Study of selective hydrogenation of biodiesel in a DBD plasma reactor
Weidong ZHAO (赵卫东),Chao HUA (华超),Xiaoyin ZHANG (张潇尹),Xiaolong QI(戚小龙),Kiatsiriroat TANONGKIAT and Junfeng WANG(王军锋)
1 School of Automotive and Traffic Engineering,Jiangsu University,Zhenjiang 212013,People’s Republic of China
2 Department of Mechanical Engineering,Faculty of Engineering,Chiang Mai University,Chiang Mai 50200,Thailand
3 School of Energy and Power Engineering,Jiangsu University,Zhenjiang 212013,People’s Republic of China
Abstract In order to achieve the selective hydrogenation of biodiesel at room temperature and under normal pressure,we researched the upgrading of soybean biodiesel using a dielectric-barrier discharge (DBD) reaction system.Using Raney-Ni as the hydrogenation catalyst,the effects of the operating parameters on the hydrogenation depth and the selectivity of biodiesel were systematically analyzed.The results show that the polyunsaturated components in soybean methyl ester were reduced by 57.04%,and that the polyunsaturated components were hydrogenated to monounsaturated components with a selectivity of 77.75%.Based on the gas chromatography and mass spectrometry(GC-MS)test results,we established a kinetic model for biodiesel hydrogenation.A comparison of the calculated and experimental results shows that the hydrogenation of the biodiesel can be described by a quasi first-order reaction model.The calculated reaction rate constants indicate that under DBD plasma reaction conditions,the hydrogenation of biodiesel has high selectivity for the formation of monounsaturated components.
Keywords: biodiesel,selectivity,hydrogenation,dielectric-barrier discharge,oxidation stability,saturation
1.Introduction
Biodiesel prepared by transesterification contains a large number of polyunsaturated components,resulting in poor oxidation stability and a low cetane number.Oxidation and polymerization are likely to occur,resulting in high molecular polymers and precipitates which are not conducive to storage,and can easily cause blockages of the engine oil circuit.On the other hand,the lower cetane number is also an obstacle to improving engine fuel economy and emission performance,so it is necessary to upgrade biodiesel.
The oxidation stability and cetane number of biodiesel could be greatly improved by the selective hydrogenation of polyunsaturated fatty acid esters to monounsaturated fatty acid esters,while not reducing low-temperature fluidity too much,and better lubrication performance could simultaneously be obtained; in doing so,the quality of biodiesel could be improved,and hydrogen consumption and cost could be reduced.Therefore,the selective hydrogenation of polyunsaturated fatty acid esters has become the main technical solution for upgrading biodiesel.
Rajkumaraet al[1] studied the physicochemical properties of hydrogenated biodiesel–diesel blends and their NOxemissions characteristics when applied to turbodiesel; the results showed that B20 blended fuel can significantly reduce NOxemissions.Nikolaouet al[2] conducted a selective catalytic hydrogenation of sunflower-oil biodiesel.Under reaction conditions of 50 bar of hydrogen pressure and a temperature of 90°C,deep hydrogenation of polyunsaturated fatty acid esters was achieved,but a large number of trans oleates and stearates with high condensation points were produced,which seriously reduced the low-temperature fluidity of the biodiesel; similar problems and results were reported in references [3,4].
In summary,the feasibility of using selective hydrogenation to improve the quality of biodiesel has been confirmed,but the massive production of fully saturated and trans fatty acid esters caused by the high hydrogen pressures of traditional thermodynamic reaction conditions is the main problem that needs to be solved.It is therefore necessary to explore biodiesel hydrogenation methods that require mild reaction conditions,in order to improve the controllability of biodiesel hydrogenation depth and selectivity.
A gas-phase reactant can be ionized by an electric field and transformed into a non-thermal plasma which contains large number of highly reactive active particles,such as excited molecules,free radicals,positive ions,negative ions,and highenergy electrons,so that chemical reactions can be initiated under mild conditions.In addition,the ionization degree and energy level of the gas-phase reactants can be changed by adjusting the working voltage,so as to realize control of the chemical reaction rate and path.Due to its low energy consumption,easy operation,and high efficiency,discharge plasma reaction technology has been widely used in material preparation,surface modification,and plasma cleaning[5–11].Recently,its application has gradually been extended to the field of oxygen-containing-compound hydrogenation,and the advantages of easy control of the reaction depth and selectivity have been further reflected in this research [12–16].
Normally,non-thermal plasmas have been produced by gas discharges; the commonly used discharge forms include coronal discharge,glow discharge,spark discharge,and dielectric-barrier discharge.The energy of free electrons generated by dielectric-barrier discharge is in the range of 1–10 eV,which is suitable for breaking most chemical bonds[17–22],and the discharge can be stable and uniformly distributed in the discharge zone,so dielectric-barrier discharge is the preferred choice for non-thermal plasma generation.
Yin [23] and Dai [24] verified the feasibility of highly selective hydrogenation of unsaturated fatty acid esters in a DBD reactor under normal pressure and at room temperature,achieving the selective hydrogenation of linolenic acid esters and linoleic acid esters in C18 enoate; the hydrogenation selectivity reached 83.5%.Liuet al[25]studied the conversion of anisole and guaiacol to benzene and toluene in a DBD reactor.They found that DBD can effectively decompose H2into active atomic hydrogen,which can effectively promote the hydrogenolysis reaction of the Caro–OR bond,and that the high selective conversion of anisole and guaiacol can be realized at room temperature by combining a hydrogen plasma with a Ni-Mo/SiO2catalyst.These applications and studies indicated the potential of efficiently achieving the selective hydrogenation of biodiesel using a discharge plasma reaction.
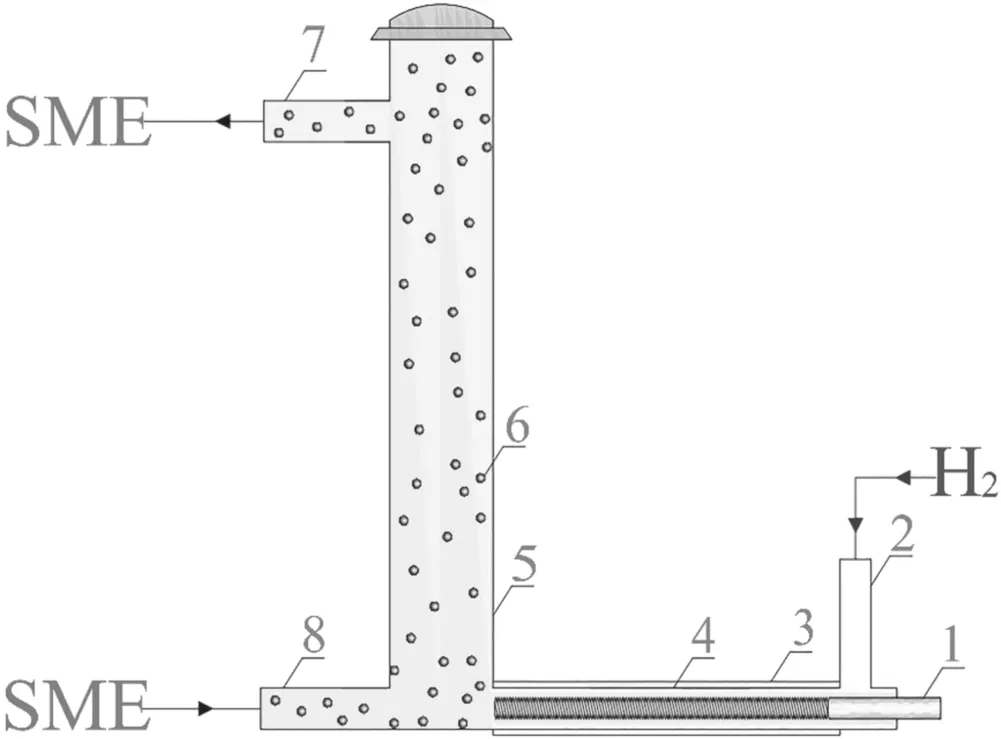
Figure 1.Schematic diagram of the DBD hydrogenation reactor.(1-inner electrode,2-gas inlet,3-outer electrode,4-discharge tube,5-reaction vessel,6-catalyst,7-SME outlet pipe,8-SME intake pipe).

Table 1.Components and contents of biodiesel.
In order to solve the problems that exist in current biodiesel upgrading,we propose a technical solution for the selective hydrogenation of biodiesel at room temperature and atmospheric pressure based on a DBD plasma reaction.Using soybean biodiesel as the raw material and hydrogen as the hydrogen donor,we conducted research into the selective hydrogenation of biodiesel.We expect that this research will provide an experimental and theoretical reference for biodiesel upgrading.
2.Experimental details
2.1.Preparation of biodiesel
According to the preparation process described in [26],the biodiesel to be used in the experiment was prepared by the transesterification of soybean oil and methanol with NaOH as a catalyst,which is called soybean methyl ester (SME).The components and component percentages of the biodiesel obtained are shown in table 1.
2.2.Experimental system and procedure
A coaxial cylindrical DBD hydrogenation reactor was designed,as shown in figure 1,for biodiesel upgrading.

Figure 2.Experimental DBD selective catalytic hydrogenation system and layout.(1-gas cylinder,2-pressure-reducing valve,3-glass rotor flow meter,4-measuring cylinder,5-micro-peristaltic pump,6-DBD reactor,7-AC high-voltage power supply,8-oscilloscope).
The device consists of a reaction vessel and a discharge tube.The reaction vessel is a quartz glass tube with a length of 300 mm and an inner diameter of 20 mm.The bottom and top of the vessel are equipped with an inlet and outlet pipe,respectively,with inner diameters of 8 mm,to circulate biodiesel into and out of the reactor during the reaction.The discharge tube is designed to be a coaxial cylindrical structure,with an inner diameter of 8 mm,a wall thickness of 1.5 mm and a length of 100 mm.An intake branch pipe with an inner diameter of 8 mm and a length of 40 mm is set perpendicular to the discharge area to supply hydrogen into the discharge area during the reaction.The inner electrode is a solid copper screw rod with a length of 100 mm,a diameter of 3.5 mm,and a pitch of 0.6 mm.An aluminum foil 0.3 mm thick,used as an outer electrode,is wrapped around the outer surface of the discharge tube.The high-voltage terminal of the power supply is connected to the inner electrode,and the outer electrode is grounded.
A schematic diagram of the experimental DBD selective catalytic hydrogenation system is shown in figure 2.
Raney-Ni (with a particle diameter of 0.1 mm,a specific surface area of 131 m2g−1,and a pore volume of 0.20 ml g−1)was selected as the hydrogenation catalyst,due to its high activity at low temperatures and large specific surface area [27–33].
After the system was built,we first opened the upper cap of the reactor and added the catalyst (3 wt.% of SME),then closed the upper cap.Next,200 ml(183 g)of prepared SME was poured into a measuring cylinder,the micro-peristaltic pump was turned on,and the flow rate was adjusted in the range of 0–50 ml min−1.SME was pumped into the oil inlet at the bottom of the reactor,and then flowed out from the oil outlet at the top of the reactor.The oil circulated from bottom to top,combined with the agitating action of the gas bubbles,enhancing mass transfer between the gas,liquid,and solid phases.A mixture of 40 vol.% H2and 60 vol.% He (Beijing Huayuan Gas Chemical Co.Ltd) was adopted as the hydrogen donor.We opened the gas cylinder and adjusted the gas flow rate through a pressure-reducing valve and the glass rotor flow meter in the range of 0–50 ml min−1,so that the mixture of H2and He gas entered the reactor from the inlet branch pipe.After the gas flow rate was stabilized,we turned on the AC high-voltage power supply (Model: CTP-2000S,Nanjing Suman Electronics Co.,Ltd) and the digital oscilloscope and adjusted the working voltage and operating frequency in the range of 10.61–21.21 kV and 5–30 kHz,respectively,to start the DBD reaction system.
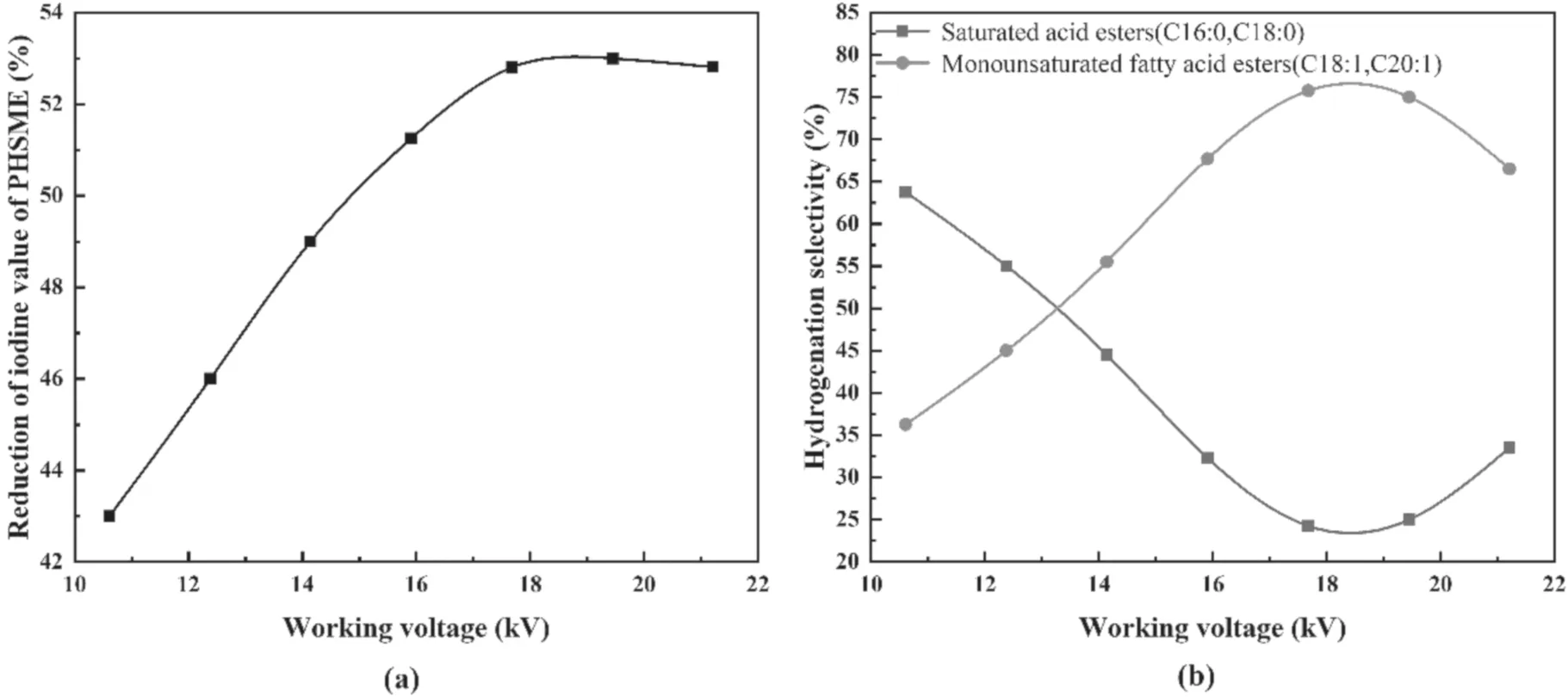
Figure 3.Effects of the working voltage on the depth and hydrogenation selectivity of SME.
Under the conditions of a room temperature of 25°C and 0.1 MPa of hydrogen pressure,hydrogen was ionized by the dielectric-barrier discharge,passed into the mixture of SME and Raney-Ni catalyst,and reacted with unsaturated fatty acid esters in the SME on the active sites of the catalyst.After the reaction was completed,the obtained solid-liquid mixture was filtered,and the liquid phase,i.e.the target product,partially hydrogenated soybean methyl ester (PHSME),was collected.
2.3.Experimental design for selective biodiesel hydrogenation
The working voltage,gas flow rate,SME circulation flow rate,and reaction time were selected as influencing factors,and single-factor experiments were conducted to investigate the effect of each factor on the depth and selectivity of SME hydrogenation.The ranges of the working voltage,gas flow rate,SME circulation flow rate and reaction time were 10.61–21.21 kV,10–40 ml min−1,0–60 ml min−1,0.5–2 h,respectively.
2.4.GC-MS detection method
The PHSME samples were analyzed by a gas chromatography mass spectrometer (GC-MS,model: Agilent 6890N-5973,USA),equipped with a HP-5MS (30 m×0.25 mm×0.25 μm) capillary column.The carrier gas was high-purity He (99.999%),and the carrier gas flow rate was 1.0 ml min−1.The injection port temperature was 280°C and the solvent delay time was 3 min.The ion source temperature and transmission line temperature of the mass spectrometer were set to 230 °C and 250 °C,respectively.The ionization potential of the EI ionization source was 70 eV,the scanning mass range was 30.00–550.00 amu,and the scanning time was set to 1 s.The temperature program was set to an initial temperature of 150 °C,held for 1 min,and then raised to 300 °C at a rate of 5 °C min−1,then held for 11 min.
3.Results and discussion
3.1.Effects of working parameters on the depth and selectivity of hydrogenation
The unsaturation degree of biodiesel is usually evaluated using the iodine value.The higher the iodine value,the higher the contents of unsaturated components in biodiesel and the worse the oxidation stability.Therefore,the iodine value reduction rate can be used to evaluate the hydrogenation depth of SME [34].
The compositions and relative contents of the PHSME samples obtained by experiment were determined by GC-MS analysis and classified according to the saturation of fatty acid esters.The hydrogenation selectivity of a fully saturated fatty acid ester is defined asSF,as shown in equation (1):
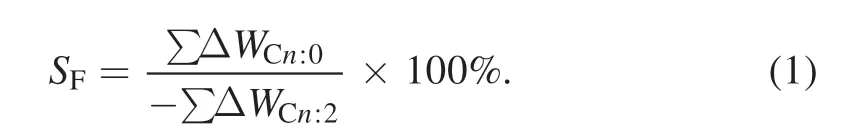
In equation (1),ΔWCn:0is the mass fraction increment of the fully saturated fatty acid ester after hydrogenation (as a percentage) and ΔWCn:2is the mass fraction increment of the polyunsaturated fatty acid ester after hydrogenation (as a percentage).
The hydrogenation selectivity of the monounsaturated fatty acid esters produced is defined asSL,as shown in equation (2):

in equation (2),ΔWCn:1is the mass fraction increment of the monounsaturated fatty acid ester after hydrogenation (as a percentage) and ΔWCn:2is the mass fraction increment of the polyunsaturated fatty acid ester after hydrogenation (as a percentage).
3.1.1.Working voltage.Figures 3(a) and (b) show the influences of the working voltage on the depth and hydrogenation selectivity of SME for a gas flow rate of 30 ml min−1,a SME circulation flow rate of 40 ml min−1,and a reaction time of 90 min.
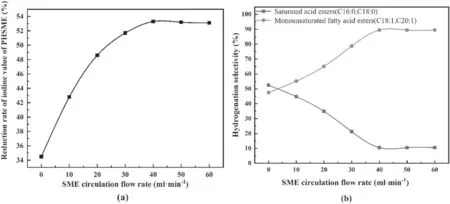
Figure 4.Effects of the SME circulation flow rate on the depth and hydrogenation selectivity of SME.
As shown in figure 3(a),with an increase in the working voltage from 10.61 kV to 17.68 kV,the hydrogenation depth gradually increased and reached a maximum value of 52.8% at 17.68 kV.When the working voltage was continuously increased to 21.21 kV,the change in the hydrogenation depth was insignificant.The reason for this is that as the voltage increased,the hydrogen ionization degree gradually reached a maximum value,as did the amount of active hydrogen in the discharge tube [35].
Figure 3(b) indicates that the working voltage has a significant effect on the hydrogenation selectivity of SME.When the working voltage was increased from 10.61 kV to 17.68 kV,SLincreased and reached a maximum value of 76.75%at a working voltage of 17.68 kV,whileSFdecreased and reached a minimum value of 23.25%at a working voltage of 17.68 kV.When the working voltage was higher than 17.68 kV,SLbegan to decrease andSFincreased.
3.1.2.SME circulation flow rate.Figures 4(a) and (b) show the influences of the SME circulation flow rate on the depth and hydrogenation selectivity of SME for a working voltage of 17.68 kV,a gas flow rate of 30 ml min−1,and a reaction time of 90 min.
As shown in figure 4(a),as the SME circulation flow rate increased to 40 ml min−1,the hydrogenation depth of SME increased,and the maximum reduction in the iodine value was 53.3%.This is because the flow of the liquid phase caused the SME at the bottom of the reactor to be pumped to the upper end.Due to the disturbance of the liquid phase,the fine Raney-Ni catalyst particles were more uniformly dispersed,enhancing the mass transfer between the gas,liquid,and solid phases,and thereby increasing the rate of SME hydrogenation.When the SME circulation flow rate was greater than 40 ml min−1,the rate of decrease in the iodine value did not change significantly,indicating that the enhancement of mass transfer due to the SME circulation flow rate was limited.
As shown in figure 4(b),when the liquid phase did not circulate,SFwas slightly higher thanSL.When the SME circulation flow rate increased to about 40 ml min−1,SLrose to the highest value of 89.48%,andSLdecreased to the lowest value of 10.52%,indicating that the polyunsaturated components were mainly converted into monounsaturated components.As the circulation flow rate continued to increase,bothSLandSFtended to be stable.Liquid-phase circulation was the main means of enhancing the interphase mass transfer in this discharge reaction system; therefore,we draw the conclusion that enhancing the interphase mass transfer of the discharge reaction system can effectively improve the selectivity of monounsaturated fatty acid esters;a similar conclusion was reported in reference [36].
3.1.3.Gas flow rate.Figures 5(a) and (b) show the influences of the gas flow rate on the depth and hydrogenation selectivity of SME for a working voltage of 17.68 kV,an SME circulation flow rate of 40 ml min−1,and a reaction time of 90 min.
As shown in figure 5(a),as the gas flow rate increased from 10 ml min−1to 34 ml min−1,the rate of decrease of the iodine value of the SME increased from 47.0% to 52.8%.The reason for this is that when the gas flow rate increased,more hydrogen was ionized in the discharge zone per unit time,thereby providing more active hydrogen for the hydrogenation reaction;the depth of hydrogenation was then increased.While the gas flow rate was continuously increased,the rate of decrease of the iodine value showed a downward trend.This is because the residence time of hydrogen molecules in the discharge zone was too short and the collision probability of high-energy electrons and ground-state molecules was reduced;meanwhile,the excited-state molecules were more likely quenched by excessive gas renewal,so that the amount of active hydrogen available for SME hydrogenation was reduced,and therefore the hydrogenation depth was also reduced [37].
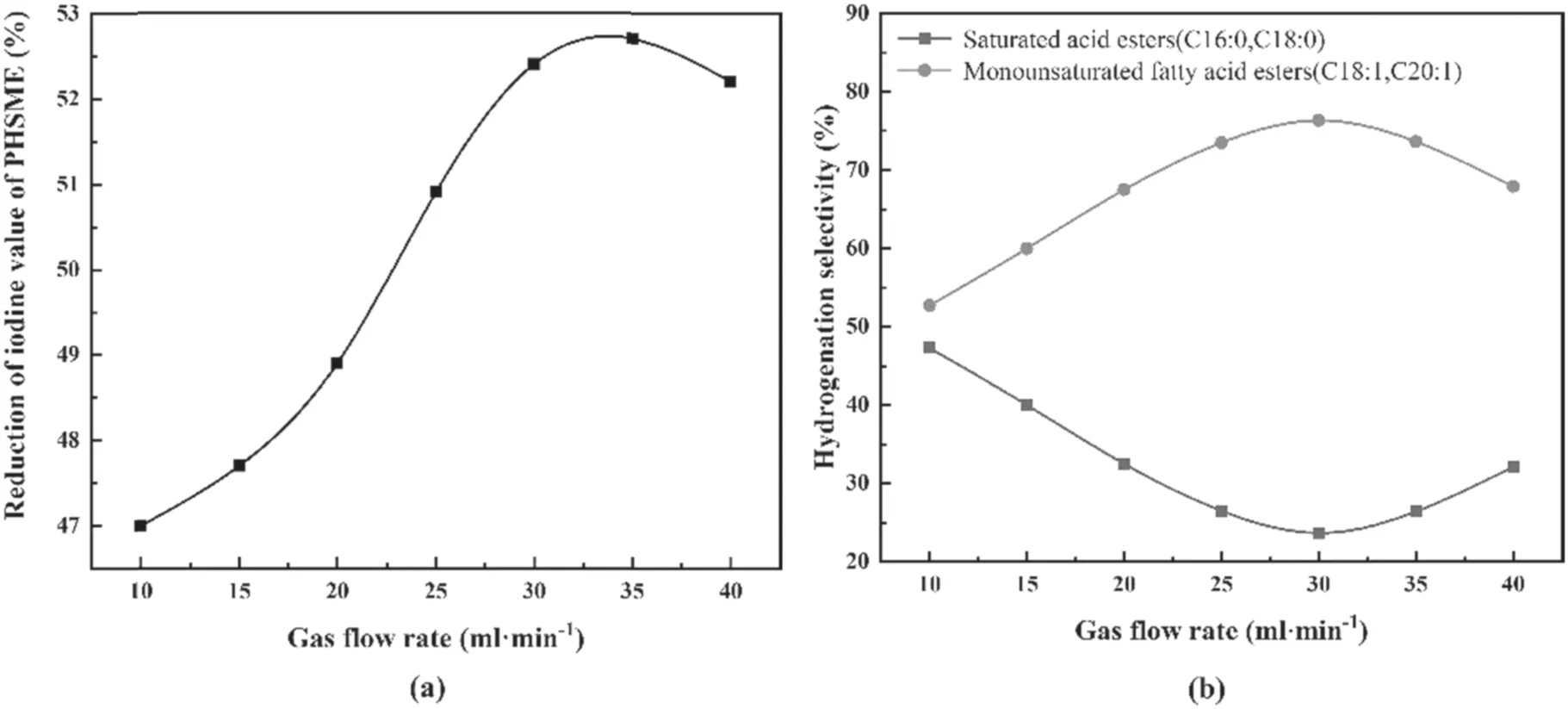
Figure 5.Effects of the gas flow rate on the depth and hydrogenation selectivity of SME.
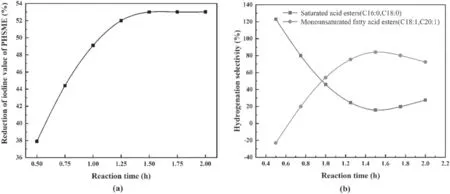
Figure 6.Effect of the reaction time on the depth and hydrogenation selectivity of SME.
Figure 5(b) shows the effect of gas flow rate on the hydrogenation selectivity of SME.With an increase in the gas flow rate,SLwas always greater thanSF.SLfirst increased and then fell and reached its highest value of 76.33%at a gas flow rate of 30 ml min−1.SFshowed the opposite trend,reaching a minimum of 23.67%at a gas flow rate of 30 ml min−1;when the gas flow continued to increase,SLshowed a reducing trend andSFbegan to increase.When the gas flow rate increased,the absolute number of highly reactive hydrogen atoms generated per unit time increased,but an excessive gas renewal rate caused a decrease in the residence time of the gas in the discharge zone,and then a decrease in the concentration of hydrogen atoms.It can be inferred that increasing the number of active hydrogen atoms is advantageous for increasing the selectivity of the monounsaturated fatty acid ester formation and suppressing the selectivity of the fully saturated fatty acid ester formation.
3.1.4.Reaction time.Figures 6(a) and (b) show the influences of the reaction time on the depth and hydrogenation selectivity of SME for a working voltage of 17.68 kV,a gas flow rate of 30 ml min−1,and an SME circulation flow rate of 40 ml min−1.
As shown in figure 6(a),as the reaction time was prolonged,the rate of decrease of the iodine value of SME gradually increased to 53.0%and then remained stable after 1.5 h.Appropriately extending the reaction time was advantageous for increasing the probability of collisions between the hydrogen plasma and unsaturated C=C bonds,and the hydrogenation reaction was also performed more fully.However,continuously prolonging the reaction time was not obviously beneficial to the hydrogenation depth,because the hydrogenation reaction between the hydrogen plasma and the SME tended to be saturated;that is,under the specific discharge reaction conditions described herein,the collision probability of the hydrogen plasma with unsaturatedcarbon bonds reached a maximum value [38].Therefore,the reaction time should be controlled from the viewpoint of improving the hydrogen utilization rate and reducing energy consumption.
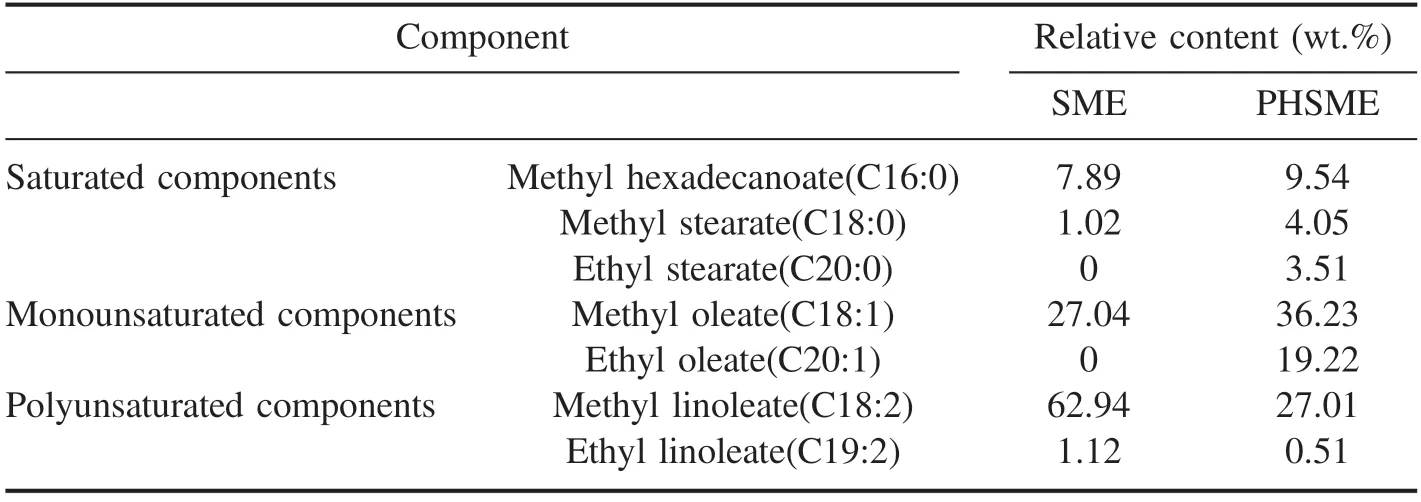
Table 2.Main composition and relative contents of SME and PHSME.
As shown in figure 6(b),at the initial stage of the hydrogenation reaction(0–0.6 h),SLwas negative andSFwas more than 100%,indicating that the number of monounsaturated components produced by hydrogenation was less than the number of monounsaturated components converted to fully saturated components.In the middle stage of the hydrogenation reaction (0.6–1.5 h),with an increase in the reaction time,SLincreased sharply,whileSFdecreased.Where the two curves intersect,SL>SF,indicating that the polyunsaturated components in SME were mainly converted into monounsaturated components.At the end of the hydrogenation reaction (1.5–2.0 h),SFincreased slightly,indicating that some of the monounsaturated components were converted into fully saturated components,and that the hydrogenation reaction proceeded to deep hydrogenation.Due to the higher melting point of fully saturated fatty acids,although an increase in their content is beneficial for improving the oxidation stability of biodiesel,the lowtemperature fluidity of biodiesel is then deteriorated.Therefore,to improve the overall quality of biodiesel,the deep hydrogenation reaction of monounsaturated fatty acid ester should be inhibited by controlling the reaction time.
3.2.Analysis of the hydrogenation depth and selectivity of biodiesel
According to the single-factor experimental results,the preferred operating parameter combination was determined,that is,a working voltage of 17.68 kV,a gas flow rate of 30 ml min−1,a SME circulation flow rate of 40 ml min−1,and a reaction time of 1.5 h.With the preferred operating parameter combination,three groups of biodiesel hydrogenation experiments were carried out.The SME and PHSME were analyzed by GC-MS; the main components of the SME and PHSME are shown in table 2.
The relative contents of saturated,monounsaturated,and polyunsaturated components in PHSME,as calculated from table 2,changed from 8.91%,27.04%,and 64.06% to 17.10%,55.45%,and 27.52%,respectively.The conversion rate of the polyunsaturated components reached 57.04% and the hydrogenation selectivity of the monounsaturated components produced was 77.75%.
Xia[39]and Yuan[40]conducted research into biodiesel upgrading using Raney-Ni as the catalyst.Their polyunsaturated disintegration rates were 100% and 75.74% at 85°C,and the hydrogenation selectivities of the monounsaturated components were 67.89% and 26.12%,respectively.
Compared with biodiesel upgraded under traditional thermodynamic reaction conditions,the 57.04% conversion rate of the polyunsaturated components described here is lower,but the 77.75% selectivity of the monounsaturated components produced is higher.The reduced catalytic activity of Raney-Ni at room temperature may be the main reason for the lower conversion rate of the polyunsaturated components.
The overall quality of the biodiesel produced under gasphase discharge reaction conditions could be further improved by moderately raising the reaction temperature to enhance the activity of the catalyst,and optimizing the design of the hydrogenation reactor to enhance the interphase mass transfer.
3.3.Mechanism of biodiesel hydrogenation under discharge reaction conditions
3.3.1.Main types of biodiesel hydrogenation reaction.The formation of atomic hydrogen with high reaction activity is of great significance for the hydrogenation of SME.Under the action of an electric field,there are five main types of ionization and excitation processes of H2,namely excitation,de-excitation,ionization,recombination,and elastic collision[41].Inelastic collisions are the main reason for the ionization of hydrogen.Strong inelastic collisions between hydrogen molecules and high-energy electrons produce a hydrogen plasma containing hydrogen radicals,positive and negative ions,and excited atoms.The reaction forms include the electron ionization reaction,the electron–neutral molecular reaction,the electron–ion reaction,the electron excitation reaction,the electron–neutral particle reaction,the positive ion–negative ion reaction,etc.The main inelastic collision reaction processes are shown in table 3.
In the hydrogenation process,a small amount of water(visible to the naked eye)and an acid substance(based on the GC-MS test results) were produced.It could be inferred that there are additional reactions of unsaturated C=C,hydrodeoxidation,and carbon chain addition taking place.Their chemical reaction formulas are as follows:
(1) Adsorption of active hydrogen on Raney-Ni

(2) Addition reaction of unsaturated C=C (C18:1,C18:2and C19:2)
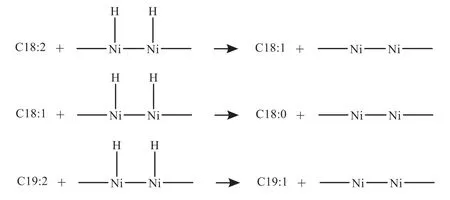
(3) Hydrodecarboxyl reaction (C18:0,C18:2)
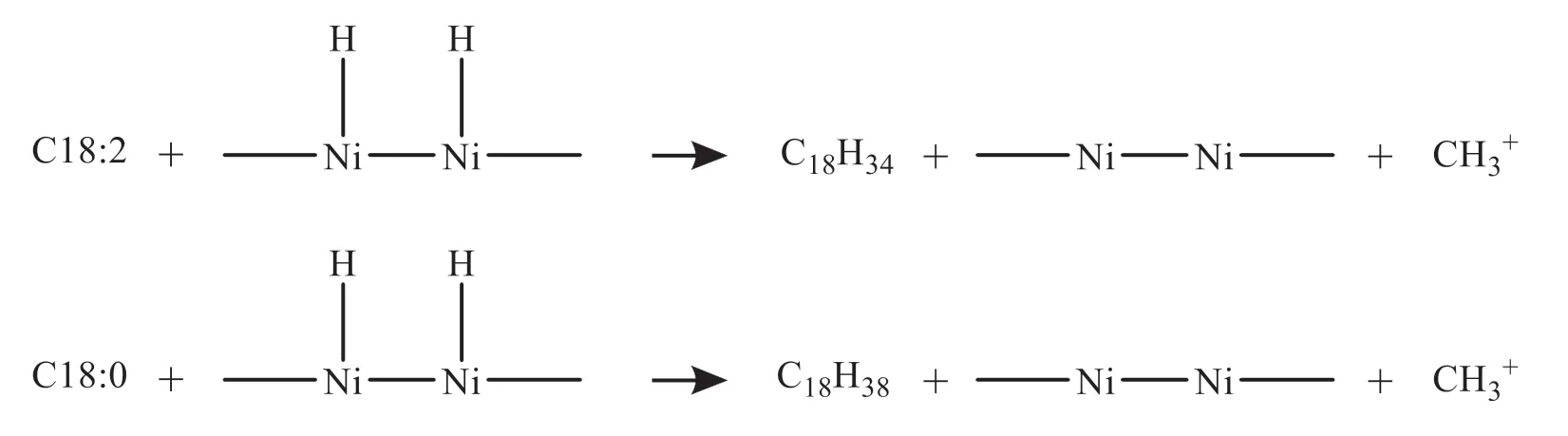
(4) Carbon chain addition reaction (C18:0,C18:1)
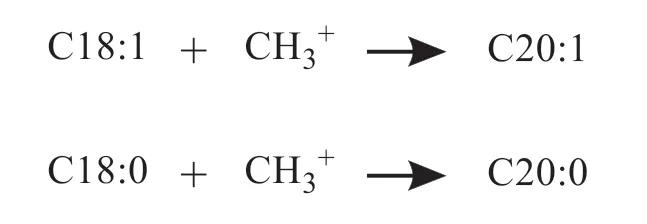
3.3.2.Kinetic model for biodiesel hydrogenation.According to the results of the GC-MS analysis,and referring to relevant research results[29],it is inferred that the main hydrogenation pathways of SME on the Raney-Ni catalyst and the discharge reaction conditions are as shown in figure 7.
In figure 7,c1,c2,c3,c4,andc5are the mass fractions of C18:2,C18:1,C18:0,C20:0,and C20:1,respectively;k1,k2,k3,andk4are the rate constants of the respective hydrogenation steps.Assuming that the catalytic hydrogenation of SME over the Raney-Ni catalyst is a kind of quasi first-order reaction,then the hydrogenation processes shown in figure 7 can be described by kinetic equations (7)–(11).

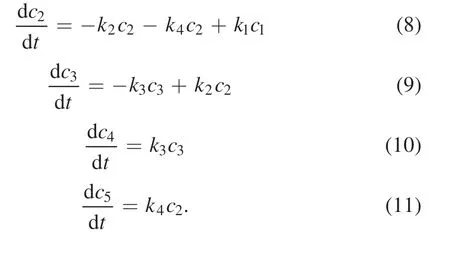
The hydrogenation rate constantsk1,k2,k3andk4can be calculated according to the GC-MS test data.According to the initial mass fraction of each component of SME and the mass fractions of the PHSME components after 90 min of reaction (table 2),it can be calculated thatk1=0.0167 min−1,k2=0.0059 min−1,k3=0.0043 min−1,andk4=0.0128 min−1.The mass fractions of C18:2,C18:1,C18:0 and C20:1 at other reaction times can be calculated using the kinetic equations above.
Figure 8 shows the calculated and experimental values of the mass fractions of C18:0,C18:1,C20:1,and C18:2.It can be seen that the calculated results are basically consistent withthe experimental values,which proves that the hydrogenation of SME on the Raney-Ni catalyst can be described by a quasi first-order reaction model.
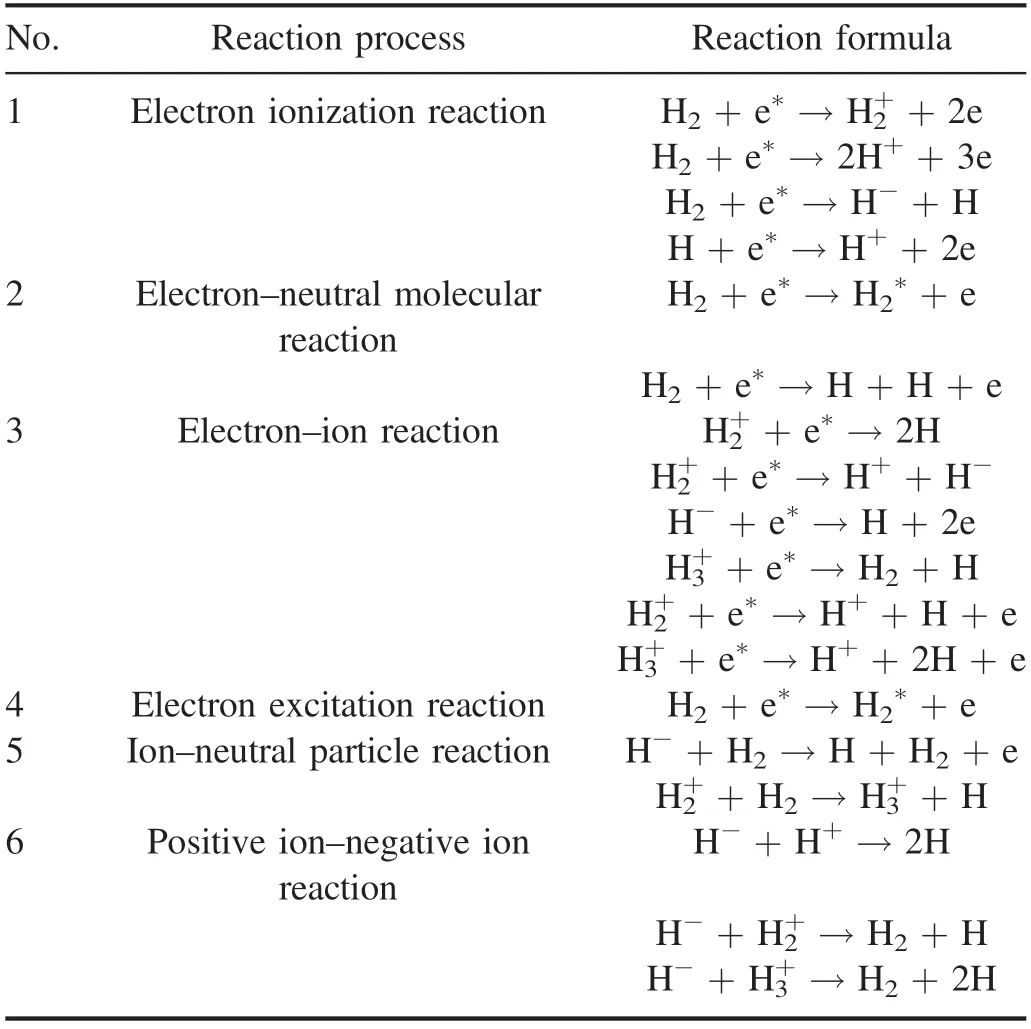
Table 3.Major ionization and excitation processes of H2 [42].
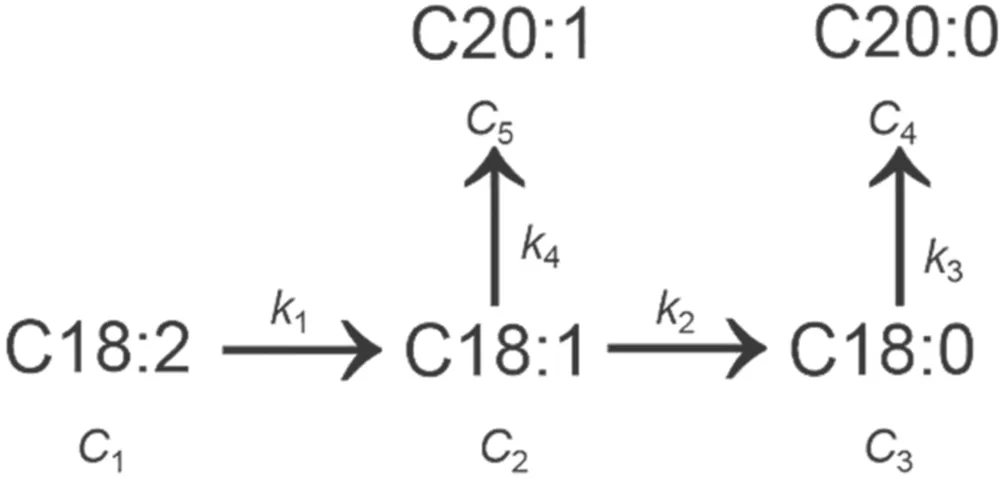
Figure 7.Hydrogenation process of SME on Raney-Ni.
The reaction rate constants obey the relationk1>k4>k2>k3,indicating that the conversion rate of C18:2 to C18:1 is faster than that of C18:1 to C18:0.It can be deduced from this that the hydrogenation rate of polyunsaturated fatty acid ester is faster than that of monounsaturated fatty acid ester,which is beneficial for increasing the content of monounsaturated components.
The hydrogenation selectivity of each component can be described by the ratio of the reaction rate constants.The ratio ofk1tok2,that is,S1=k1/k2=2.83,which indicates that the formation rate of C18:1 by C18:2 hydrogenation is 2.83 times that of C18:0 from C18:1.C18:1 has two conversion methods;one is that the carbon chain length increases but the number of unsaturated carbon bonds remains the same,resulting in conversion into C20:1; the other conversion path is deep hydrogenation to the fully saturated component C18:0.The ratio ofk4tok2isS2=k4/k2=2.17,which indicates that the conversion from C18:1 to C20:1 has a higher probability and that deep hydrogenation can be avoided.Some of the C18:0 components were converted to C20:0 due to carbon chain growth,and the selectivity coefficientS3=k2/k3=1.37,indicating that the formation rate of C18:0 from C18:1 hydrogenation is 1.37 times that of C20:0 from C18:0.
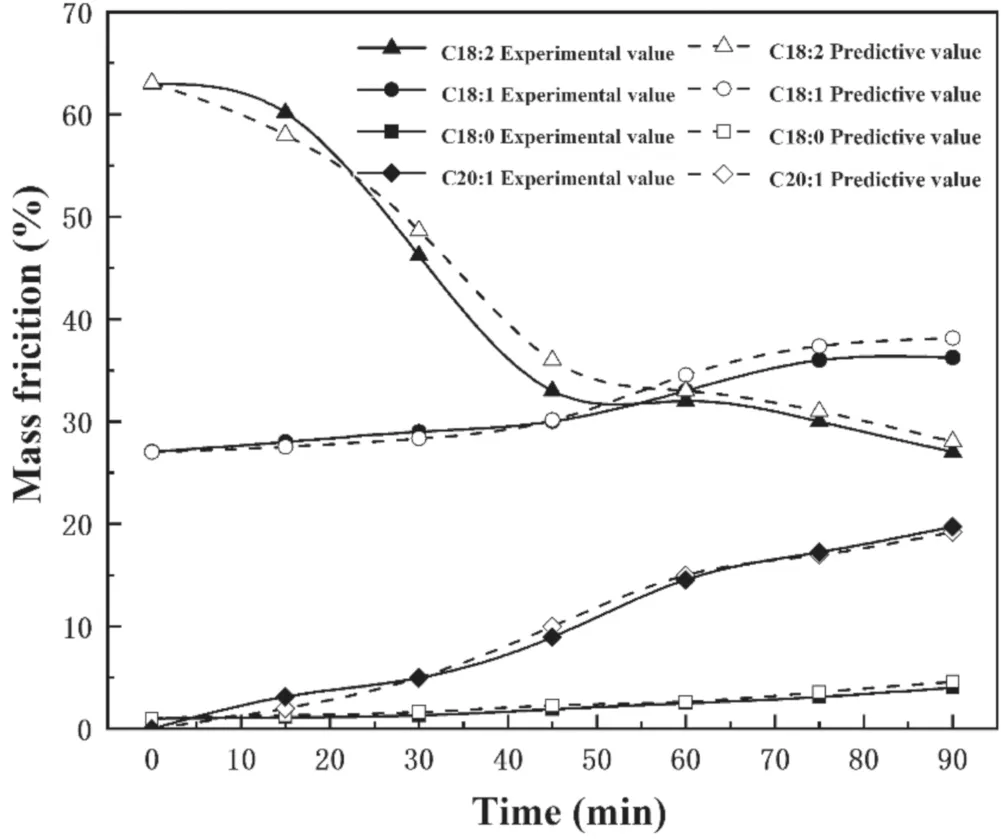
Figure 8.Comparison of the experimental and calculated values of the C18:2,C18:1,and C18:0 mass fractions of PHSME with reaction time.
In conclusion,in the discharge reaction system,the hydrogenation rate of polyunsaturated fatty acid esters is larger than that of monounsaturated fatty acid esters,and it has good selectivity for the formation of monounsaturated products,which is very beneficial for the comprehensive quality improvement of biodiesel.
4.Conclusions
A DBD hydrogenation reaction system was constructed to achieve the selective hydrogenation of biodiesel at low temperatures and under normal pressure,using soybean methyl ester as the raw material and Raney-Ni as the catalyst.Research into biodiesel upgrading was carried out,and the main conclusions are as follows:
(1) The hydrogenation depth of biodiesel first increased and then decreased with an increase in the gas flow rate; it increased and then remained stable with an increase in the working voltage,the SME circulation flow rate and the reaction time.With an increase in the working voltage,gas flow rate,and reaction time,the hydrogenation selectivity of the monounsaturated components produced showed a trend of first increasing and then declining; it first increased and then remained stable with an increase of the SME circulation flow rate.With the preferred operating parameter combination,the polyunsaturated components in SME were reduced by 57.04% and hydrogenated to monounsaturated components with a selectivity of 77.75%.
(2) The hydrogenation reaction of SME can be described by a quasi first-order reaction model.The calculated reaction rate constants indicate that under DBD reaction conditions,the hydrogenation of biodiesel has high selectivity for the formation of monounsaturated components.
Acknowledgments
This work was supported by National Natural Science Foundation of China (No.51761145011),the Priority Academic Program Development of Jiangsu Higher Education Institutions(PAPD),and the Jiangsu University Senior Talent Fund Project (No.12811020026).
 Plasma Science and Technology2021年9期
Plasma Science and Technology2021年9期
- Plasma Science and Technology的其它文章
- Recent results of fusion triple product on EAST tokamak
- Suppression and mitigation of inter-ELM high-frequency Alfvén-like mode by resonant magnetic perturbation in EAST
- Simulations of NBI fast ion loss in the presence of toroidal field ripple on EAST
- Reconstructions of velocity distributions from fast-ion D-alpha (FIDA) measurements on EAST
- Tomography of emissivity for Doppler coherence imaging spectroscopy diagnostic in HL-2A
- Comparison of natural grassy ELM behavior in favorable/unfavorable Bt in EAST
