Effect of moxibustion at Shenque (CV 8) on myocardial remodeling and function in exercise-induced fatigue rats
Zhang Zhi-fang (张治方), Liang Yu-lei (梁玉磊), Lü Tian-yuan (吕天元), Shen Zheng-xian (沈正先), Wang Xin (王鑫),Zhu Jie (祝婕), Li Wen-li (李文丽), Lü Shi-ling (吕诗灵), Sun Dong-yun (孙东云)
1 Hebei University of Chinese Medicine, Shijiazhuang 050091, China
2 Langfang Traditional Chinese Medicine Hospital, Langfang 065000, China
3 Hebei Key Laboratory of Chinese Medicine Research on Cardio-cerebrovascular Disease, Shijiazhuang 050091, China
4 Hebei Province Traditional Chinese Medicine Hospital, Shijiazhuang 050091, China
Abstract
Keywords: Moxibustion Therapy; Moxa Stick Moxibustion; Point, Shenque (CV 8); Point, Zusanli (ST 36); Fatigue; Myocardium;Rats
Exercise-induced myocardial remodeling is a process of significant cardiac structure and function changes caused by long-term endurance exercise. High-intensity endurance exercise results in acute and reversible damage to the myocardium. If the damage is repaired,long-term endurance exercise will cause adaptability enlarged heart, which is manifested as physiological hypertrophy of the myocardium, increased myocardial oxygen uptake and cardiac output, which is often referred to as ‘athlete’s heart’[1]. However, long-term fatigue exercise makes these reversible micro-injures unable to be effectively repaired, resulting in accumulated injury. The irreversible pathological remodeling of the myocardium will cause decreased cardiac function, induce cardiovascular diseases such as arrhythmia and atrial fibrillation, and even develop into congestive cardiac failure, causing sudden cardiac death[2]. It has been reported that endurance athletes have a higher arrhythmia incidence, which is positively correlated with the exercise intensity and duration[3-4].Fatigue-induced myocardial remodeling has become the focus of today's society with the accelerating life rhythm.How to effectively improve such myocardial remodeling has become a common issue in sports and clinical medicine.
Preliminary experiments confirmed that moxibustion at Shenque (CV 8) increased dopamine (DA)concentration, and the activities of free radical superoxide dismutase (SOD), glutathione peroxidase(GSH-Px) and total antioxidant capacity (T-AOC) in hippocampus tissues of long-term exercise rats; reduced the concentrations of central monoamine neurotransmitter 5-hydroxytryptamine (5-HT) and free radical malondialdehyde (MDA); inhibited the concentrations of serum MDA and blood urea nitrogen(BUN), and the activities of aspartate transaminase (AST),alanine transaminase (ALT) and lactate dehydrogenase(LDH), thus effectively relieving exercise-induced fatigue by regulating the central and peripheral nervous systems[5-8]; at the same time, it was found that moxibustion at Shenque (CV 8) can regulate the oxygen free radical production and antioxidant capacity of myocardium in exercise-induced fatigue rats, and reduce the myocardial damage[9]. This experiment was designed to further clarify the effect of moxibustion at Shenque(CV 8) on the exercise-induced fatigue myocardial structure and function. The report is as follows.
1 Materials and Methods
1.1 Experimental animals and groups
Seventy male, specific pathogen free (SPF), 7-weekold Sprague-Dawley rats weighing (200±10) g were purchased from Beijing Weitong Lihua Laboratory Animal Technology Co., Ltd., China [License No. SCXK(Beijing) 2012-0001]. Rats were adaptively fed for 7 d at the Experimental Animal Center of Hebei University of Chinese Medicine, with temperature of 20-26 ℃,12 h/12 h light-dark cycle, distilled feeding water, and free access to food and water. The adaptive treadmill exercise was performed for 3 d on the 5th, 6th, and 7th day, 15-30 min each time, and 10 unqualified rats (those who cannot run for 30 min at 20 m/min) were excluded.The remaining rats were divided into 6 groups according to the random number table method, a blank group, a control group, a model group, a non-meridian nonacupoint, a Zusanli (ST 36) group and a Shenque (CV 8)group, with 10 rats in each group. Rats were raised in separate cages with 5 in each cage. All animal experiments followed the relevant regulations of Hebei University of Chinese Medicine for the management of experimental animals.
1.2 Main reagents and instruments
Serum C-reactive protein (CRP) kit (Batch No.20190523); serum myoglobin (Mb) kit (Batch No.20190528); serum creatine kinase-myocardial band (CKMB) kit (Batch No. 20190527); serum cardiac troponin I(cTnI) kit (Batch No. 20190523); serum cardiac troponin T (cTnT) kit (Batch No. 20190523); myocardial tissues cTnT kit (Batch No. 20191107). All kits were purchased from Nanjing Jiancheng Institute of Biological Engineering (China).
Moxa sticks (7 mm in diameter, 11.7 cm in length,Henan Nanyang Hanyi Moxa Co., Ltd., China);homemade moxibustion special rat box (National Utility Model Patent No. ZL201120193244.8)[5]; DB030 animal experiment treadmill (Beijing Zhishu Duobao Biotechnology Co., Ltd., China); VEVO 2100 highresolution small animal ultrasound system (VisualSonics Inc., Canada); AU400 automatic biochemical analyzer[Kelegewani Analytical Instrument Co., Ltd. (Shanghai),China]; AUY120 electronic analytical balance (SHIMADZU Company, Japan); 2233MK-2 high-speed lowtemperature centrifuge (HEMNLE Company, Germany);Thermo Multiskan GO microplate reader (Shanghai Danding International Trade Co., Ltd., China).
1.3 Model preparation
After adaptive feeding for 7 d, the model was prepared by referring the exercise program of Bedford TG,et al[10]. The details were as follows: treadmill speed was 28 m/min, and treadmill slope was 10° [equivalent to (81.0±3.5) % of maximum oxygen uptake (VO2Max)load intensity]; first run for 5 min at 15 m/min, then gradually increased to 28 m/min within 5 min,maintained until the end of the training, 1 h/d, for 5 consecutive days a week at a 2-day interval, for 12 weeks.
1.4 Intervention in each group
Blank group: Rats without exercise-induced fatigue training were placed in a moxibustion-specific rat box[11]for 15 min, once a day for 5 consecutive days a week at a 2-day interval, 60 times in total.
Control group: Rats without exercise-induced fatigue training were placed in a moxibustion-specific rat box to receive mild moxibustion at Shenque (CV 8) with moxa sticks for 15 min, once a day for 5 consecutive days a week at a 2-day interval, 60 times of moxibustion in total.
Model group: After each exercise-induced fatigue training, the rats were placed in a moxibustion-specific rat box for 15 min but without moxibustion, once a day for 5 consecutive days a week at a 2-day interval,60 times in total.
Non-meridian non-acupoint group: After each exercise-induced fatigue training, rats were placed in a moxibustion-specific rat box to receive mild moxibustion at bilateral non-meridian non-acupoint points (25 mm above the iliac crest below the costal region, 20 mm away from the posterior midline)[12]with moxa sticks for 15 min, once a day for 5 consecutive days a week at a 2-day interval, 60 times of moxibustion in total.
Zusanli (ST 36) group: After each exercise-induced fatigue training, rats were placed in a moxibustionspecific rat box to receive mild moxibustion at bilateral Zusanli (ST 36) with moxa sticks for 15 min, once a day for 5 consecutive days a week at a 2-day interval,60 times of moxibustion in total.
Shenque (CV 8) group: After each exercise-induced fatigue training, rats were placed in a moxibustionspecific rat box to receive mild moxibustion at Shenque(CV 8) with moxa sticks for 15 min, once a day for 5 consecutive days a week at a 2-day interval, 60 times of moxibustion in total.
1.5 Test items
1.5.1 Myocardial morphology and function-related items
Rats were subjected to shallow anesthesia with isoflurane before treadmill running training and 1 h after the last treadmill running training. Each rat was fixed in a supine position on a 37 ℃ constant temperature heating plate, and tested with a VEVO2100 highresolution small animal ultrasound system after the left chest was depilated. The short-axis view of the left ventricle was revealed by 2D ultrasound. At the papillary muscle level, movement of the left ventricle was recorded using M-mode ultrasound; the left ventricular end-diastolic diameter (LVEDd), the left ventricular endsystolic internal diameter (LVESd), the left ventricular diastolic volume (LVDv), the left ventricular systolic volume (LVSv), the ejection fraction (EF), the stroke volume (SV), the early diastolic peak flow velocity of mitral valve (E), and the late diastolic peak flow velocity of mitral valve (A) were measured; the E/A and the left ventricular fractional shortening (LVFS) were calculated.LVFS = (LVEDd - LVESd) ÷ LVEDd.
1.5.2 Serum indicators
Six hours after exercise-induced fatigue training, the rats were anesthetized by intraperitoneal injection of 10% chloral hydrate [10 mL/(kg·bw)]; 5 mL blood was collected immediately from the femoral artery,centrifuged at 3 000 r/min for 10 min, and the supernatant was stored in -80 ℃ freezer. An automatic biochemical analyzer was used to determine the serum CRP, Mb, CK-MB, cTnI and cTnT levels.
1.5.3 Myocardial items
After the blood was collected from the femoral artery,the chest was opened to separate heart; the heart mass was weighed after blood was cleaned with saline and the residual water was blotted to dry with filter paper; and the heart mass index (HMI) was calculated. HMI = Heart mass (HM) ÷ Body mass (BM). After weighing, the left ventricle was quickly separated; each ventricle was divided into two parts on the sagittal axis: one part was stored at -80 ℃ for cTnT concentration detection(determined by Thermo Multiskan GO microplate reader), and the other part was fixed in 4 ℃glutaraldehyde phosphate buffer solution for transmission electron microscopy observation.
All biochemical tests were completed by the Scientific Research Center of Hebei University of Chinese Medicine.
1.6 Statistical analysis
Data were processed with SPSS 21.0 statistical software. The measurement data were presented as mean ± standard deviation (±s). Paired-samplet-test was used for comparison before and after treatment in the same group, and one-way analysis of variance was used for between-group comparisons.P<0.05 indicated that the difference was statistically significant.
2 Results
2.1 Changes in the heart internal diameter
Compared with the same group before treatment:the cardiac LVEDd and LVESd of rats in the model group and the non-meridian non-acupoint group were increased after treatment (allP<0.01), and LVFS was decreased (bothP<0.01). Comparisons among groups after treatment: compared with the blank group, the changes of LVEDd, LVESd and LVFS in the control group were not significant (allP>0.05), the LVEDd and LVESd were increased (bothP<0.01), while the LVFS was decreased (P<0.01) in the model group; compared with the model group, the LVEDd, LVESd and LVFS in the nonmeridian non-acupoint group were not significantly changed (allP>0.05), the LVEDd and LVESd were all decreased (allP<0.01), the LVFS was increased (bothP<0.01) in both the Zusanli (ST 36) group and the Shenque (CV 8) group; compared with the non-meridian non-acupoint group, the LVEDd and LVESd were all decreased (P<0.01 orP<0.05), and the LVFS was increased (bothP<0.01) in the Zusanli (ST 36) group and the Shenque (CV 8) group; the LVEDd, LVESd and LVFS of the Shenque (CV 8) group were not significantly different from those of the Zusanli (ST 36) group (allP>0.05),(Table 1).

Table 1. Comparison of heart inner diameters of rats before and after treatment ( x ±s)
2.2 Changes in the heart volume
Compared with the same group before treatment: the cardiac SV, LVDv and LVSv of rats in the model group,non-meridian non-acupoint group and Zusanli (ST 36)group were all increased after treatment (allP<0.01), and the LVDv of the Shenque (CV 8) group was increased(P<0.01). Comparisons among groups after treatment:compared with the blank group, the SV, LVDv and LVSv in the control group did not change significantly (allP>0.05),and the SV, LVDv and LVSv of the model group were increased (allP<0.01); compared with the model group,the SV, LVDv and LVSv of rats in the non-meridian nonacupoint group were not significantly changed (allP>0.05), while those in the Zusanli (ST 36) group and the Shenque (CV 8) group were all reduced (allP<0.01);compared with the non-meridian non-acupoint group,the SV, LVDv and LVSv of rats in the Zusanli (ST 36) group and the Shenque (CV 8) group were all decreased (allP<0.01); there were no significant differences in the SV,LVDv and LVSv between the Shenque (CV 8) group and the Zusanli (ST 36) group (allP>0.05), (Table 2).
2.3 Changes in the cardiac function
Compared with the same group before treatment: E and A of rats were all increased after treatment (allP<0.01), and both E/A and EF were decreased (allP<0.01)in the model group, non-meridian non-acupoint group and Zusanli (ST 36) group; E and A were increased(P<0.01,P<0.05), and E/A was decreased (P<0.01) in the Shenque (CV 8) group. Comparisons among groups after treatment: compared with the blank group, the changes of E, A, E/A and EF in the control group were not obvious(allP>0.05), while E and A were all increased (bothP<0.01), and both E/A and EF were decreased (bothP<0.01) in the model group; compared with the model group, the changes of E, A, E/A and EF in the nonmeridian non-acupoint group were not obvious (allP>0.05); E and A were all decreased (allP<0.01), and both E/A and EF were increased (allP<0.01) in the Zusanli(ST 36) group and the Shenque (CV 8) group; compared with the non-meridian non-acupoint group, E and A were all decreased (allP<0.01), and E/A and EF were both increased (allP<0.01) in the Zusanli (ST 36) group and the Shenque (CV 8) group; compared with the Zusanli (ST 36) group, A was decreased (P<0.01), and both E/A and EF were increased (P<0.01,P<0.05) in the Shenque (CV 8) group (Table 3).
2.4 Changes in the HM and HMI
Compared with the blank group, the HMI of rats in the control group did not change significantly (P>0.05), while the HMI of rats in the model group was increased significantly (P<0.01); compared with the model group,the HMI of the rats in the non-meridian non-acupoint group did not change significantly (P>0.05), and the HMI of the rats in the Zusanli (ST 36) group and the Shenque(CV 8) group was significantly reduced (bothP<0.01);compared with the non-meridian non-acupoint group,the HMI of rats in the Zusanli (ST 36) group and the Shenque (CV 8) group was significantly lower (bothP<0.01); there was no statistical difference in HMI between the Shenque (CV 8) group and the Zusanli(ST 36) group (P>0.05), (Table 4).

Table 2. Comparison of heart volume before and after treatment among groups ( x ±s, μL)

Table 3. Comparison of cardiac function before and after treatment ( x ±s)
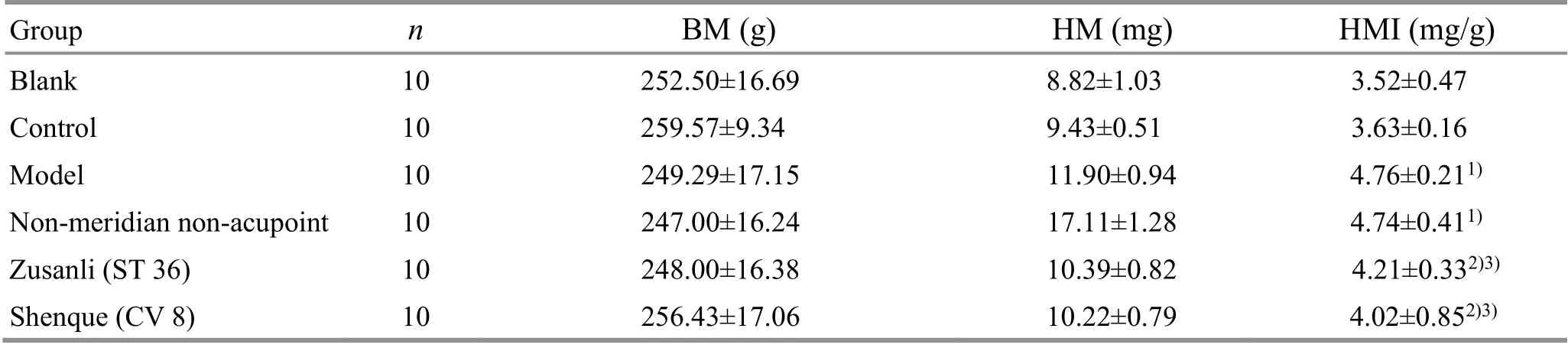
Table 4. Comparisons of HM and HMI of rats among groups ( x ±s)
2.5 Changes in the serum CRP, Mb, CK-MB, cTnI and cTnT levels
Compared with the blank group, the levels of serum CRP, Mb, CK-MB, cTnI and cTnT in the control group did not change significantly (allP>0.05), while those in the model group were all significantly increased (allP<0.01).Compared with the model group, the serum CRP, Mb,CK-MB, cTnI, and cTnT levels of rats in the non-meridian non-acupoint group did not change significantly (allP>0.05, while those in the Zusanli (ST 36) group and the Shenque (CV 8) group were significantly reduced (allP<0.01). Compared with the non-meridian non-acupoint group, the serum CRP, Mb, CK-MB, cTnI, cTnT levels in the Zusanli (ST 36) group and the Shenque (CV 8) group were significantly reduced (allP<0.01). Compared with the Zusanli (ST 36) group, the serum Mb level was significantly reduced (P<0.01), while no statistically significant differences in CRP, CK-MB, cTnI and cTnT levels were found in the Shenque (CV 8) group (allP>0.05),(Table 5).
2.6 Changes of the cTnT level in myocardium
Compared with the blank group, the cTnT level of the myocardial tissue was not significantly changed in the control group (P>0.05), while it was significantly increased in the model group (P<0.01). Compared with the model group, the cTnT level of the myocardial tissue in the non-meridian non-acupoint group did not change significantly (P>0.05), while it was significantly reduced in the Zusanli (ST 36) group and the Shenque (CV 8)group (bothP<0.01). Compared with the non-meridian non-acupoint group, the cTnT levels of myocardial tissue in the Zusanli (ST 36) group and the Shenque (CV 8)group were significantly reduced (P<0.05,P<0.01). The cTnT level in myocardial tissue of the Shenque (CV 8)group was not significantly different from that of the Zusanli (ST 36) group (P>0.05), (Table 6).
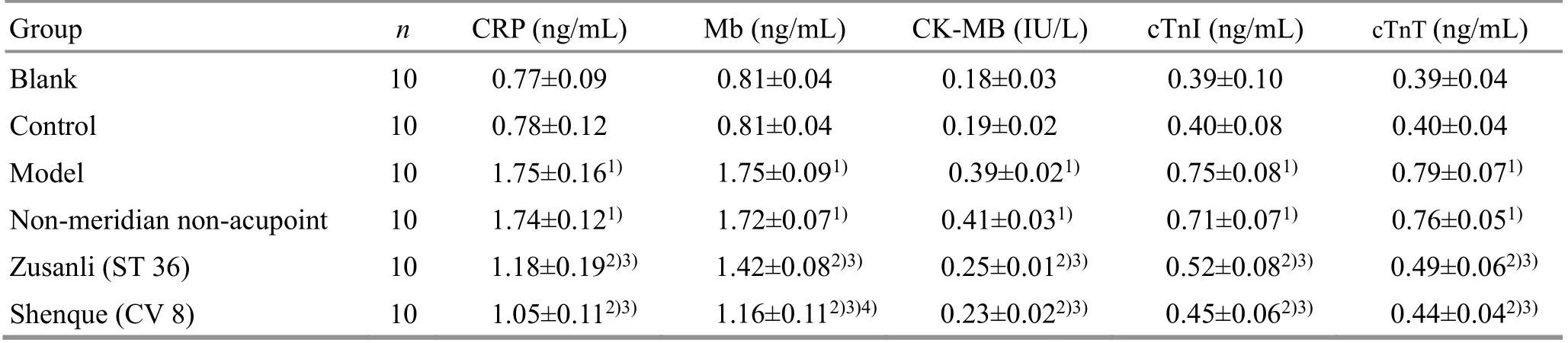
Table 5. Comparisons of the serum CRP, Mb, CK-MB, cTnI and cTnT levels after treatment among groups ( x ±s)

Table 6. Comparison of the cTnT level in myocardial tissues after treatment among groups ( x ±s, ng/L)
2.7 Comparison of ultrastructure of left ventricle among groups after treatment
Observed under the electron microscope, the myofibrils of the blank group and the control group were arranged neatly with clear light and dark bands; different degrees of disintegrated and ruptured myofibrils, as well as increased and aggregated mitochondria with different sizes and increased electron density were showed in both the model group and the non-meridian nonacupoint group; the myofibrils were arranged neatly with clear light and dark bands, and compensatory mitochondrial hyperplasia in the Shenque (CV 8) group and the Zusanli (ST 36) group, while there was no significant difference between the Zusanli (ST 36) group and the Shenque (CV 8) group (Figure 1), indicating that a 12-week treadmill running training remodeled ultrastructure of the left ventricle; moxibustion at Zusanli(ST 36) and Shenque (CV 8) effectively prevented or repaired micro-injuries caused by long-term exerciseinduced fatigue training, thereby effectively intervening myocardial remodeling.

Figure 1. Ultrastructure of rat left ventricle among groups (×2 000)
3 Discussion
Exercise-induced myocardial remodeling is mainly characterized by decompensated changes in morphology,structure, and functional metabolism of myocardial cells and sub-cells, which may be an important cause of exercise-induced arrhythmia[13-14]. Studies have shown that a 7-week treadmill running training causes evacuated, disorder direction, or even partially broken myofibrils of rat ventricular muscles; transparent, and generally decreased electron density mitochondrial matrix, even accompanied by the breakage or loss of mitochondrial cristae[15]. At the same time, it increases the expression of corresponding genes in the myocardium, such as activation of some embryonic genes; overexpression, inactivation, deletion or mutation of some genes, thus to regulate protein synthesis and change cell growth[16], which promotes changes in cardiac structure and function, that is,remodeling the myocardial structure and function[17].Changes in rat myocardium can be clearly observed by a small animal ultrasound instrument; meanwhile, HMI is also an important indicator for judging cardiac hypertrophy.
In this study, compared with the blank group, the results in the model group showed that LVEDd, LVESd, E,A and HMI were increased; LVFS, E/A and EF were decreased; under the electron microscope, the myocardial myofibrils were disintegrated and ruptured,and the mitochondria were aggregated with different sizes and increased electron density of rat left ventricle,indicating that 12-week treadmill running training caused a thickened heart wall, decreased heart output,and myocardial remodeling in the rats.
Long-term fatigue exercise causes relatively mild myocardial injury. Unlike the pathological myocardial injury, there is no large-scale myocardial necrosis and characteristic ECG changes[18]. This kind of myocardial injury mainly happens in cardiac cytoskeletons, including myosin, C-protein, M-protein, tubulin, actin, desmin and so on. Currently, dynamic detection of serum Mb and cTnI is the main method for clinical diagnosis of pathological myocardial injury. Mb is an oxygen-binding hemoglobin protein that exists in the cytoplasm of the myocardium cell. After myocardial injury, Mb enters into blood and reaches the peak earlier, but its specificity is poor[19]. cTnI is a calcium-mediated protein that regulates the interaction of actin and myosin in the myocardium. Its specificity in myocardium is much higher than other markers, and it is not affected by diseases of other organs such as skeletal muscle.However, the increasing time of cTnI in blood comes later.Combined application of Mb and cardiac troponin (cTn)is called the ‘gold standard’ for the diagnosis of myocardial injury in clinical practice[20]. The results of our study showed that the serum CRP, Mb, CK-MB, cTnI, cTnT,and myocardial cTnT levels in the model group were significantly increased versus the blank group, indicating that the 12-week treadmill running training resulted in rat myocardial cell injury.
The myocardial remodeling caused by exerciseinduced fatigue belongs to the category of ‘deficiency’ or‘palpitations’ in Chinese medicine, and the treatment should be based on warming and nourishing.Moxibustion has the functions of warming the meridians for dispelling cold, strengthening yang to avoid dehydration. Modern studies have proved that moxibustion has a protective effect on myocardial injury caused by exercise-induced fatigue. Xiong Y,et al[21]found that moxibustion at Zusanli (ST 36) and other acupoints has a protective effect on myocardial injury caused by acute force exhaustion exercise, which may be achieved by preventing the overexpression and activation of protein kinase C. Zhang HR,et al[22]also confirmed that pre-moxibustion at Zusanli (ST 36) and other acupoints may prevent myocardial tissue damage in rats suffering one-time exhaustive exercise by regulating adenosine monophosphate-activated protein kinase (AMPK) and mammalian target of rapamycin(mTOR) expression to induce corresponding signal cascade. Therefore, this study used moxibustion at Zusanli (ST 36) as a positive control.
The results of this study found that the LVEDd, LVESd,SV, LVDv, LVSv, E, and A levels, the serum CRP, Mb, CK-MB,cTnI, and cTnT, as well as the myocardial tissue cTnT concentration and HMI of rats were decreased; while the LVFS, E/A and EF were elevated; the myocardial myofibrils were arranged neatly with clear light and dark bands under the electron microscope in the Shenque(CV 8) group and Zusanli (ST 36) group, indicating that moxibustion at Zusanli (ST 36) and Shenque (CV 8) can alleviate myocardial injury caused by exercise-induced fatigue via effectively preventing the occurrence of myocardial remodeling. At the same time, we found that Mb and A were significantly decreased, and E/A and EF were significantly increased in the Shenque (CV 8) group,suggesting that the effect of moxibustion at Shenque(CV 8) was better than at Zusanli (ST 36) in improving cardiac function. In addition, it was found that there was no statistically significant difference in cardiac structure and function between the non-meridian non-acupoint group and the model group after intervention. The nonmeridian non-acupoint points and Shenque (CV 8) are in the charge of the same spinal nerve segment. Therefore,we speculated that moxibustion at Shenque (CV 8) did not or did not mainly play a therapeutic role through spinal nerves. There was no statistically significant difference in cardiac structure and function between the control group and the blank group after intervention,suggesting that moxibustion at Shenque (CV 8) had no significant effect on normal myocardial structure and function.
In conclusion, moxibustion at Shenque (CV 8) can effectively prevent cardiac structure changes caused by exercise-induced fatigue and enhance the cardiac function without affecting the normal organism. It is safe and effective. We will focus on exploring the mechanism of myocardial remodeling, and the possible mechanism of moxibustion at Shenque (CV 8) in intervening cardiac structural remodeling caused by exercise-induced fatigue in our future study.
Conflict of Interest
The authors declare that there is no potential conflict of interest in this article.
Acknowledgments
This work was supported by Hebei Key Laboratory of Chinese Medicine Research on Cardio-cerebrovascular Disease (河北省心脑血管病中医药防治研究重点实验室开放课题, No. 201802); Key Research Projects of Higher Education in Hebei Province (河北省高等学校科学技术研究项目, No. ZD2019061); Undergraduate Innovation and Entrepreneurship Training Program of Hebei University of Chinese Medicine (河北中医学院大学生创新创业训练计划项目, No. 201914432021).
Statement of Human and Animal Rights
The treatment of animals conformed to the ethical criteria in this experiment.
Received: 22 July 2020/Accepted: 30 October 2020
 Journal of Acupuncture and Tuina Science2021年4期
Journal of Acupuncture and Tuina Science2021年4期
- Journal of Acupuncture and Tuina Science的其它文章
- Regulatory effect of mild moxibustion on P2X3 receptors in spinal cord, anterior cingulate cortex and thalamic ventral posterolateral nucleus of rats with IBS visceral hyperalgesia
- Clinical study on long-snake moxibustion plus Western medicine in treating chronic heart failure due to heart-kidney yang deficiency
- Muscle regions of meridians warm needling method plus pricking Jing-Well points for blood-letting in the treatment of shoulder-hand syndrome after stroke
- Adjunctive effects of acupressure therapy on pain and quality of life in patients with knee osteoarthritis: an interventional study
- Efficacy and effect on serum VEGF-C of mild moxibustion plus functional exercise for upper-limb lymphedema after breast cancer surgery
- Effects of acupuncture plus MOTOmed intelligent motor training in treating children with spastic cerebral palsy
