Fabrication of sulfur-doped cove-edged graphene nanoribbons on Au(111)*
Huan Yang(杨欢) Yixuan Gao(高艺璇) Wenhui Niu(牛雯慧) Xiao Chang(常霄)Li Huang(黄立) Junzhi Liu(刘俊治) Yiyong Mai(麦亦勇)Xinliang Feng(冯新亮) Shixuan Du(杜世萱) and Hong-Jun Gao(高鸿钧)
1Institute of Physics and University of Chinese Academy of Sciences(CAS),Beijing 100190,China
2Center for Advancing Electronics Dresden(cfaed)&Faculty of Chemistry and Food Chemistry,Technische Universit¨at Dresden,01062 Dresden,Germany
3School of Chemistry and Chemical Engineering,Frontiers Science Center for Transformative Molecules,Shanghai Key Laboratory of Electrical Insulation and Thermal Ageing,Shanghai Jiao Tong University,Shanghai 200240,China
4Department of Chemistry and State Key Laboratory of Synthetic Chemistry,The University of Hong Kong,Hong Kong,China
5CAS Center for Excellence in Topological Quantum Computation,University of Chinese Academy of Sciences,Beijing 100190,China
Keywords: on-surface synthesis, sulfur-doped cove-edged graphene nanoribbons, scanning tunneling microscopy,non-contact atomic force microscopy
1. Introduction
Graphene has attracted intensive attentions due to its unique properties and potential applications in nanoelectronics.[1-7]Graphene nanoribbons (GNRs), which have an energy bandgap and tunable electronic structures,[4,8-11]that can be directly determined by widths, edge configurations,and dopants are an important branch in graphene research as promising candidates for future electronic devices.[12-18]The fabrication of GNRs generally includes top-down and bottom-up approaches,[19-22]among which the latter is renowned for its capabilities to yield structurally well-defined GNRs.[19,23,24]With specifically designed organic precursors,scientists have achieved various kinds of GNRs, including armchair-edged GNRs with different widths and their corresponding heterostructures,[15,19,25,26]zigzag-edged GNRs,and cove-edged GNRs.[16,27-29]Among those, cove-edged GNRs as a representative semiconductor enrich the families of GNRs,which could benefit to explore exotic physical properties of GNRs containing distinct edge configurations.[27,30,31]Furthermore, GNRs doped with heteroatoms have been successfully synthesized and investigated during the past few years.[12,32-34]For instance, the introduction of nitrogen (N)atoms into the GNRs can shift the bandgap position without changing its magnitude;[12,33,35]while sulfur (S) and boron(B) atoms can lead to the change of the bandgap values of GNRs.[36-40]Particularly, bandgaps of GNRs can be further tailored by modulating sulfur configurations,which may yield S-doped GNRs containing a sequence of multiple heterojunctions with distinct bandgaps.[37,38]
In this work,we report the bottom-up synthesis of sulfurdoped cove-edged graphene nanoribbons (S-CGNRs) on Au(111)using a uniquely designed thiophene-ring containing precursor,4,4’-dibromo-10,10’-biphenanthro[2,1-b]thiophene(DBDPT). Scanning tunneling microscopy (STM) measurements verified the successful fabrication of GNRs,while noncontact atomic force microscopy(nc-AFM)images confirmed the existence of S dopants at the cove edges. Scanning tunneling spectroscopy (STS) spectra demonstrate the conduction band minimum of the S-CGNR locates at 1.2 eV.Density functional theory (DFT) calculations on the wellordered S-CGNRs show a bandgap of 1.17 eV, significantly narrower than that of the pristine carbon-based cove-edged CNRs (CGNRs) (1.7 eV).[27]Due to the partial cleavage of C-S bonds and different chirality of the precursor on the surface, the involved Ullmann-type polymerization and cyclodehydrogenation yielded S-CGNRs with branched configurations. By increasing the precursor coverage from submonolayer to monolayer,the cross-link of S-CGNRs was evidently reduced,providing linear-shaped S-CGNRs arrays.
2. Methods
2.1. Sample preparation,STM/STS measurement
All experiments were performed in a CreaTec ultra-high vacuum (UHV) STM/nc-AFM system with a base pressure of 2×10-10mbar (1 bar=105Pa). Au(111) substrate was cleaned by repeated cycles of Ar+ion sputtering and annealing at 700 K for 20 min. The precursor was thermally sublimated at 560 K onto the Au(111) substrate, which was held at room temperature during molecular deposition. Subsequently, the sample was annealed at 500 K and 700 K for 40 min each, which led to the surface-assisted polymerization and cyclodehydrogenation to form corresponding polymers and S-CGNRs,respectively. All STM and nc-AFM measurements were carried out at 5 K using a qPlus tuning. STM images were obtained in constant current mode and the voltages referred to the bias on samples with respect to the tip.AFM images were acquired in the frequency modulation mode with a CO-functionalized tip.[41,42]Differential conductance(dI/dV) spectra were acquired by using a lock-in technique at a frequency of 973 Hz.
2.2. DFT calculations
The structural relaxations and electronic-structure calculations were performed within density functional theory calculations by using the Viennaab initiosimulation package.[43,44]A generalized gradient approximation (GGA)in the form of Perdew-Burke-Ernzerhof (PBE) generalized gradient approximation was adopted for the exchangecorrelation functional.[45]The wave functions were expanded using a planewave basis set with an energy cutoff of 500 eV.The thickness of vacuum layer was larger than 13 ˚A to avoid interactions between neighboring nanoribbons. AΓ-centered 6×1×1k-point sampling in the first Brillouin zone was used during structural optimization of the GNR. The structures were relaxed until the residual forces were smaller than 0.01 eV/˚A.The band structure was calculated along high symmetry directions in the Brillouin zone.
3. Results and discussion
Figure 1(a) illustrates the synthesis of S-CGNRs on Au(111) from a specifically designed precursor containing fused thiophene rings (4,4’-dibromo-10,10’-biphenanthro[2,1-b]thiophene,DBDPT,synthesis method described in Section 2 and Figs. S1-S6 of supplementary material). In principle,the precursors could undergo dehalogenation,polymerization,and cyclodehydrogenation when annealed on surface, resulting in the formation of GNRs with sulfur-doped cove edges as shown in Fig.1(a).
The DBDPT molecules are prochiral when deposited on Au(111)substrate,as illustrated in Fig.S7(a).Due to the steric hinderance between the two groups of hydrogen atoms highlighted by the green ovals in Fig. 1(a), the precursors are not planar on the substrate. At low coverage, the precursors tend to form clusters that adsorb on fcc and elbow regions of the reconstructed Au(111)surface as shown in Fig.1(b). Most of the clusters consist of 10 bright protrusions with a height of 320 pm (Fig. S7(c)) and assemble into rhomboids. The apparent lateral size of one bright protrusion in STM images is about 1.95 nm(Fig.S7(c)),which is comparable to the lateral size of a precursor (1.1 nm) considering the dispersed electronic states of molecules. Therefore,we attribute each bright protrusion in Fig.1(b)corresponds to a single molecular precursor. By annealing the sample to 500 K,the precursors went through Ullmann coupling and formed polymers. The polymers defused into islands on fcc regions of Au(111) surface and modified the Au(111)surface reconstruction as shown in Fig. 1(c), suggesting a strong interaction between the polymers. Figure 1(d) shows an STM image of the sample after annealing to 700 K, which results in the formation of GNRs with a height of 180 pm(Fig.S8). Most nanoribbons exhibit an appearance of branched structures that indicate complex inter-ribbon cross-coupling during the cyclodehydrogenation stage. The straight ribbon segments generally have the lengths ranging from 2 nm to 9 nm.The strong interaction between the polymers in previous step is likely the reason for the formation of the branch-shape GNRs.
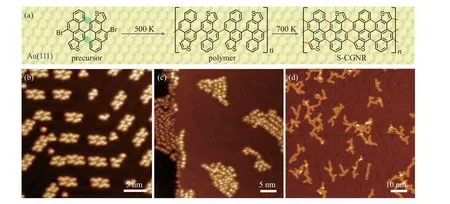
Fig.1. On-surface synthetic process of S-CGNR and the corresponding STM images on Au(111). (a)Structure model of the precursor 4,4’-dibromo-10,10’-biphenanthro[2,1-b]thiophene monomer, the polymer after Ullmann reaction, and the resultant S-CGNR. (b) STM image of the precursors adsorbed on Au(111)substrate at a coverage of 0.2 ML(V =-1 V,I=10 pA).(c)STM image of the polymers after annealing the sample to 500 K(V =-1 V,I=10 pA).(d)STM image of the S-CGNRs after annealing the sample to 700 K(V =-1 V,I=10 pA).
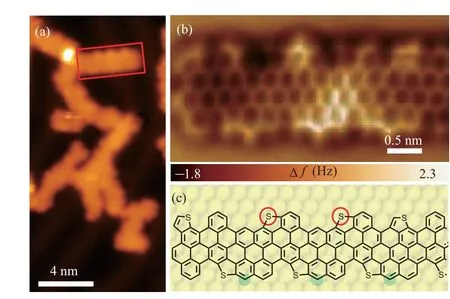
Fig. 2. A segment of branched S-CGNRs. (a) STM image of S-CGNRs(V =-1 V, I =10 pA). (b) Chemical-bond-resolved nc-AFM image of the S-CGNR segment obtained on the area marked by a red rectangle in panel(a).(c)Structure model of the S-CGNR segment. The segment is originated from isomers with the same chirality.
Despite the branch-like morphology,it is possible to find short straight segments of GNRs that have ordered peripheries.To gain further insight into the atomic structure of the resultant ribbons, nc-AFM measurements were carried out with a COfunctionalized tip.[46]The AFM image in Fig.2(b)is obtained on the area marked by the red rectangle in Fig.2(a). Combining the AFM image and the configurations of the precursors,we infer that the segment in Fig. 2(b) arises from precursors with the same chirality. To guide our eyes,the corresponding structure model of the nanoribbon is provided in Fig.2(c).The cove-shaped edges are clearly visualized, but not as uniform as illustrated in Fig.1(a). The thiophene rings observed in the AFM image prove the existence of sulfur atoms. However,some C-S bonds have been cleaved due to annealing at high temperature and the unsaturated S atoms form new bonds with adjacent carbon atoms marked with red circles in the structure model in Fig.2(c). Carbon atoms detached from other precursors may also be involved in the reaction to form the ribbons,as referred by the green circles(Fig.2(c)).
For the purpose of unveiling the electronic properties of S-CGNRs, differential conductance (dI/dV) spectra were performed to obtain the local density of states(LDOS)of the ribbon. Figure 3(a)shows an STM image of a short segment from a S-CGNR,which was used to acquire the representative dI/dVspectra. In Fig. 3(b), the red curve was taken on the S-CGNR as marked by the red dot in Fig.3(a),while the black curve taken on bare Au(111)surface for comparison with the same tip. The significantly increased intensity of LDOS at 1.2 V is attributed to the conduction band minimum(CBM)of the S-CGNR.The valence band maximum of the S-CGNR is not detected due to the strong coupling between S-CGNRs and metallic substrate,which is similar to the situation for zigzagedges graphene nanoribbons on Au(111).[16]Meanwhile, the spatial distribution of the CBM on the ribbon is displayed in the dI/dVmap in Fig. 3(c), exhibiting pronounced LDOS along the coved edges of the ribbon.
To explore the effect of S atoms on the electronic structures of CGNRs,DFT calculations were also carried out based on the perfect structure of S-CGNR in Fig.1(a). Figures 3(d)shows the DFT calculated density of states(DOS)of S-CGNR,indicating a bandgap of 1.17 eV.Compared to its corresponding pristine CGNR that has been reported to have a calculated bandgap of 1.70 eV,[27]the doping of S atoms significantly reduces the energy bandgap.
In order to tune the morphology of S-CGNRs, we tried to adjust the coverage of DBBPT molecules to near monolayer. At this coverage, the precursors form self-assembled structures along the Au(111) surface reconstruction, as displayed in Fig. 4(a). Magnified STM image shows detailed well-ordered monolayer structure in Fig. 4(b), in which the DBBPT molecules regularly assemble to form the periodical nanoporous structures. After annealing the sample to 700 K,S-CGNRs can be obtained as shown in Fig.4(c). Long-curved ribbons are formed comparing with the branch-like structures in sub-monolayer coverage. The zoom-in STM image in Fig.4(d)shows that well-aligned linear-shaped S-CGNRs can also be formed in this condition,as highlighted in the red rectangle.

Fig.3. Electronic structure of S-CGNR.(a)STM image of a short segment from a S-CGNR(V =1 V,I=50 pA).(b)Differential conductance(dI/dV)spectra taken on a S-CGNR(red)and on bare Au(111)(black). The conduction band minimum(CBM)of the S-CGNR locates at 1.2 eV(open-feedback parameters: V =1.00 V,I =50 pA;modulation voltage Vrms =10 mV).(c) dI/dV map at the energy of 1.2 eV(CBM)(open-feedback parameters:V =1.2 V,I=600 pA,modulation voltage Vrms =20 mV).(d)DFT calculated density of states(DOS)of the S-CGNR.The DFT calculations show that the S-CGNR has a bandgap of 1.17 eV,while the pristine CGNR possesses a bandgap of 1.7 eV.
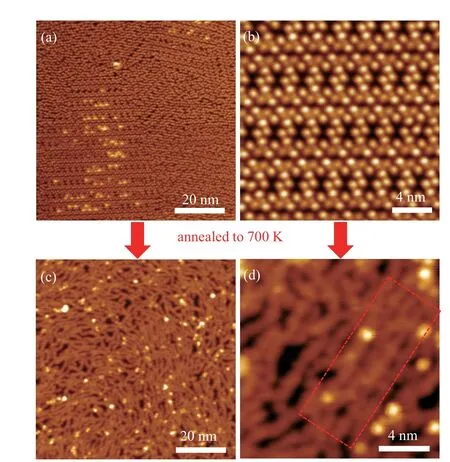
Fig. 4. STM images of monolayer precursors and the corresponding SCGNRs on Au(111). (a) Monolayer precursors deposited on Au(111) substrate(V =-1 V,I=10 pA).(b)Magnified STM image of monolayer precursors(V =1 V,I=60 pA).(c)STM image of the S-CGNRs shows linear structures after annealing to 700 K (V =-1 V, I =30 pA). (d) Zoom-in STM image on the S-CGNRs presents the well-aligned linear-shaped structures(V =-1 V,I=30 pA).
4. Conclusion
We have successfully synthesized sulfur-doped coveedged GNRs on Au(111)substrate. Nc-AFM image confirms the formation of S-CGNRs and resolves the atomic structures with non-uniform edge configurations due to the C-S bond cleavage at elevated temperature.STS spectra demonstrate the conduction band minimum of the S-CGNR locates at 1.2 eV.DFT calculations reveal that the well-ordered,S-CGNRs have a bandgap of 1.17 eV,which is much smaller than that of the pristine carbon-based GNRs with the similar structure. The SCGNRs produced with low precursor coverage cross-couple into branched structures. With increasing the precursor coverage to near monolayer, linear-shaped ribbons have been successfully obtained. This bottom-up synthesis of S-CGNRs promotes future applications of heteroatom-doped graphene nanoribbons in nanoelectronics.
Acknowledgements
Part of the research was performed in the Key Laboratory of Vacuum Physics,Chinese Academy of Sciences. Computational resources were provided by the National Supercomputing Center in Tianjin Municipality, China. The Instrumental Analysis Center of Shanghai Jiao Tong University is also acknowledged for some measurements.
- Chinese Physics B的其它文章
- Projective representation of D6 group in twisted bilayer graphene*
- Bilayer twisting as a mean to isolate connected flat bands in a kagome lattice through Wigner crystallization*
- Magnon bands in twisted bilayer honeycomb quantum magnets*
- Faraday rotations,ellipticity,and circular dichroism in magneto-optical spectrum of moir´e superlattices*
- Nonlocal advantage of quantum coherence and entanglement of two spins under intrinsic decoherence*
- Universal quantum control based on parametric modulation in superconducting circuits*

