Micro-positron emission tomography imaging of angiogenesis based on 18 F-RGD for assessing liver metastasis of colorectal cancer
Ming-Yu Zhng , ,Hui-Jie Jing , ,Ho Jing ,Rong-Jun Zhng ,Zhen-Chng Wng ,
a Department of Nuclear Medicine, Beijing Friendship Hospital, Capital Medical University, Beijing 10 0 050, China
b Department of Radiology, The Second Affiliated Hospital of Harbin Medical University, Harbin 150086, China
c Jiangsu Institute of Nuclear Medicine, Wuxi 214063, China
d Department of Radiology, Beijing Friendship Hospital, Capital Medical University, Beijing 10 0 050, China
Keywords:RGD peptide Positron emission tomography Colorectal liver metastases Vascular endothelial growth factor
ABSTRACT Background: Positron emission tomography (PET) imaging is a non-invasive method to visualize and quantify the tumor microenvironment.This study aimed to explore the feasibility of 18 F-AIF-NOTAE[PEG4 -c(RGDfk)]2 (denoted as 18 F-RGD) PET quantitative parameters to distinguish the angiogenesis in colorectal cancer (CRC) mice which has different metastatic potential.Methods: Twenty LoVo and twenty LS174T of CRC liver metastases animal models were established by implantation of human CRC cell lines via intrasplenic injection.Radiotracer-based micro-PET imaging of animal model was performed and the uptake of 18 F-RGD tracer in the tumor tissues was quantified as tumor-to-liver maximum or mean standardized uptake value (SUVmax or SUVmean) ratio.Pearson correlation was used to analyze the relationship between radioactive parameters and tumor markers.Results: The SUVmax and SUVmean ratios of LoVo model were significantly higher than those of LS174T in both liver metastasis and primary tumor lesions (P < 0.05).A significant difference was observed in both vascular endothelial growth factor (VEGF) and Ki67 expressions between LoVo and LS174T primary tumors (P < 0.05).The tumor-to-liver SUVmax or SUVmean ratio of 18 F-RGD showed a moderate correlation with VEGF expression (r=0.5700,P=0.001 and r=0.6657,P < 0.001,respectively),but the SUVmean ration showed a weak correlation with Ki67 expression (r=0.3706,P < 0.05).The areas under the receiver operating characteristic (ROC) curves of 18 F-RGD SUVmean ratio,SUVmax ratio for differentiating LoVo from LS174T tumor were 0.801 and 0.759,respectively.Conclusions: The tumor-to-liver SUVmean ratio of 18 F-RGD was a promising image parameter for the process of monitoring tumor angiogenesis in CRC xenograft mice model.
Introduction
Colorectal cancer (CRC) is the third most common cancer worldwide and liver metastasis is one of the main causes of CRCrelated death [1,2].Although the progression-free survival of multiple cancer has been significantly increased due to early detection and precise medicine,metastasis still means incurable for most cancers.Almost all of the CRC patients with liver metastasis have poor prognosis.
The accumulation of tumor metastasis molecular mechanisms over the past decade has given us an in-depth understanding of the biological behavior of metastatic progression in various kinds of cancers [3,4].Angiogenesis is defined as the formation of new vessels from the pre-existing microvasculature.The supplement of neovascularization is the key factor in malignant tumor growth,progression,and metastasis [5,6].The neovascularization in tumor is complex and disorganized.Vascular endothelial growth factor (VEGF) has been identified as the most important regulator of tumor angiogenesis,which is widely expressed in a variety of tumors.The overexpressed VEGF in tumor cells or tumor microenvironment can promote CRC tumor growth and hematogenous metastasis by stimulating angiogenesis [7,8].Integrins are cell adhesion receptors for extracellular matrix proteins,and are highly expressed on the surface of various tumor cells and neovascular endothelial cells.Tumor cells can migrate effectively on extracellular matrix substrates,and the multiple integrin functioning contributes to this process and plays an important role in tumor angiogenesis.Among all integrins,integrinαvβ3 was most strongly involved in the regulation of angiogenesis [9] .The specific interaction between integrinαvβ3 and VEGF receptors (VEGFRs) is considered crucial for tumor growth and metastasis [10] .
Positron emission tomography (PET) imaging is a non-invasive method to visualize and quantify the tumor microenvironment,such as proliferation and angiogenesis.Arginine-glycine-aspartic acid (RGD)-based peptides are well-known to specifically bind with integrinαvβ3 and considered a promising PET tracer for monitoring the status of tumor angiogenesis [11] .However,there are only a few reports on the prediction of tumor metastatic potential based on assessment of tumor angiogenesis status.The present study investigated the feasibility of dimeric RGD peptides-based PET quantitative parameters to distinguish the angiogenesis in CRC mice.
Materials and methods
Cell culture
The human CRC cell lines LoVo and LS174T were purchased from the Type Culture Collection of the Chinese Academy of Sciences,Shanghai,China.LS174T cell line was derived from the primary colorectal adenocarcinoma,Dukes’ type B;LoVo cell line was derived from the left supraclavicular metastatic site of colorectal adenocarcinoma,Dukes’ type C.The LoVo and LS174T cell lines were maintained in Dulbecco’s Modified Eagle Medium (DMEM,Gibco Corporation,Grand Island,NY USA) supplemented with 10%fetal bovine serum (Hyclone,Logan,Utah,USA) and 1% penicillinstreptomycin (Beyotime Biotechnology,Shanghai,China).All cells were cultured in a humidified atmosphere containing 5% CO 2 at 37 °C.
Animal model
Five-week-old female BALA/C nude mice (weighing 16-18 g)were purchased from Animal Laboratory of Cavens Corporate of Changzhou (Changzhou,China).Colorectal liver metastases (CLM)xenograft models were established by injecting LoVo or LS174T cells (2.0 × 107cells in 0.15 mL of phosphate-buffered saline) into spleen which anesthetized by intraperitoneal injection of 10% chloral hydrate,respectively (n=20).All the animals were housed in an environment with temperature of 22 ± 1 °C,relative humidity of 50% ± 1% and a light/dark cycle of 12/12 h.All animal experiments were conducted in compliance with the protocols approved by the Institutional Animal Care and Use Committee (IACUC) of Jiangsu Institute of Nuclear Medicine.The body weight of nude mice was recorded every three days.
Radiopharmaceutical preparation
18F-AIF-NOTA-E[PEG 4 -c(RGDfk)] 2 (denoted as18F-RGD) was acquired by Jiangsu Key Laboratory of Molecular Nuclear Medicine,Jiangsu Institute of Nuclear Medicine (Wuxi,China).Synthesis of 18 F-RGD has been described previously [12] .18 F-RGD was a glutamic acid linked dimeric RGD labeled with NOTA-18F-AIF that has been proved to be safe and stable for PET imaging [11] .
Cellular uptake
The cellular uptake was carried out according to our previous study [13] .LoVo and LS174T cells were maintained in corresponding medium for 24 h before18F-RGD cellular uptake.For experimental group,the cells,18F-RGD and buffer (DMEM containing 0.2% bovine serum albumin) were mixed into a glass test tube(2 mL).For block group,the cells,18F-RGD and inhibitor buffer(0.045 μg unlabeled RGD diluted with 100μL DMEM buffer which contains 0.2% bovine serum albumin) were mixed into a glass test tube (2 mL).All the glass test tubes were incubated at 37 °C for 1 h.The tubes were divided into three groups:Group O was used as a control tube,composing of 100μL radionuclides and 200μL buffer (DMEM buffer with 0.2% bovine serum albumin);Group T was used to measure the radionuclide dose,containing 100μL radionuclides;Group X,was composed of 100μL radionuclides,100μL cell suspension and 100μL corresponding buffer.The cellular uptake was normalized to 5 × 105cells/tube;each cell line was tested three times.After 1 h incubation,the O and X groups were centrifuged at 1000 rpm for 5 min and the supernatants were removed.The radioactivity of each tube was accurately measured using Automatic Gamma Counter (PerkinElmer 2480,Waltham,MA,USA).The cellular uptake ratio was calculated using the following formula:[X(cpm) -O(cpm)]/T(cpm) × 100%.cpm:counts per minute.Liver metastasis rate was defined as:the number of mice with liver metastasis divided by the number of mice with primary tumor in spleen.
Micro-PET imaging and analysis
18F-RGD PET imaging of LoVo (n=20) and LS174T (n=20)CLM mice models was performed seven weeks after implantation.Six-minute static18F-RGD PET image was acquired 1 h (radiopharmaceutical administration time in mice) after injection via tail vein (about 9.25 MBq,250μCi).All the mice were anesthetized with 2% isoflurane in 100% oxygen with a flow rate of 2 L/min prior to imaging.Micro-PET scans were acquired in 3-dimensional mode using an Inveon micro-PET scanner (Siemens Medical Solutions,Erlangen,Germany) with an ordered-subset expectation maximization/maximum:matrix,128 × 128 × 159;pixel size,0.86 × 0.86 × 0.8 mm;β-value,1.5,with uniform resolution.PET images were reconstructed and postprocessed using Inveon Acquisition Workplace software (version 2.0,Siemens Preclinical Solutions).
Regions of interests (ROIs) were drawn on images around the entire liver metastasis or primary tumor lesions and normal liver tissue using ASI Pro VM 6.8.6.9 software (Concorde Microsystems,Knoxville,TN,USA).The maximum or mean standardized uptake value (SUVmax or SUVmean) ratio was calculated as the tumorto-liver SUVmax or SUVmean (T/L ratio).All micro-PET imaging procedures were conducted according to protocol approved by the Jiangsu Institute of Nuclear Medicine Animal Care and Use Committee.
Immunohistochemical staining
The tumor specimens were fixed in 10% formalin for 48 h,paraffin-embedded,and cut into 3μm-thick sections.Immunohistochemical staining was performed as previously described [14] .Briefly,the slides were incubated with anti-Ki67(1:100,ab16 6 67,Abcam,Cambridge,UK) or anti-VEGF (1:200,ab46154,Abcam) at 4 °C overnight.Next,the slides were incubated with horseradish peroxidase-labeled goat anti-mouse or anti-rabbit secondary antibody (Boster,Wuhan,China) at room temperature followed by counterstaining with hematoxylin.The staining was observed under a BX53 Olympus microscope (Olympus,Tokyo,Japan) at magnification × 200.A brown-yellow staining was defined as positive expression.Ki67 or VEGF protein were quantitated by Image-J software (NIH,Bethesda,MD,USA).
Statistical analysis
Data were expressed as mean ± standard deviation (SD).Statistical analyses were performed with R version 3.6.1 (www.R-project.org).The difference between two groups was assessed by using Student’s unpairedt-test.The Fisher exact test was used for comparing differences in liver metastatic potentialinvivo.The difference between survival curves of two groups was assessed by using Gehan-Breslow-Wilcoxon test.The correlation between the RGD parameters and tumor marker was analyzed based on Pearson correlation analysis.The receiver operating characteristic (ROC) curve was used to differentiate LoVo tumor from LS174T tumor.APvalue<0.05 was considered statistically significant.
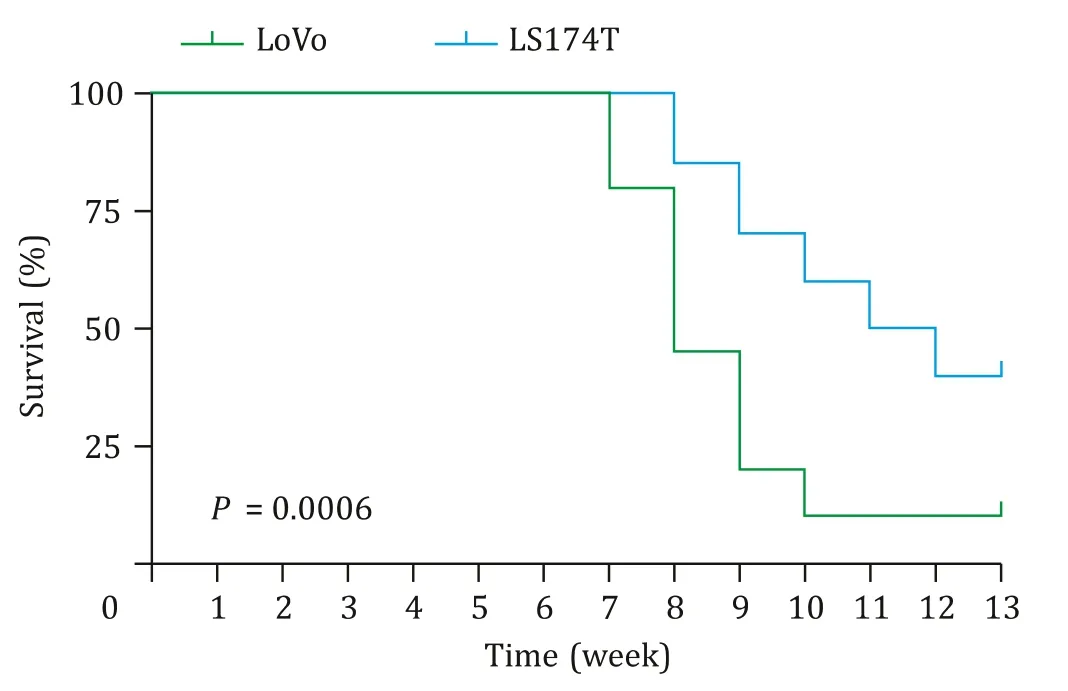
Fig.1.Survival analysis of LoVo (n=20) and LS174T (n=20) liver metastasis model.
Results
Comparison of metastatic potential and malignancy between the two models
Firstly,we calculated the tumor formation rate (including primary tumor and liver metastasis) of CLM model mice to evaluate theinvivometastatic potential of LoVo and LS174T CRC cells(Table 1).Primary tumor and liver metastasis were confirmed by pathological.The liver metastasis rate of LoVo-CLM mice was significantly higher than that of LS174T mice (66.67% vs.41.67%,P=0.039).In addition,the median survival time of LoVo and LS174T CLM models were 8.0 and 11.5 weeks,respectively (P<0.001,Fig.1).
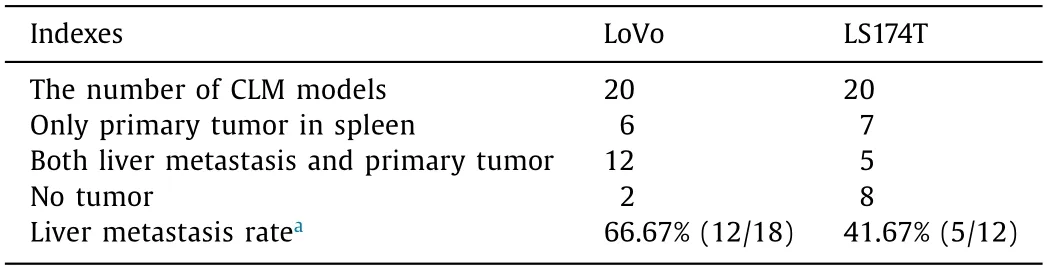
Table 1 The liver metastasis rate of CRC models.
In vitro cellular 18 F-RGD uptake analysis of LoVo and LS174T cell lines
The cellular uptake of LoVo (3.07% ± 0.28%) at 1 h tended to be higher than that of LS174T (2.43% ± 0.63%),however,there was no significant difference (P>0.05).Compared with the data of 1 h experimental group,the cellular uptake of both LoVo (1.61%± 0.04%) and LS174T (0.88% ± 0.06%) cells in 1 h block group were decreased significantly (P<0.05).
Comparison of 18 F-RGD parameters between LoVo and LS174T models
The liver metastasis and primary tumor in spleen were confirmed by HE staining (Fig.2 A).The 6-minute static scan of whole-body18F-RGD PET was shown in Fig.2 B.LoVo CLM model had a significantly higher SUVmean ratio and SUVmax ratio values in both liver metastasis and primary tumor than LS174T model (P<0.05) (Table 2,Fig.2 C).In addition,the18F-RGD parameters of SUVmean ratio and SUVmax ratio in LoVo and LS174T primary tumor were higher than those of corresponding liver metastasis tumor.
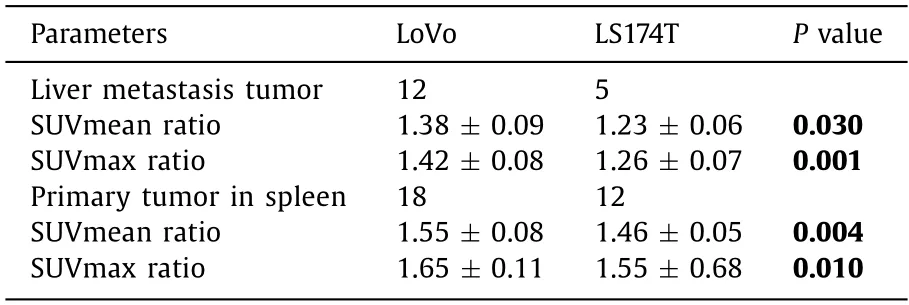
Table 2 The18 F-RGD parameters in LoVo and LS174T CLM models.
Tumor markers of VEGF and Ki67 expressions in LoVo and LS174T primary tumor
Immunohistochemical staining demonstrated that VEGF protein was mainly expressed in the cytoplasm and occasionally in the nucleus,whereas Ki67 protein was expressed in cell nucleus (Fig.3 C).Both VEGF and Ki67 expressions in LoVo tumor were higher than those in LS174T tumor (Fig.3 A and B,P<0.05).
Correlation analysis
No correlation was seen between18F-RGD SUVmax ratio and Ki67 expression (r=0.3334,P=0.0718).However,a weak correlation was found between18F-RGD SUVmean ratio and Ki67 expression (r=0.3706,P=0.0438) (Fig.4).On the contrary,a moderate correlation was found between18F-RGD SUVmax ratio or SUVmean ratio and VEGF expression in primary tumor (r=0.5700,P=0.001 andr=0.6657,P<0.001,respectively).
Diagnostic performance of 18 F-RGD parameters to differentiate LoVo from LS174T animal models in tumor angiogenesis
The ROC curve analysis was summarized in Table 3 and Fig.5 .The areas under the ROC curves (AUC) of18F-RGD SUVmean ratio,SUVmax ratio and SUVmean ratio combined with SUVmax ratio for differentiate LoVo from LS174T animal models were 0.801,0.759 and 0.787,respectively.The optimal cut-off value to differentiate LoVo from LS174T animal models was 1.551 for SUVmean ratio,1.629 for SUVmax ratio and 0.759 for SUVmean ratio combined with SUVmax ratio.In addition,the sensitivity and specificity of SUVmean ratio to differentiate LoVo from LS174T tumors in mice models were 100% and 61.1%,respectively,the same as SUVmean combined with SUVmax ratio.
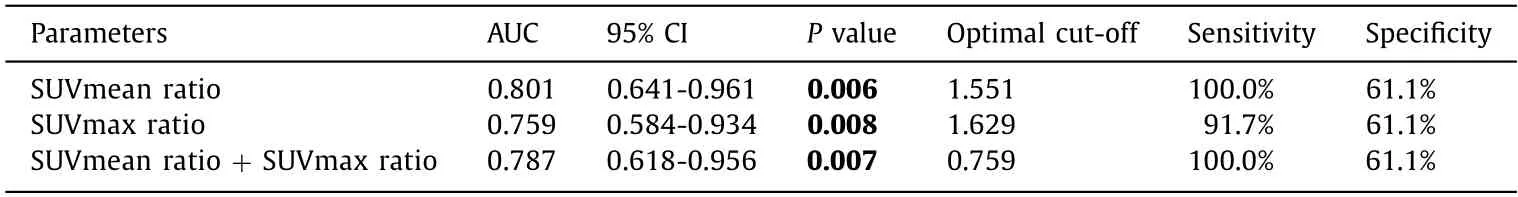
Table 3 ROC curve analysis for 18 F-RGD parameters in LoVo and LS174T tumors.
Discussion
Tumor metastasis is the leading cause of CRC-related death.Liver is the most common site of CRC metastasis [15] .Understanding the biological characteristics of tumor metastasis is the essential prerequisite for predicting CRC metastasis.Angiogenesis provides favorable conditions for tumor growth and metastasis.Folkman [16] suggested that tumors transform into metastatic potential must undergo an“angiogenic switch”by perturbing the balance of proangiogenic and antiangiogenic factors.Tumors with VEGF-overexpression was more prone to“angiogenic switch”,consequently converting to metastatic potential.
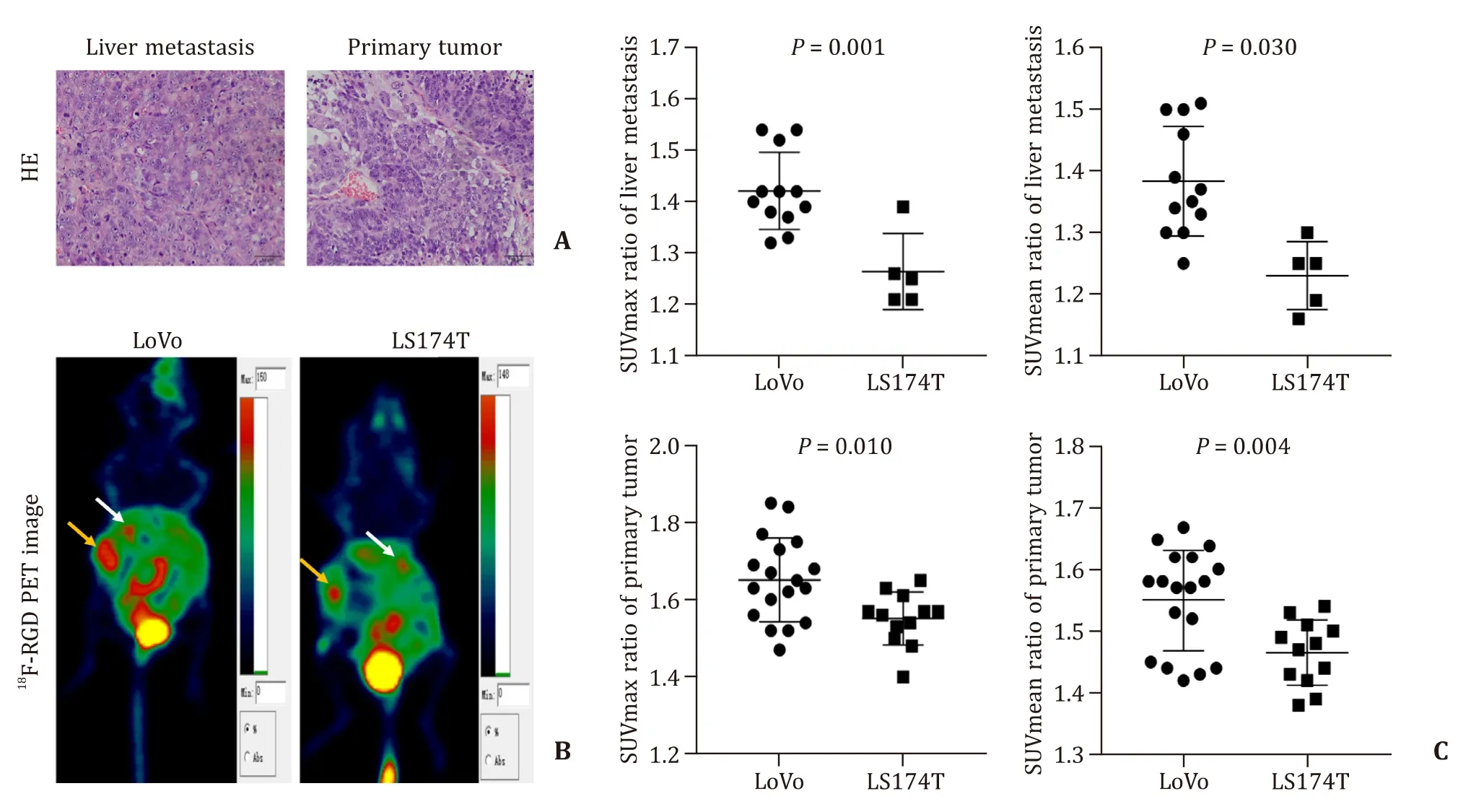
Fig.2.In vivo whole-body micro-PET images of 18 F-RGD.A:HE staining of liver metastasis tumor and primary tumor (original magnification × 200).B:White arrows show the uptake of 18 F-RGD in liver metastasis.Yellow arrows show the uptake of 18 F-RGD of the primary tumor in spleen.C:The comparison of SUVmax ratio and SUVmean ratio between the two cell lines.
In recent years,advances in the field of anti-angiogenesis therapy in oncology have provided more options for the treatment of cancer patients.More and more preclinical and clinical researches focused on how to monitor the tumor angiogenesis and treatment response by using multiple imaging modalities such as PET,SPECT,molecular MRI,targeted ultrasound,or optical imaging [17] .Integrins are strongly involved in mediating adhesion events during tumor metastasis by activating many cellular signaling pathways,and the expression of integrinαvβ3 is mostly correlated with tumor metastatic potential [18,19].RGD peptides-based PET imaging for evaluation tumor angiogenesis and proliferation is a promising non-invasive method for understanding the tumor biological behavior [20] .In addition,integrinαvβ3 plays an important role in regulating tumor angiogenesis and proliferation.Our results evidenced a significant correlation between the18F-RGD parameters and VEGF expression in primary CRC tumor.Moreover,the SUVmean ratio of18F-RGD in primary tumor has the ability to distinguish LoVo tumor from LS174T tumor in mice model,which had different tumor metastatic potential.
In this study,we first compared the liver metastatic potential of LoVo and LS174T cellsinvivoby establishing a liver metastasis model.The results showed that LoVo-CLM model had a higher liver metastatic rate than LS174T model (66.67% vs.41.67%).In addition,LoVo-CLM mice has a shorter survival time compared to LS174TCLM mice (8 vs.11.5 weeks),suggesting that LoVo cells exhibited a stronger metastatic capability than LS174T cells.The result was the same with our previous study [21] .
The18F-RGD radiological parameter was a potential image marker to quantify the angiogenesis of CRC in mice model.For the cellular uptake of18F-RGD,a low RGD uptake was found in two cell lines and there was no statistical difference between LoVo and LS174T cells at 1 h post-incubation.As we know integrinαvβ3 is an extracellular matrix adhesion receptor,and it is overexpressed in neovascularization.In cellular18F-RGD uptake assay,LoVo and LS174T cells were suspended in buffer that lack of extracellular matrix and expressed low integrinαvβ3,hence the results showed a weak18F-RGD uptakeinvitro.The reason for this phenomenon could be explained as an inactive or“off”state of integrins expressed on cell surfaces,in which they do not bind ligands [22] .In order to explore the status of tumor neovascularization in solid of CRC mice,we performed18F-RGD PET imaging 1 h post-injection and compared the radiological parameters between LoVo and LS174T mice with different metastatic potential.We delineated the ROI in liver metastases and primary splenic tumors according to corresponding anatomical locations.The encouraging data showed that the uptake of18F-RGD in tissue level was superior to that in cellular level,and the parameters of18F-RGD SUVmean/SUVmax ratio were capable of distinguishing the differences in angiogenesis between LoVo and LS174T animal models.Our data are consistent with Avraamides et al.that tumor tissues with neovascular networks have a good uptake of18F-RGD [23] .Our results demonstrated that tumors with high metastatic potential are prone to high18F-RGD uptake,and acquisition of angiogenic properties accelerate the change from a quiescent to an invasive phenotype known as“angiogenic switch”[24] .In addition,we also found that the18F-RGD parameters in primary tumors were higher than those in liver metastases in the same animal model.Primary tumor formed earlier than liver metastasis and has a more complete neovascularization network,therefore a higher uptake of18F-RGD.
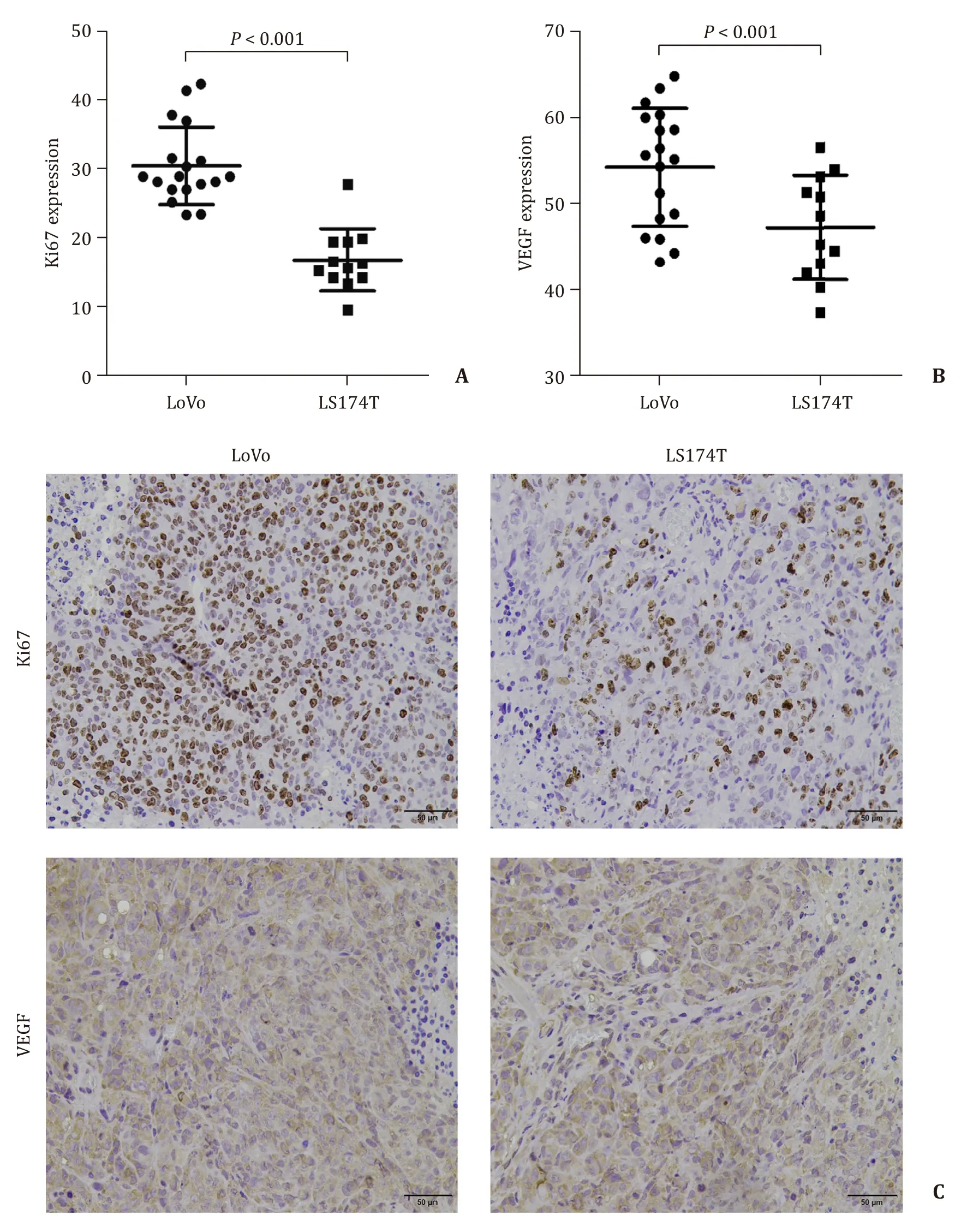
Fig.3.Comparison of Ki67 and VEGF expression protein between LoVo and LS174T primary tumors.Quantification of Ki67 (A) and VEGF (B) from immunohistochemical staining.C:Immunohistochemical staining for Ki67/VEGF (brown) in LoVo (left side) and LS174T (right side) primary tumor tissues,respectively (original magnification × 200).
VEGF,which is an important factor in tumor angiogenesis,and Ki67,which is a nucleolar protein widely appreciated as a cell proliferation,are both associated with tumor growth and metastasis [25,26].Our quantification of immunohistochemical staining in primary tumor showed that the expression of VEGF and Ki67 in LoVo tissues were stronger than that in LS174T tissues,combined with the results of correlation analysis,a weak correlation between18F-RGD SUVmean ratio and Ki67 expression and a moderate correlation between18F-RGD SUVmax ratio/SUVmean ratio and VEGF expression in primary tumor,indicating that the SUVmax ratio/SUVmean ratio of18F-RGD may be recognized as an ideal radiation maker to reflect tumor angiogenesis in CLM mice model.Our data were consistent with previous studies that18F-RGD is a promising tracer for tumor angiogenesis imaging and monitoring anti-angiogenesis therapy in solid tumor [27,28].A study from Isal et al.also verified that the expression of integrinαvβ3,which is targeted by RGD-based peptides tracer,was associated with cell proliferation in glioblastoma [29] .
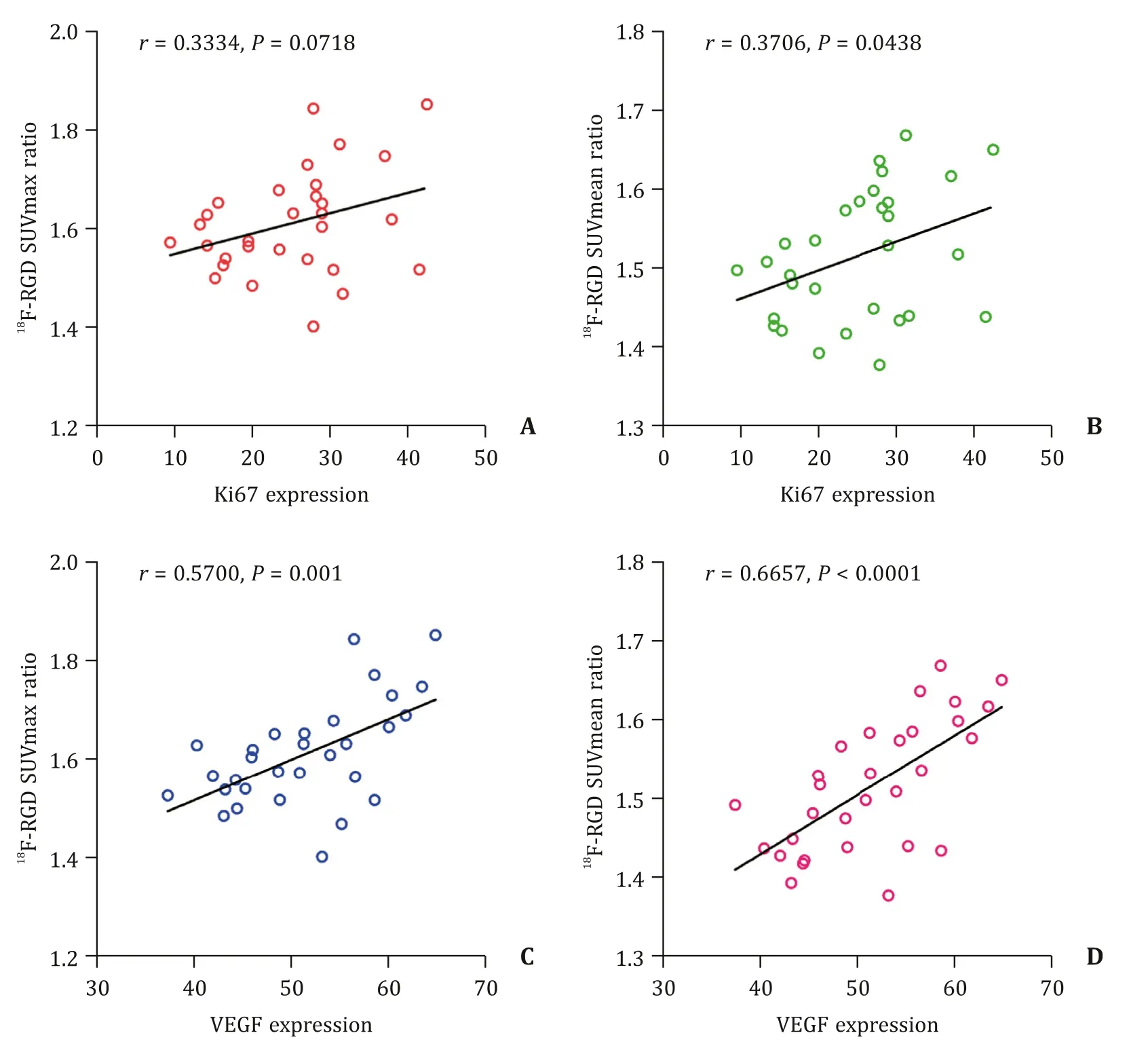
Fig.4.Correlation analysis between RGD parameters and biomarkers.A:correlation of 18F-RGD SUVmax ratio and Ki67 expression;B:correlation of 18 F-RGD SUVmean ratio and Ki67 expression;C:correlation of 18 F-RGD SUVmax ratio and VEGF expression;D:correlation of 18 F-RGD SUVmean ratio and VEGF expression.
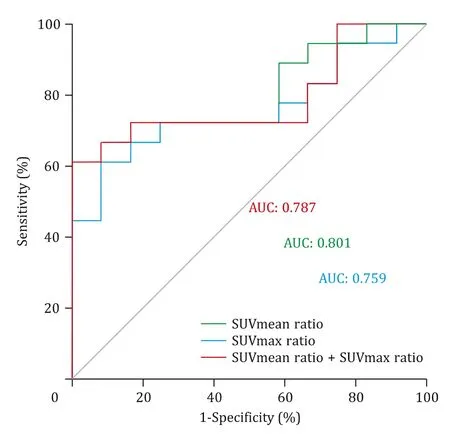
Fig.5.Receiver operating characteristic (ROC) curves analysis of different RGD parameters to distinguish LoVo from LS174T animal models.AUC:the areas under the ROC curves.
According to the ROC curve analysis,18F-RGD parameters of SUVmean ratio and SUVmax ratio had the capacity to distinguish LoVo tumor from LS174T tumor,among these parameters,SUVmean ratio showed the highest AUC.Our results were in line with the literature as recent research showed that the contrast-to-noise indexes of the Gd-RGD tracers in MRI were suitable for differentiating hepatocellular carcinoma tissues with high metastatic potential from those with low metastatic potential [30] .The present study had limitations.On the one hand,only two kinds of CRC cell lines with different metastatic potentials were included in the comparative study.On the other hand,due to the individual differences in nude mice model,eight mice in LS174T group and two mice in LoVo group failed in tumor formation.Future research is needed with more types of CRC cell lines and enlarged model size to reveal the effect of18F-RGD on predicting the metastatic potential in CRC.
In conclusion,18F-RGD was a promising tracer for tumor imaging and monitoring angiogenesis microenvironment in CRC xenograft mice model.SUVmean ratio of18F-RGD could be used for differentiating LoVo tumor with high metastatic potential from LS174T tumor with low metastatic potential.It was a suitable parameter for predicting the metastatic potential of CRC in animal models.
Acknowledgments
None.
CRediTauthorshipcontributionstatement
Ming-YuZhang:Conceptualization,Data curation,Formal analysis,Funding acquisition,Investigation,Methodology,Writing -original draft.Hui-JieJiang:Conceptualization,Funding acquisition,Project administration,Writing -original draft,Writing -review &editing.HaoJiang:Formal analysis,Investigation,Methodology,Writing -review &editing.Rong-JunZhang:Conceptualization,Investigation,Methodology,Writing -review &editing.Zhen-ChangWang:Conceptualization,Funding acquisition,Project administration,Resources,Supervision,Writing -review &editing.
Funding
This study was supported by grants from the National Key Research and Development Program of China (2019YFC0118100),the National Natural Science Foundation of China (81671760,81873910 and 82001860),Scientific Research Transformation Special Fund of Heilongjiang Academy of Medical Sciences (2018415),Scientific Research Project of Health and Family Planning Commission of Heilongjiang Province (201812 and 201622),Beijing Scholars Program ([2015] 160),Beijing Friendship Hospital Seed Project of Capital Medical University (YYZZ201919),and Beijing Postdoctoral Research Foundation (2020-Z2-022).
Ethicalapproval
This study was approved by the Ethics Committee of the Beijing Friendship Hospital of Capital Medical University.All animal experiments were conducted in compliance with the protocols approved by the Institutional Animal Care and Use Committee (IACUC) of Jiangsu Institute of Nuclear Medicine.
Competinginterest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
 Hepatobiliary & Pancreatic Diseases International2021年4期
Hepatobiliary & Pancreatic Diseases International2021年4期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- Recurrence and survival following microwave,radiofrequency ablation,and hepatic resection of colorectal liver metastases:A systematic review and network meta-analysis
- Mitochondria:A critical hub for hepatic stellate cells activation during chronic liver diseases
- Symptomatic Val122del mutated hereditary transthyretin amyloidosis:Need for early diagnosis and prioritization for heart and liver transplantation
- The growth rate of hepatocellular carcinoma is different with different TNM stages at diagnosis
- Overexpression of anillin is related to poor prognosis in patients with hepatocellular carcinoma
- Fisetin mitigates hepatic ischemia-reperfusion injury by regulating GSK3 β/AMPK/NLRP3 inflammasome pathway
