低表达食管鳞癌细胞株后,E-cadherin表达升高,而Vimentin表达下降,并且细胞迁移、侵袭能力下降。这表明在食管鳞癌细胞中,抑制STAT3表达可下调间质性标志物Vimentin表达而上调上皮性标志物E-cadherin表达,从而逆转EMT过程,促进细胞形成复合体,降低食管鳞癌迁移侵袭能力。进一步明确了STAT3在食管鳞癌细胞EMT中的重要作用,当STAT3信号通路被多种因素激活后,不仅使食管鳞癌E-cadherin表达下降,促使细胞间黏附能力降低,而且促使Vimentin进入细胞质,激活EMT相关的信号途径,进而提升了细胞的迁移和侵袭能力[20-22]。提示联合检测STAT3、E-cadherin、Vimentin可能有助于食管鳞癌的诊断、分级及预后。随着细胞生物学和生物技术的不断发展,笔者希望通过使患者体内维持在一个较低水平表达的STAT3,进而抑制或逆转食管鳞癌细胞的EMT过程,改变食管鳞癌细胞的生物学特性,影响其侵袭迁移能力,从而降低食管鳞癌的复发率,提高患者的生存率。这为进一步深入研究STAT3在影响食管鳞癌患者预后方面,其在提高分子生物学治疗等方面提供了一定依据。
參考文献
[1] Roseweir A K,Kong C Y,Park J H,et al.A novel tumor-based epithelial-to-mesenchymal transition score that associates with prognosis and metastasis in patients with stage Ⅱ/Ⅲ colorectal cancer[J].International Journal of Cancer,2019,144(1):150-159.
[2] Rodriguez-Barrueco R,Yu J,Saucedo-Cuevas L P,et al.
Inhibition of the autocrine IL-6-JAK2-STAT3-calprotectin axis as targeted therapy for HR-/HER2+breast cancers[J].Genes Dev,2015,29(15):1631-1648.
[3] Y Liu,F Luo,B Wang,et al.STAT3-regulated exosomal miR-21 promotes angiogenesis and is involved in neoplastic processes of transformed human bronchial epithelial cells[J].Cancer Letters,2016,370(1):123-125.
[4] Wang Y,Guo W Y,Li Z Q,et al.Role of the EZH2/miR-200 axis in STAT3-mediated OSCC invasion[J].International Journal of Oncology,2018,52(4):1149-1164.
[5] Ji-Hoon J,Jin J H,Sungmin K,et al.Novel TGF-β1 inhibitor antagonizes TGF-β1-induced epithelial-mesenchymal transition in human A549 lung cancer cells[J].Journal of Cellular Biochemistry,2019,120(1):997-987.
[6] Orlandini L,Reis F,Da Silveira W,et al.Identification of a Subtype of Poorly Differentiated Invasive Ductal Carcinoma of the Breast Based onVimentin and E-cadherin Expression[J].Revista Brasileira Ginecologia Obstetrícia,2018,40(12),779-786.
[7] Takahashi K,Menju T,Nishikawa S,et al.Tranilast Inhibits TGF-β1 -induced Epithelial-mesenchymal Transition and Invasion/Metastasis via the Suppression of Smad4 in Human Lung Cancer Cell Lines[J].Anticancer Research,2020,40(6):3287-3296.
[8] Jrgensen C,Forsare C,Bendahl P O,et al.Expression of epithelial-mesenchymal transition-related markers and phenotypes during breast cancer progression[J].Breast Cancer Research and Treatment,2020,181(4):369-381.
[9] Franke F C,Müller J,Abal M,et al.The Tumor Suppressor SASH1 Interacts With the Signal Adaptor CRKL to Inhibit Epithelial-Mesenchymal Transition and Metastasis in Colorectal Cancer[J].Cellular and Molecular Gastroenterology and Hepatology,2019,7(1):33-53.
[10] Bacalini M G,Franceschi C,Gentilini D,et al.Molecular Aging of Human Liver: An Epigenetic/Transcriptomic Signature[J].Journals of Gerontology Series A-biological Sciences and Medical Sciences,2019,74(1):1-8.
[11] Wang Zongjie,Xia Fan,Labib M,et al.Nanostructured Architectures Promote the Mesenchymal-Epithelial Transition for Invasive Cells[J].ACS Nano,2020,14(5):5324-5336.
[12] Liu X L,Zhang X T,Meng J,et al.ING5 knockdown enhances migration and invasion of lung cancer cells by inducing EMT via EGFR/PI3K/Akt and IL-6/STAT3 signaling pathways[J].Oncotarget, 2017, 8(33):54265-54276.
[13] Liu Z,Zhang Y,Chen Y,et al.STAT1 inhibits STAT3 activation in esophageal squamous cell carcinoma[J].Cancer Management and Research,2018,10:6517-6523.
[14] Miao Z,Yu F,Ren Y,et al.d,l-Sulforaphane Induces ROS-Dependent Apoptosis in Human Gliomablastoma Cells by Inactivating STAT3 Signaling Pathway[J].International Journal of Molecular Sciences,2017,18(1):72.
[15] Hajimoradi M,Mohammad Hassan Z,Ebrahimi M,et al.
STAT3 is Overactivated in Gastric Cancer Stem-Like Cells[J].Cell J,2016,17(4):617-628.
[16] Walz J Z,Saha J,Arora A,et al.Fatty acid synthase as a potential therapeutic target in feline oral squamous cell carcinoma[J].Veterinary and Comparative Oncology,2018,16(1):99-108.
[17] Kim C,Kim J H,Oh E Y,et al.Blockage of STAT3 Signaling Pathway by Morusin Induces Apoptosis and Inhibits Invasion in Human Pancreatic Tumor Cells[J].Pancreas,2016,45(3):409-419.
[18] Zhang C,Guo F,Xu G,et al.STAT3 cooperates with Twist to mediate epithelial-Mesenchymal transition in human hepatocellular carcinoma cells[J].Oncol Rep,2015,33(4):1872-1882.
[19] Guo L M,Gao R,Gan J C,et al.Downregulation of TNFRSF19 and RAB43 by a novel miRNA,miR-HCC3,promotes proliferation and epithelialmesenchymal transition in hepatocellular carcinoma cells[J].Biochemical and Biophysical Research Communications,2020,525(2):425-432.
[20] Tsai P C,Lin Y L,Fu Y S,et al.Cardiotoxin Ⅲ suppresses the invasiveness of MDA-MB-231 cells by targeting proto-oncogene tyrosine-protein kinase Src and reversing mesenchymal-to-epithelial transition[J].Toxicological and Environmental Chemistry,2016,98(7):942-958.
[21] Sonnenblick A,Brohée S,Fumagalli D,et al.Constitutive phosphorylated STAT3-associated gene signature is predictive for trastuzumab resistance in primary HER2-positive breast cancer[J].BMC Med,2015,13(1):177.
[22]高遠,轩小燕,张红燕,等.STAT3信号蛋白与食管鳞癌上皮间质转化的关系及其意义[J].世界华人消化杂志,2010,18(5):447-448.
(收稿日期:2020-07-20) (本文编辑:张爽)

