Development of Helmholtz free energy equation of state for hydrogen/methanol binary mixture
Yan Jiwei Gao Neng
(1 Shanghai Velle Automobile Air Conditioner Co.,Ltd,Shanghai 201615,China)
(2 Ningbo Technology University,Ningbo 315100,China)
(3 Zhejiang Engineering Research Center for Intelligent Marine Ranch Equipment,Ningbo 315100,China)
Abstract: A multi-parameter Helmholtz energy explicit equation of state for hydrogen/MeOH was proposed,and four reducing parameters was obtained based on experimental data collected.It was shown that the new equation of state predicted the VLE and statured liquid density very well.The average reduced deviations(ARD)of liquid mole fraction and vapor mole fraction were 9.37 and 3.66,respectively.The maximum density deviation is 1.48%.
Key words: reducing parameters;equation of state;hydrogen-rich mixtures;thermodynamic properties
1 Introduction
Due to great concerns of climate change,decreasing the carbon emissions from all walks of industries is a common consensus of the world[1].Many countries,global industrial associations and international organizations are raising their initiatives and fundings to promote the utilization of decarbonization technologies.Among which,carbon dioxide(CO2)hydrogenation is considered one of most notable carbon utilization technologies and,implementation with blue or green hydrogen technologies,it has great potential to decrease and even achieve negative emissions[1].Methanol is a very common product and feedstock of the following more complex CO2hydrogenation process.Thermodynamic properties and phase behavior are fundamental to the design,optimization and maintenance of the processes involved in CO2hydrogenation.Unfortunately,there are still no consistent and reliable theoretical models covering wide operating regimes,especially,the hydrogen/MeOH binary are far more been studied.
To evaluate the thermodynamic properties,the development of equation of state is very essential[2].Generally,based on exclusive equation of state,the density,enthalpy,entropy,heat capacity,speed of sound,Joule-Thomson coefficient,etc.can be well predicted at given temperature and pressure.Currently,most widely used equation of states in chemical industrial clusters to calculate the properties are cubic equation of state,Cubic +Association(CPA)equation of state and SAFT equation of state.Nevertheless,they only present very good accuracy in certain ranges.A more accurate equation of state acceptable in wide range is critically needed.The multifluid Helmholtz energy approximation(MFHEA)Equation of state mostly used in natural gas refrigeration industries[3].Due to the combination of ideal gas contribution heat capacity,it is a more “fundamental” equation of state.It has great flexibility,high accuracy,and potential to correlate the binary mixtures of hydrogen/MeOH reliably.Nevertheless,it has not been fully parameterized.
In this paper,a multi-parameter Helmholtz energy explicit equation of state for hydrogen/MeOH was proposed.Four reducing parameters was obtained based on collected experimental data.The paper is expected to provide supports for the thermodynamic properties’ evaluation of hydrogen/MeOH binary and the development of more comprehensive equation of state.
2 Development of equation of state for binary mixture
2.1 Equation-of-state
As mentioned in the introduction section,a multiparameter equation of state,which is explicit of Helmholtz energy,is drawing wide attention due to its great flexibility and accuracy.The equation-of-state is given by reduced Helmholtz energy as[3]:

whereα,A,Ris reduced Helmholtz energy,Helmholtz energy and universal gas constant,respectively.δandτare reduced density and reciprocal reduced temperature and given by:

The reduced Helmholtz energy in equation(1)is separated into an ideal gas partα0and a residual partαr.The ideal part describes the properties of ideal gas at the same temperature and pressure,while the residual part considers the deviation between the ideal gas and the real fluid.They are presented as[4]:

In whichare the ideal Helmholtz energy and residual Helmholtz energy of pure hydrogen and methanol,and departure function,respectively.Therefore,the reduced Helmholtz energy can be predicted by properties of each component and binary-specific departure function.Generally,for pure hydrogen and methanol,their equation of states have been well developed.To determine the reduced Helmholtz energy of their binary,the reduced density,reciprocal reduced temperature and binary-specific departure function need to be determined.
The reduced density and temperature are the functions of density,temperature and composition.They are presented by:


In whichβT,ij,γT,ij,βV,ij,γV,ijare adjustable binaryspecific parameters and fitted from experimental data.Based on the setting of their values,Equations(5) -(6) allow both asymmetric and symmetric reducing functions.The critical parameters of hydrogen and methanol are listed in Table 1.

Table 1 Critical parameters of hydrogen and methanol
The departure function is introduced enabling the modeling of non-ideal behavior of binary mixtures,and is given by:

WhereFijis scaling factor.αrij(δ,τ) consists of polynomial,exponential,and Gaussian bell-shape terms,and it is depicted as[4]:

Depending on the desired accuracy of the equation of state and availability of experimental data,the departure function may not be involved in the model.In this work,only reducing parameters are fitted by the experimental data.
2.2 Data collection and uncertainty analysis
The binary-specific reducing parameters in equation(5) -(6)are fitted from the experimental data.A total of 245 data were collected.Table 2 presents the details of the collected data current available in the literature.Generally,the current data cover a wide range regarding the temperature and pressure.Meanwhile,the current data also involve the mole fractions of liquid and vapor in equilibrium,and liquid density data.
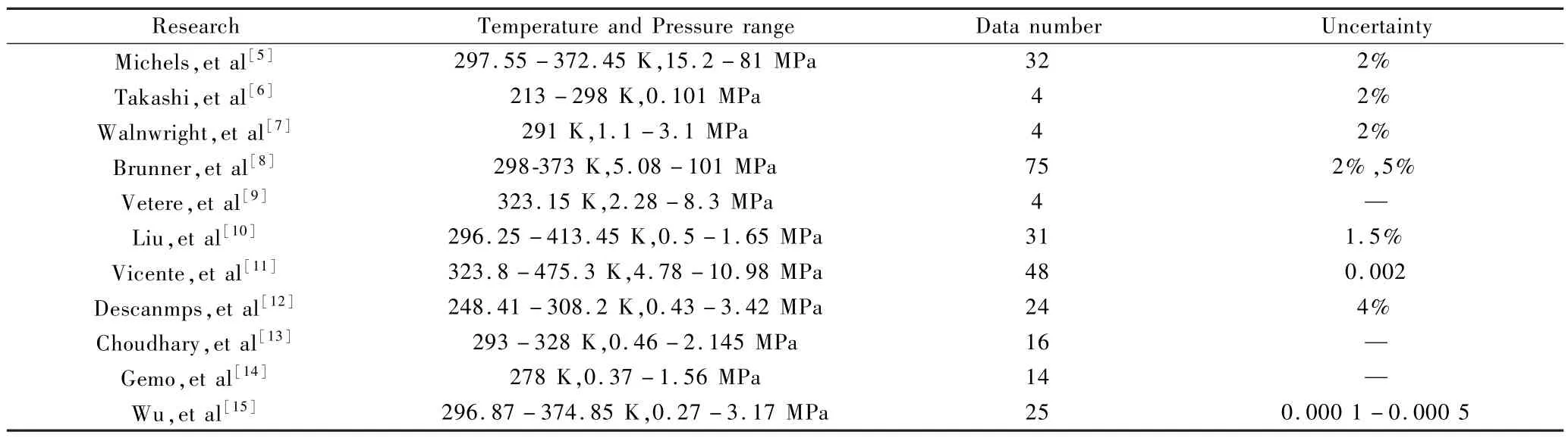
Table 2 The data bank of hydrogen/MeOH binary mixture
2.3 Optimization algorithm
Based on the experimental data collected in Table 2,four reducing parameters for hydrogen/MeOH were obtained.As the data has different uncertainties,the objective function(OF)was weighted with their uncertainties and given by:
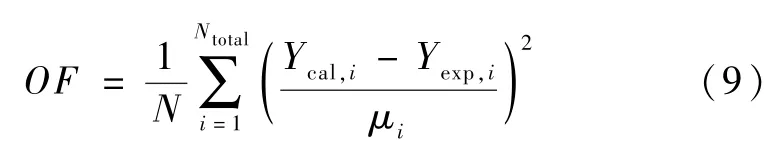
Whereμis the uncertainty of each experimental data point.WhenOF~1,the average prediction deviations are at the same magnitude of the uncertainty,which suggests a quite good prediction.The optimization of reducing parameters is highly non-linear,and the objective function is not smooth.Furthermore,the calculation of flash points may divergent even with mature algorithms used in commercial software[3].therefore,the following algorithm was developed to address the fitting.
(1)Map the error matrix with gridded data pints,to identify the vicinity of potential local optimum.
(2)Find the local optimum by optimizing the gridded local optimum.
(3)Compare various local optimums to obtain the global optimum.
To optimize the gridded local optimum,the Levenberg-Marquardt algorithm was used due to its great flexibility,along with the penalty function defining by:

Whereffail,nfailare failure index and penalty sharpness exponent,respectively.In this work,there were set as:
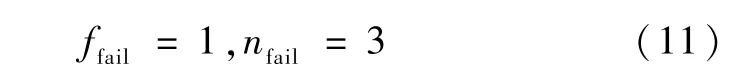
To evaluate the calculation results,average reduced deviation (ARD) and maximum reduced deviation(MRD)is defined by:

The overall calculation diagram is shown in Fig.1.

Fig.1 Algorithm to obtain reducing parameters
3 Results and Discussions
Due to the limited availability of experimental data,and most of the collected data are VLE,therefore,only reducing parameters are fitted and their results are given in Table 3.

Table 3 Reducing parameters of hydrogen/MeOH
With the optimized reducing parameters,the phase diagram was theoretically calculated against the experimental data.Fig.2 shows the phase diagrams of hydrogen/MeOH binary mixture at~298.15 K,~323.25 K,~348.15 K and~372.15 K,respectively.For the liquid fraction,the deviations between the prediction and experimental data are not significantly,suggesting a quite good agreement.TheARDof liquid fraction prediction is 9.37.The influence of the pressure and temperature on reduced deviations for the liquid phase is further presented in Fig.3.The deviation is generally positive at lower pressures and then become negative at higher pressures.Furthermore,most big deviations fall within 300 -380 K.

Fig.2 Pressure-composition diagram of hydrogen/MeOH binary at different temperatures

Fig.3 Influence of parameters on liquid-composition predicted accuracy
When it comes to the vapor phase mole fraction prediction,the deviation is larger,as shown in Fig.2.Nevertheless,most predictions for vapor mole fraction still around the uncertainty of experimental data.It is because for the vapor fraction,it is very difficult to obtain accurate measurement and usually with large uncertainty.TheARDfor vapor fraction calculation is 3.66.Fig.4 shows the influence of pressure and temperature on the reduced deviations for vapor fraction.Great deviations occur at higher pressures and intermittent temperatures.

Fig.4 Influence of parameters on vapor-composition predicted accuracy
The density of saturated liquid was also calculated and compared with the experimental data,as shown in Fig.5.The prediction of density generally well agreed with the experimental data.The maximum relative deviation of the prediction is 1.48%.The temperature has not apparent influence on the prediction accuracy.

Fig.5 Prediction of saturated liquid density
4 Conclusions
A Helmholtz energy equation-of-state for hydrogen/MeOH binary mixture was developed.Based on data collected,four reducing parameters were fitted.The prediction results agreed with the experimental data very well.TheARDof the liquid mole fraction,vapor mole fraction predictions are 9.37 and 3.66,respectively.The maximum relative deviation of statured liquid density prediction is 1.48%.Despite the work presented in this work,a more comprehensive equation of state for hydrogen/MeOH with departure function will need to be developed given more experimental data(including pressure-density-temperature(pρT),heat capacity(cp) and speed of sound(w))are available in the future.
5 Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could influence the work reported in this paper.
6 Acknowledgement
This work is financially supported by the National Natural Science Foundation of China(No.51906216).

