Potential for Control of A. Flavus and Aflatoxin B1 in Vitro and in Stored Egyptian Shelled Peanuts using Gaseous Ozone Treatment (英文原文)
Yousef Sultan, Naresh Magan✉
(1. Applied Mycology Group, Environment and AgriFood Theme, Cranfield University,Cranfield, Beds., MK43 0AL, U.K.; 2.Department of Food Toxins and Contaminants,National Research Centre, Dokki, Cairo 12622, Egypt.)
Abstract: The objective of this study was to examine the effect of gaseous ozone (O3) on (a) germination, (b)mycelial growth, (c) aflatoxin B1 (AFB1) production by strains of Aspergillus flavus at different water activity(aw, 0.89~0.97=13.1%~24% moisture content) and 25 oC in vitro. In addition, taking Egyptian peanuts as a research object, shelled peanuts at 0.93 aw were inoculated with two concentrations of A. flavus conidia (103,105 conidia/g) and exposed to gaseous O3 and then stored. In all cases, exposure was for 30 min at 6 L/min.Generally, >100 ppm O3 significantly inhibited conidial germination of A. flavus strains (EGP-B07; SRRC-G 1907) on a defined yeast sucrose medium within 48 hrs. However, exposure of growing colonies of A. flavus to O3 with up to 300 ppm, had no effect on subsequent colony extension. The same concentration significantly affected AFB1 production, but only at 0.89 aw. Populations of both A. flavus strains in stored shelled peanuts exposed at 0.93 aw were significantly decreased at 100~400 ppm O3. However, AFB1 production was only significantly reduced in the 400 ppm O3 treatments at both inoculum load levels. These results are discussed in the context of O3 gas use for controlling A. flavus populations and AFB1 in shelled peanuts post-harvest.
Key words: ozonation; Egyptian peanuts; storage; aflatoxins; germination; growth; toxin
1. INTRODUCTION
Fungal growth on stored cereals and nuts causes significant reductions in both the quality and nutritional value of the commodity. In peanuts, fungal spoilage can also result in contamination with toxic secondary metabolites, especially aflatoxins. Aflatoxin B1(AFB1) is a Class 1a carcinogen. Drought stress prior to harvest or poor drying and storage can result in colonisation byAspergillus flavusandA.parasiticusand concomitant AFB1contamination of both peanut and tree nuts. Indeed, 20%~25% of quality losses can be due to mycotoxigenic spoilage moulds and mycotoxins[1-2].
The use of modified atmosphere storage,monitoring of volatile organic compounds and chemical fumigation, especially during post-harvest storage have all been examined for use especially in silos, for hygiene purposes to eliminate both fungal contaminants and pests[3]. However, use of gaseous fumigants are limited because of the problems of residues and consumer acceptance from a food safety perspective. Thus alternative approaches have been considered to reduce fungal contamination by mycotoxigenic species and try and reduce post-harvest losses[3].
The key gaseous systems examined have been sulphur dioxide (SO2) and ozone (O3). Sulphur dioxide (SO2) is a gaseous food additive, which has been used as a disinfectant agent historically for many years by burning sulphur and using the resultant fumes[4-5]. SO2is also classified as a food grade preservative (E220) because of its antimicrobial properties. It has been used as an anti-fungal agent for preservation of grapes and a range of processed foods including dried fruits, bakery products,breakfast cereals and beverages including white wine[6]. However, high concentrations of SO2can have human impacts including liver and cardiovascular disease, skin rashes and respiratory problems[7]. For controlling moulds and mycotoxins in cereals, Serre[8]showed that treatment of wheat grain with SO2required much higher concentrations because of absorption and binding of the SO2to the wheat grain, which minimised the antifungal activity.
Magan[4-5]showed that growth of mycotoxigenic fungi such asA. flavus,A. ochraceus, andA. terreuswas prevented by 50 ppm dissolved SO2at 0.995 and 0.95 water activity (aw). However, often at least 200 ppm SO2was necessary for a 1~2 log CFUs decrease in fungal populations in cereals at 0.96 and 0.92 aw. Pezley[9]found that binding of SO2to nutritional components of a food matrix enabled yeasts to become more resistant to higher concentrations of SO2. The products of solubility of SO2in water are very dependent on the pH level[10].They suggested that the effective dose of sodium metabisulphite used for the production of SO2for OTA control were about 650~700 ppm at 0.985 aw,400~500 ppm at 0.965 aw400~450 ppm at 0.93 aw.
Ozone (O3) is a powerful oxidant agent and unstable triatomic compound that breaks down to normal oxygen (O2) without any residue in the lower atmosphere[11].Gaseous O3is produced naturally as a colourless gas and has been used as a highly powerful oxidation agent and disinfectant of microorganisms and chemicals in the food industry[12].O3gas is produced naturally by two methods including the ultra-violet rays of the sun and lightning. It can also be produced artificially by three methods including UV-light, corona discharge method (CDM) and electrolytic O3generation(EOG). The corona-discharge method is the most common method for O3generation today[3,13].
O3gas has been commonly used in the food industry for sanitising packaging materials, raw materials, and storage facilities[14]. The most important advantage of O3over other remedial chemicals used in foodstuffs is that it is residue-free. It decomposes to diatomic oxygen rapidly due to its short half-life,which is about 20~50 min in the atmosphere and 1~10 min in water[15]. O3was also found to be effective in the destruction and detoxification of mycotoxins, including aflatoxins in some cases[16].
While effective against microorganisms,especially when applied to water-based systems care is needed when used in food production chains.O3can cause negative changes in the quality and nutritional value, especially related to oxidation of lipids, colour loss, degradation of some vitamins and phenolic compounds[16-17]. It has also been used for medical applications including treatment of foot ulcers in patients with diabetes[18]. However, care is needed as O3exposure at high doses above natural levels can cause harmful effects on health such as respiratory[19]and cardiovascular diseases[20]. The FDA legislative limit for exposure to O3is 0.1 ppm for eight hrs continuously or 0.3 ppm for 15 min according to Federal Occupational Safety and Health Administration (OSHA)[21].
Fungal species have variable sensitivity and tolerance to exposure of O3depending on concentration(ppm) x exposure time, spore morphology and also influenced by the type and moisture content of substrate[11]. El-Desouky et al.[22]found that the amount of AFB1production byA.flavusin (PDA)media were gradually decreased when increased the O3concentration time and exposure time. They also showed that the AFB1production byA. flavusin cereals such as wheat was degraded by 55%~77% after exposure to 40 ppm O3for 5~20 mins. Sahab et al.[23]reported that exposure of peanuts seeds to 40 ppm O3for 10 min inhibited AFB1and AFB2by 95% and 99%. However, the water availability conditions were not controlled in these studies. In recent studies of O3treatment of coffee to reduce populations ofAspergillus westerdijkiaeandAspergillus carbonariusand ochratoxin A (OTA) contamination it was found that even at 600 ppm concentration, it was diffiuclt to effectively reduce levels of OTA in this commodity[11].
The objectives of this study were (a) to investigate the efficacy of gaseous O3on germination, growth and AFB1production of two strains ofA. flavus(EGP-B07 isolated from peanuts; SRRC-G 1907, a reference strain) on a defined YES medium under different water activity (aw) conditions, (b) to evaluate the effect of O3treatment on theA. flavuspopulations in stored peanuts inoculated with different initial spore concentrations (103, 105CFUS/mL), and (c) quantification of the effect on AFB1contamination of the stored peanuts.
2. MATERIALS AND METHODS
2.1 Fungal strains
Aspergillus flavusEGP-B07 was isolated from Egyptian peanuts was used for inoculation either inin vitroorin situexperiments. The fungal strain was sub-cultured before examination on MEA. The spores were gently dislodged from the colony surface into suspensions of 10 mL sterile distilled water containing 0.05% Tween-20 in 25 mL Universal bottles. The resulting spore suspension was filtered through two layers of Miracloth (Merck, Darmstadt,Germany). Fungal spore concentration was determined using a hemocytometer and adjusted to 106spores/mL. This was used for inoculation of either media or peanuts. A type strain ofA. flavusSRRCG1907 was also used in these studies for comparison.
2.2 Media used in this study
Malt extract agar (MEA): Commercial MEA(Oxoid, Basingstoke, Hampshire, UK) was made using the supplier’s recommendation. A small amount of chloramphenicol (250 μg/mL) was added to inhibit bacterial growth and once the components were mixed, the substrate was autoclaved at 121 ℃and 1 atm for 15 min. The autoclaved medium was allowed to cool to approximately 50 ℃ before being aseptically poured into sterile 90mm diameter Petri dishes. Cooled solidified plates were kept at 4 ℃ in sealed polyethylene bags for a maximum period of 21 days.
Yeast extract sucrose agar (YES): YES was prepared by using 20 g of each yeast extract and agar, 150 g sucrose and 0.5 g MgSO4·7H2O in 885 mL distilled water. Once the components were mixed, the substrates were autoclaved and poured in 55 mm Petri plates. These were kept at 4 ℃ in closed polyethylene bags until required. Prior to use they were equilibrated at 25 ℃.
The water availability of the YES was adjusted using glycerol (9.2, 23 and 36.8 g/100 mL). These were mixed well and then used like water to obtain 0.95, 0.92 and 0.89 aw(= 19.5%~13.1% m.c.). The media were poured into 55 mm diameter Petri dishes(Fisher Scientific Ltd., Loughborough, Leicestershire,UK). All media awlevels were checked with an Aqualab TE3 instrument and found to be within 0.003 aw.
2.3 Ozonation method used in these studies
Ozone gas was generated from a laboratory corona discharge ozone generator (C-Lasky, Model CL-010-DS, AirTree Ozone Technology Co., Ltd.,Sijhih City, Taipei County 22150, Taiwan) using air at a flow rate 6 L/min[11]. Either YES plates or vessels containing peanuts (without lids) were placed in an O3exposure chamber (Kilner glass jar)and tightly closed. Ozone gas was applied through one tube and associated valve in the lid of the chamber obtain the required concentrations. The concentrations of O3in the exit gas from the chamber was measured via a second tube and valve O3Analyzer (Model UV 100, Eco Sensors Inc.,Santa Fe, New Mexico 87505 USA). This ensured that the treatments received the accurate concentrations of O3during the exposure period.
2.4 Effects of Ozone exposure on A. flavus conidial germination
A 100 µL aliquot ofA. flavus106spores/mL was spread plated onto YES medium at different awlevels (0.89, 0.92, 0.95 and 0.97 aw) and the plates were aseptically placed in the O3reactor without lids. The O3generator was adjusted to obtain 20, 40,100 and 250 ppm in the chamber for 30 min. at 6 L/min flow rate. After the treatment, plates were covered and incubated at 25 ℃ for 48 h.Periodically every 12 h two plugs (0.8 cm diameter)were removed and place don glass slides and stained with lacotophenol cotton blue, a cover slip carefully placed on the surface and germination examined. Four random microscopic fields per plug were examined to determine the spore germination of both strains. All experiments were carried out with three replicates and repeated once. Spores were considered to have germinated when the germ tube length was equal to or greater than the spore diameter[24]. Germinated spores were counted and recorded as a percentage of the total spore number.
2.5 Effects of ozone exposure on mycelial growth and AFB1 production
Duplicate Petri plates of YES medium at different awlevels were centrally inoculated with 5µL of the spore suspension (106/mL) of each strain and incubated at 25 ℃. When they had reached 0.6~0.8 cm colony diameter for the two strains ofA.flavusthe colnies were exposed to O3at 75, 100,150 and 300 ppm at 6 L/min for 30 mins. Treated plates were subsequently incubated again at 25 ℃for 72 h. The colony diameters of replicates were measured in two directions at right angles every 12 h. The growth against time and the slope of the linear growth phase was used to obtain the radial growth rate mm/day.
2.6 In situ effect of O3 on the population of A.flavus EGP-B07 and AFB1 production on shelled peanuts
The shelled peanuts were initially gamma irradiated at 12~15 KGys (Ref). This resulted in all contaminant microorganisms being killed by the treatment. A moisture adsorption curve was made to calculate the exact amount of water needed to modify the peanut samples to the target awlevels[25].The shelled peanut samples (25 gms) were placed in glass jars with microporous lids. They were divided into 2 groups and water added to obtain 0.93 aw(=16.5% m.c.). These were equilibrated in sealed environmental chambers overnight at 4 ℃ with regular mixing to allow effective equilibration. They were then equilibrated at 25 ℃ and inoculated with either 103or 105spores/g peanuts. Three replicate samples were aseptically exposed at 25 ℃ to gaseous O3at 100, 200 and 400 ppm at 6 L/min for 30 mins in a fume cupboard. The control was exposed to air in a similar way for 30 min. The treatments were stored separately in sealed environmental chambers.Each environmental chamber included 2 × 500 mls of a glycerol/water solution of 0.93 awto maintain the equilibrium relative humidity of the atmosphere the same as that of the shelled peanuts.
Populations ofA. flavusand AFB1levels in the stored shelled peanuts were measured before and after treatment and storage for 4 days at 25 ℃.Enumeration was done on MEA plates using serial dilution of 5 gm sub-samples of each replicate,spread plating of the washings in sterile water +tween 80 (0.125%) and plating 0.1 mL on each of three MEA Petri plates for each dilutions (10-2~10-6).These plates were incubated at 25 ℃ for 5~7 days and the populations ofA. flavusenumerated and the populations quantified on a dry weight basis (g/peanuts).
2.7 Extraction and quantification of AFB1 from media and peanut samples using HPLC
2.7.1 From agar media
The agar discs (5~6, 5 mm diameter) across the mycelial colonies on YES media exposed to different O3concentrations were transferred to 2mL Eppendorf tubes and weighed[26]. An aliquot of 800 μL chloroform was added to each Eppendorf and shaken for 1 hr using KS 501 digital orbital shaker (IKA(R)Werke GmbH & Co. KG, Germany). The chloroform extract was transferred to a new vial and dried gently under air for derivatization using the method of AOAC[27]and then analysed quantitatively using HPLC. A 200 µL stock solution of Aflatoxin mix standard in methanol (Supelco, Bellefonte, PA, USA), containing 200 ng B1, 60 ng B2, 200 ng G1and 60 ng G1, was dried under nitrogen gas and derivatized as for samples. Four concentrations were prepared for HPLC injection to make the standard curve. Detection limit of AFB1using HPLC was 0.8 ng/g.
2.7.2 Analysis of aflatoxin B1in peanuts
Three replicates of the peanut samples from each treatment were analysed for AFB1using an aflaprep column (Neogene Europe, wide bore). Five grams of ground sample was blended (1 min at high speed) with 1 g salt (NaCl) and 25 mL of 60% methanol. This was then filtered through Whatman No.1 filter paper and the filtrate was collected in a clean vessel. Ten millilitres of filtered extract was diluted 1:1 with phosphate buffered saline (PBS).The diluted extract (20 mL=2 g sample) was passed through an aflaprep column without drying via a 50 mL glass syringe reservoir attached to the column through an adaptor at a rate of about 1.5 to 2.0 mL per min. By removing the 50 mL glass syringe and the adaptor, the column was filled completely with 25% methanol then the reservoir was reattached to the adaptor. Twenty millilitres of 25% methanol was passed through the column at a rate of 2 mL per min(until air comes through the column). Two millilitres of methanol was pipetted into the column and carefully pushed through the column and collected in a 2 mL Eppendorf tube. The elution was dried gently under nitrogen for derivatization and then HPLC analysis. Detection limit of AFB1was 0.012 ng/g peanut seeds.
2.7.3 Derivatization of aflatoxins
Two hundred microlitre hexane and 50 μL trifluoroacetic acid (TFA) were added to the vial containing the sample or standard. The vial was capped, its contents vigorously mixed (30 s) and the mixture was left to stand for 5 min. To the mixture,950 mL H2O: acetonitrile (9:1) was then added and shaken vigorously for 30 s and left for 10 min to form two separate layers. The lower aqueous layer was filtered and used for analyses (AOAC, 2005).
2.7.4 HPLC conditions
The HPLC system used for Aflatoxins was an Agilent 1200 series system (Agilent, Berks., UK)with a fluorescence detector (FLD G1321A), an auto sampler ALS G1329A, FC/ALS therm G1330B,Degasser G1379B, Bin Bump G1312A and a C18(Phenomonex, Luna 5 micron, 150 × 4.6 mm) column joined to a pre-column (security guard, 4×3 mm cartridge, Phenomenex Luna). The mobile phase was methanol:water:acetonitrile (30:60:10, v/v/v)using an isocratic flow rate of 1 mL/min at 360 nm excitation and 440 nm emission wavelengths and a 25 min run time for AFs analyses. For OTA analyses,acetonitrile (57%): water (41%): acetic acid (2%)were isocratically used at the same flow rate at 333 nm excitation, 460 nm emission wavelengths.The run time for samples was 15 min with OTA being detected at 5.75 min.
2.8 Statistical analyses
Statistical significance was determined using Statistica version 9 (StatSoft Inc., Tulsa, OK, USA).The logarithmic values for CFUs ofA. flavusand AFB1levels (ng/g) were used for analyses. Means of growth rate, log10CFUs ofA. flavusand log10AFB1concentrations were determined by analysis of variance (ANOVA, two and three ways analyses)(P<0.05). Fisher’s LSD Method (α=0.05) was applied as well to compare significant differences between treatments and controls.
3. RESULTS
3.1 Efficacy of exposure to ozone on germination of A. flavus spores on media
Figure 1 shows the effect of exposure to different concentrations of O3for 30 min prior to incubation for up to 48 hrs for one strain. Similar results were obtained for both strains. In general, the percentage germination decreased gradually with the increase in O3dose and increased with the incubation time except at 0.92 awand especially for theA. flavusSRRC-G 1907 strain. Within 36 h incubation, the germination of this strain at 40 ppm (46%~64%)was higher than that at 20 ppm (23%~34%). This was confirmed by microscopic examination and plates of the germinating spores of both strains after 24 h at 0.92 aw. Spore germination ofA. flavusSRRC-G 1907 was more sensitive to ozonation thanA. flavusEGP-B07. The spores failed to germinate at 100 and 250 ppm during the incubation time for both strains at all awlevels except at 0.89 awand 100 ppm after 48 h. Statistically, incubation time(hrs), aw, strain type and their two, three and four way interactions onA. flavusgermination (%) were found to be significant. Exposure to gaseous O3,time and awhad the most significant impact on germination rates for both strains.
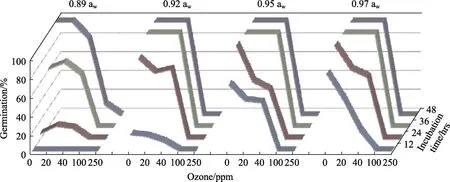
Fig.1 Effect of ozone concentration (ppm) on spore germination of A. flavus EGP-B07 on YES media over periods of 48 hrs. Ozone exposure was for 30 min at 6 L/min prior to incubation
3.2 Effects of ozone exposure on mycelial growth
Figure 2 shows the effect of 100 and 300 ppm O3on mycelial extension of growing colonies of both strains ofA. flavus. This showed that there was relatively little effect of O3treatment on the mycelial extension of the two strains over a period of 3 days after exposure. The effect was more noticeable at the lower awlevels (0.89 and 0.92 aw). Significant reductions in growth rate ofA. flavusEGP-B07 was observed as a result of O3treatment at 0.89 and 0.92 awand only at 0.92 awfor growth ofA. flavusSRRC-G 1907. The statistical results suggest that aw,type of strain and then only O3were significant factors affecting mycelial extension.
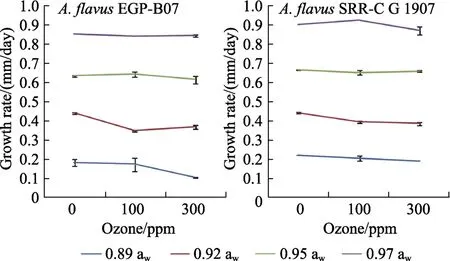
Fig.2 Relative growth rate of A. flavus EGP-B07 and strain SRRC-G 1907 on YES media at 25 ℃ for 3 days
Figure 3 shows the effect of treatment of growing colonies ofA. flavusEGP-B07 and SRRC-G 1907 with O3on AFB1production over 3 days incubation under different awconditions. When compared with controls (0 ppm), significant reduction of AFB1using the dose 300 ppm was observed at 0.89, 0.95 awbyA. flavusEGP-B07 and at 0.89, 0.97 awbyA.flavusSRRC-G 1907. The best reduction of AFB1(2.3 log10ng/g) was obtained at 0.89 awbyA. flavusEGP-B07.Statistical analyses of AFB1levels produced byA. flavusin YES media showed that ozonation,aw, type of strain, two and three way interactions were statistically significant except awx strain interaction. The major effects were produced by strain, awand then O3.
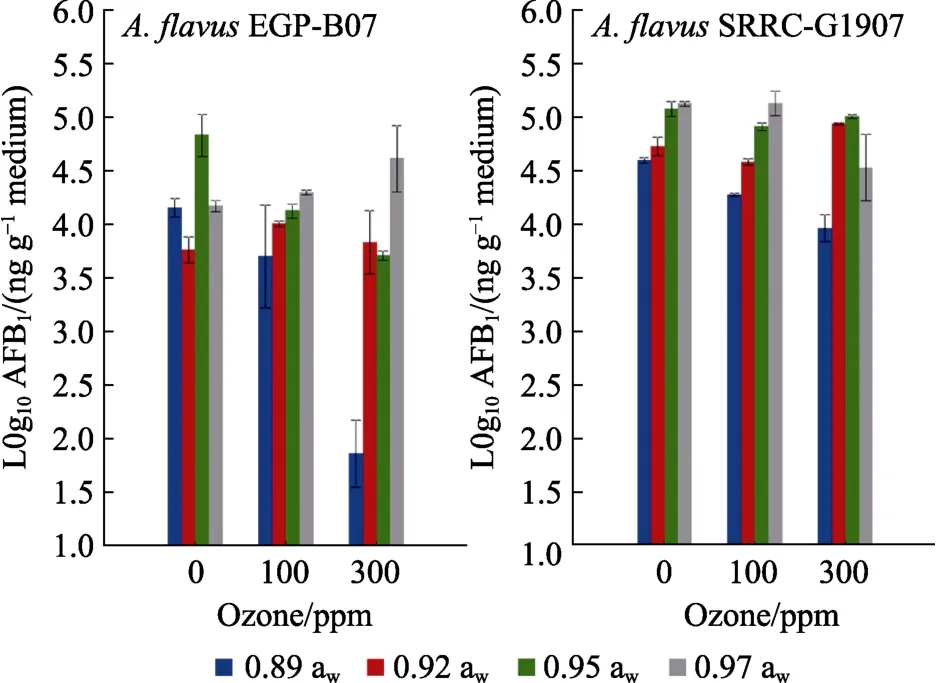
Fig.3 Effect of ozone exposure on AFB1 produced by A. flavus EGP-B07 and SRRC-G 1907 strains on YES media at 25 ℃ after 3 days incubation
3.3 In situ effect of ozone on A. flavus populations on stored shelled peanuts and on aflatoxin B1 contamination
Figure 4 shows the changes inA. flavuspopulations when inoculated with different initial loads in relation to gaseous O3treatments. In stored shelled peanuts there was a significant decrease in the populations of this mycotoxigenic species isolated,especially at 200 and 400 ppm O3. This reduction ranged from 0.8 to 2.2 log CFUs/g, especially in the shelled peanuts inoculated with the higher initial inoculum (105spores/g) when compared with the populations before treatment. Statistically, effects of factors showed that all factors and their interactions significantly affected populations ofA. flavus(log10CFUs/g). The difference in initial inoculum concentration was the main factor affecting the populations, and subsequently by O3treatment.
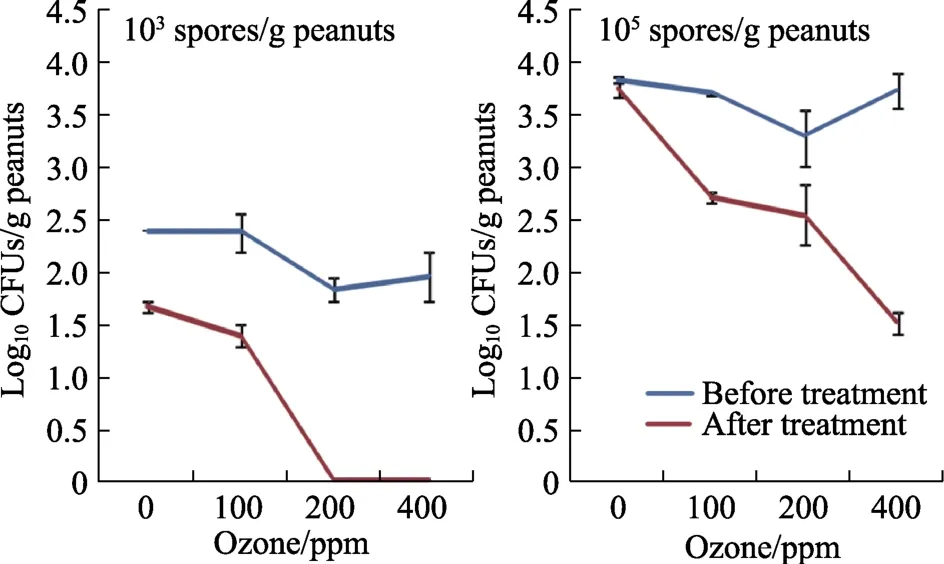
Fig.4 Effect of ozone treatment for 30 min at 6 L/min on isolation of viable populations of A. flavus EGP-B07 from shelled peanuts before and after storage for 4 days
Figure 5 illustrates effect of pre-storage O3treatment on AFB1contamination of shelled stored peanuts inoculated with two different initial concentrations ofA. flavusspores (103, 105spores/g).The doses 100 and 400 ppm significantly decreased AFB1in samples inoculated with 105spores/g.However, only exposure to 400 ppm O3was required to obtain a significant decrease in samples inoculated with 103spores/g. Statistically, only incubation time and the interaction of O3x time significantly (P<0.05) affected AFB1production.
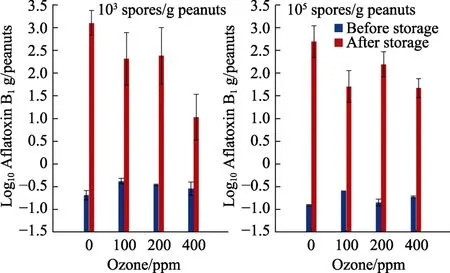
Fig.5 Effect of gaseous ozone exposure of shelled peanuts for 30 min at 6 L/min on AFB1 contamination with different initialinoculums after 4 days storage at 0.93 water activity
4. DISCUSSION
4.1 Effects on germination
Two strains ofA. flavuswere used in this study to evaluate the efficacy of gaseous O3on inhibition of spore germination.A. flavusEGP-B07 was isolated from Egyptian peanuts andA. flavusSRRC-G 1907 was a reference strain. Exposure to gaseus O3inhibited conidial germination, with concentrations>100 ppm completely inhibiting germination over 48 hrs. Generally, the strainA. flavusEGP-B07 was more resistant to O3exposure at doses <100 ppm thanA. flavusSRRC-G 1907.
Very few studies have examined thein vitroeffect of O3on filamentous fungi at different awlevels and rarely onA. flavus.Zottiet al.[28]reported that treatment of fungal colonies ofA. flavus(3 days old) with O3for 3 h completely inhibited viability of conidia. However, the efficacy of O3treatment decreased with increasing colony age (6 and 9 days old). In addition,A. nigerwas found to be more sensitive thanA flavusto O3treatment. However,more recent studies showed that species from theAspergillussectionCircumdatiand sectionNigerwere very tolerant of up to 500 ppm O3[11]. Hudson and Sharma[29]exposed 13 different fungal species includingA. flavusandA. nigeron sterile plastic tray lid surfaces to ozone (35 ppm) for 20 min. They found that this inactivated 3 log10CFUs of most of the fungi after 48 hrs incubation. Beuchat et al.[30]investigated the inactivation of toxigenicA. flavusandA. parasiticusin suspensionby ozone. They found that D-values (the time required to kill 90% of the conidia) ofA. flavuswere 1.72 min and 1.54 min at pH 5.5 and 7.0, respectively; D-values ofA.parasiticuswere 2.08 and 1.71 min, respectively.However, liquid suspensions are very different from dry conidia or those exposed at different aw levels which has only been done for very few species[11,31].Indeed, Mylona et al.[31]found that while initial inhibition of spore germination of microconidia ofFusarium verticillioidesby O3exposure, they could subsequently recover via DNA repair mechanisms,develop hyphal growth and produce fumonisins. So longer term experiments might be necessary to examine such behaviour inA. flavus.
4.2 Effect on in vitro growth and AFB1 production
Generally, growth rate of both strains was relatively unaffected by exposure to O3, regardless of concentration, regardless of the awlevel which appeared to be a more important abiotic factor. AFB1production was significantly affected at the higher dose of O3(300 ppm) at 0.89, 0.95 awbyA. flavusEGP-B07 and at 0.89, 0.97 awbyA. flavusSRRC-G 1907. ExposingA. flavusEGP-B07 on YES at 0.89 awto O3at 300 ppm gave the best inhibition (2.3 log10ng/g). Previous studies by Mason et al.[15]applied O3(5 days) to agar cultures ofA. flavusandF. verticillioides. They found that sporulation and hyphal growth above the surface of the agar were completely inhibited by the O3. Vijayanandraj et al.[32]reported that O3inhibitedA. nigervegetative mycelial growth but did not have any effect on spore germination. This could partially be due pigmentation which may protect the conidia from damage by O3. It has been suggested that a reduction in O3effectiveness can occur in the presence of dissolved organic and inorganic substances because they have a protective effect on the microorganisms against the action of O3[21].
In terms of effects on AFB1production, Zotti et al.[28]reported that exposingA. flavuscolonies to O3can decrease toxin production indirectly by the bleaching effect on the yellow pigments. This was based on the findings of Shier et al.[33]that the yellow anthraquinone pigments of the colonies were either biosynthetic intermediates needed to synthesise aflatoxins, or unutilized branch pathway products.
4.3 In situ effects of ozone on A. flavus populations and AFB1 production in stored peanuts
In this study, the efficacy of O3treatment on appeared to be influenced by the relative initial spore inoculum load on the shelled peanuts (103and 105condia/g). In general, the lower the initial inoculum and the higher the O3concentration,resulted in better inhibitory effects. The doses of 200 and 400 ppm completely inhibited the isolation of conidia from stored shelled peanuts at the lower inoculums level. At the same time, 400 ppm gave a significant reduction of 2.2 log CFUs/g in peanuts inoculated with 105spores/g. Recent studies by Giordanoet al.[34]showed that O3treatment for 5 h at 31 ppm was able to successfully inhibit the viability of aflatoxigenicAspergillusspecies on Brazil nuts from the day of application. Previously,studies with maize showed that treatment of maize with 50 ppm O3for 3 days resulted in a 63% reduction in contamination byA. parasiticuson the kernel surfaces[35].
Treating the raw shelled peanut samples with O3immediately after inoculation with 103/105conidia/g had relatively little effect on the AFB1contamination level immediately after inoculation and exposure to O3. However, after storage for 4 days and due to colonisation byA. flavusinoculum, there was a significant increase in AFB1contamination.Using a 400 ppm O3dose pre-storage did significantly inhibited AFB1production of shelled peanuts with both initial inoculum levels. Previous studies with brazil nuts using low O3concentrations of 14 and 32.5 ppm were found to completely inhibited AFB1production, but the treatment was applied for 24 hrs which completely inhibited aflatoxigenic fungal growth[34]. However, effects of initial awand interactions with O3exposure were not investigated.
Studies by Wang et al.[36]showed that the wet method of O3fumigation of grains was more effective than a dry method for degrading ochratoxin A (OTA)formation. OTA was degraded after exposure to 30 ppm O3for 120 min or exposure to 60 ppm O3for 90 min[37]. The degradation percentages of AFB1in contaminated wheat artificially inoculated (10 µg/kg)were 84%~86% when exposed to 20~40 ppm O3for 20 min[37].
O3has been affirmed as GRAS for use for food processing[3,38]. Also, O3does decompose rapidly(half-life of 20~50 min) to molecular oxygen without leaving any residue[35]. However, it is effective for increasing the storage life of different commodities[39].Care may be needed with lipid rich commodities where some tainting may occur at high O3treatment concentrations. Potentially it would be better to treat such commodities with lower concentrations but longer periods of time to obtain the required efficacy. However, O3is not very user friendly and requires effective safety protocols and equipment which will not degrade during use. O3can be very corrosive to rubber tubing and seals thus PTFE tubing and stainless steel or similar container materials for effective use commercially[3].

