Early gastric cancer: A challenge in Western countries
Maria Michela Chiarello, Valeria Fico, Gilda Pepe, Giuseppe Tropeano,Neill James Adams,Gaia Altieri,Giuseppe Brisinda
Abstract Early gastric cancer (EGC) is an invasive carcinoma involving only the stomach mucosa or submucosa, independently of lymph node status. EGC represents over 50 % of cases in Japan and in South Korea, whereas it accounts only for approximately 20 % of all newly diagnosed gastric cancers in Western countries. The main classification systems of EGC are the Vienna histopathologic classification and the Paris endoscopic classification of polypoid and non-polypoid lesions. A careful endoscopic assessment is fundamental to establish the best treatment of EGC. Generally, EGCs are curable if the lesion is completely removed by endoscopic resection or surgery. Some types of EGC can be resected endoscopically; for others the most appropriate treatment is surgical resection and D2 lymphadenectomy, especially in Western countries. The favorable oncological prognosis, the extended lymphadenectomy and the reconstruction of the intestinal continuity that excludes the duodenum make the prophylactic cholecystectomy mandatory to avoid the onset of biliary complications.
Key Words: Early gastric cancer; Diagnosis; Treatment; Endoscopic resection; Surgery;Lymph nodes metastases
INTRODUCTION
In its initial stages, gastric cancer tends to spread in the lamina propria, infiltrating the muscularis mucosae and the submucosal layer of the gastric wall[1 -4 ]. The Japanese Society for Gastroenterological Endoscopy’s definition of early gastric cancer (EGC) is an invasive gastric cancer that invades no more deeply than the submucosa, irrespective of lymph node metastases (LNM). Thanks to rigorous screening programs in the Asian countries, up to 50 % of patients treated for a gastric cancer have EGC[5 ,6 ]. Such a high incidence is not found in European countries, where advanced gastric cancer is prevalent.
The difference in incidence between Asian and Western countries may be due to a wide use of magnifying upper endoscopy with high-resolution images and chromoendoscopy in Japan and South Korea.
A correct classification and an accurate diagnosis are fundamental to plan the most effective treatment[7 -10 ]. Some forms of EGC can be treated by endoscopic resection; in other forms surgical treatment is mandatory[10 -14 ].
EGC carries an excellent prognosis if the lesion is completely removed by endoscopic resection or surgery[15 -17 ]. Most of the Japanese studies have reported 5 -year and 10 -year survival rates of more than 90 % for the patients with EGC. In the Western studies, 5 -year survival rates are variable, ranging from 68 % to 92 %. EGCs recur in at least 1 .9 % of cases after resection with time intervals ranging from 4 mo to more than 10 years. Important risk factors for the recurrence are the presence of submucosal invasion, LNMs and undifferentiated histology.
CLASSIFICATION
The most common classification is the TNM, which relates information about the primitive lesion (T),node involvement (N) and distant metastases (M) thus providing information about the diffusion of the disease.
The macroscopic classification of EGC, as defined by the Japanese Gastric Cancer Association (JGCA),identifies different subtypes: type 0 -I (protruding-polypoid tumors); type 0 -IIa (superficial elevated tumors); type 0 -IIb (tumors without elevation or depression); type 0 -IIc (slightly depressed tumors); and type 0 -III (excavated tumors), referring to the morphological features of the lesion on the mucosal surface[18 ].
The Vienna classification (Table 1 ) is a histologic classification. While the TNM defines EGC as a cancer invading no more deeply than the submucosa, irrespective of LNMs, the Vienna classification is the most exhaustive[19 ,20 ]. In particular, the intramucosal EGC is classified in 3 categories, while the submucosal EGC in 2 , according to the depth of infiltration. This distinction is not present in the TNM system and is fundamental for the treatment planning. In fact, it allows the option for an endoscopic therapy [(endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD)] or surgery if mandatory.
The Paris classification of polypoid and non-polypoid lesions including flat lesions is useful to evaluate possible endoscopic treatments. This classification was created for the superficial colon neoplasms, undoubtedly the most frequent in the Western World and with some small changes was adopted to the superficial gastric neoplasms. One of the changes is the thickness of the wall layer, as in the submucosa, are thinner in the stomach.

Table 1 Vienna classification
DIAGNOSIS
A correct endoscopic diagnosis is fundamental in EGC[21 ]. The Japanese Society for Gastroenterological Endoscopy suggests that the length of the endoscopy, the accurate stomach wall distension and the further removal of mucous from the stomach lining are proportional to diagnostic accuracy and lesion detection rate[22 ]. As far as the length of the procedure is concerned, the most appropriate protocol is the Systematic Screening Stomach, which requires careful vision of the gastric lumen and acquisition of 22 pictures (12 in anterograde vision and 10 in retro vision) with a global length of the exam not inferior to 12 min[22 ]. Sedation is advised, while trans-nasal endoscopy is not.
Regarding diagnostic accuracy, the use of traditional or virtual cromo-endoscopy is advised to look for the “minimal changes” of the Japanese authors to evaluate the size and morphology of the lesion and the glandular and vascular pattern. Lesions should be described according to the Paris classification(Type 0 I protruding, Type 0 -IIa elevated, Type 0 -IIb flat, Type 0 -IIc depressed, Type 0 -III excavated)[23 ].
TREATMENT
Endoscopic resection must be taken into account when the risk of LNMs is low or when the location and size of the tumor allows a safe “en-bloc” complete resection[24 ,25 ]. Selected EGCs can be treated with EMR or ESD with good results in Western patients[26 -28 ]. Absolute indications for endoscopic resection, according to the Japanese gastric cancer treatment guidelines, are: cT1 a differentiated-type adenocarcinoma without ulcerative findings or cT1 a differentiated-type adenocarcinoma with ulcerative findings and a diameter 3 cm. Endoscopic resection for cT1 a undifferentiated carcinoma without ulcerative findings and with a diameter 2 cm is considered an expanded indication. Therefore,endoscopic resection should be considered for elderly patients with a high operative risk (relative indication)[18 ,29 ].
According to JGCA guidelines (Table 2 ), en-bloc endoscopic resection for differentiated pT1 a cancer without ulcerative findings (UL0 ), negative horizontal margin (HM0 ), negative vertical margin (VM0 )and no lymphovascular infiltration (Ly0 , V0 ) or en-bloc endoscopic resection for pT1 a cancer with ulcerative findings, tumor size 3 cm, HM0 , VM0 , Ly0 and V0 are classified as endoscopic curability A.However, the resection is classified as endoscopic curability B for undifferentiated pT1 a cancer resected en-bloc, tumor size 2 cm, without ulcerative findings, HM0 , VM0 , Ly0 and V0 or for en-bloc resected pT1 b differentiated cancer, with a depth of infiltration < 500 µm, tumor size 3 cm, HM0 , VM0 , Ly0 and V0 . When the resection fulfills criteria to be classified as endoscopic curability A or endoscopic curability B, but the cancer was not resected en-bloc or has positive horizontal margin, it is classified asendoscopic curability C-1 . All other resections are classified as endoscopic curability C-2 .
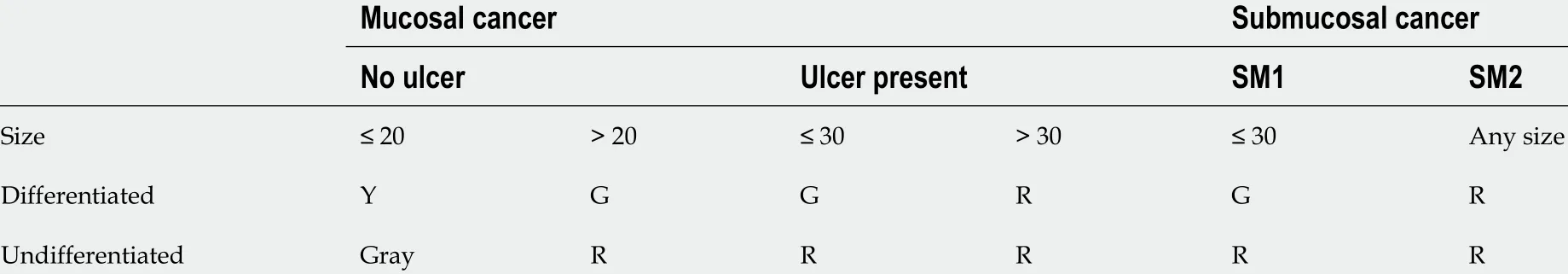
Table 2 Criteria for endoscopic mucosal resection, endoscopic submucosal dissection and surgery
Both endoscopic curability A and endoscopic curability B are considered to have low risk of LNMs and do not require additional surgery, so the patient can go to follow-up. Endoscopic curability C-1 has a low risk of LNMs but should be aware of residual or local recurrence, so the patient could be treated with another ESD, surgical resection, close observation or endoscopic coagulation. After an endoscopic resection classified as endoscopic curability C-2 , gastrectomy with lymphadenectomy should be considered the standard-of-care[18 ].
The results of endoscopic treatment (EMR and ESD) have been compared to traditional surgical treatment in patients with EGC in several meta-analyses[30 ]. Overall survival after EMR or ESD is not significantly different when compared to surgical resection (hazard ratio: 0 .995 , 95 % confidence interval:0 .836 -1 .185 ; P = 0 .9 ). As for recurrence free survival, it has been proved that the recurrence risk after EMR is significantly higher compared to surgical resection (hazard ratio: 3 ,946 , 95 % confidence interval:1 .233 -12 .632 , P = 0 .02 )[30 ]. However, it has been noted that in adequately selected patients endoscopic resection offers results that are similar to those of traditional surgery[31 ]. In patients who underwent EMR/ESD, strict endoscopic surveillance is mandatory, given the higher risk of endoluminal recurrence.
Complications of EMR or ESD for EGC include pain, bleeding and perforation[32 ]. Pain after resection is typically mild. Bleeding occurs in up to 8 % of patients undergoing endoscopic treatment.Immediate bleeding appears more common with EMR/ESD of tumors located in the upper third of the stomach. Perforation occurs in 4 % of the patients during ESD[15 ].
Directly the last mouthful had disappeared she was seized with such violent thirst that she caught up a great pot full of water and drank it at a single draught18
An innovative procedure called laparoscopic endoscopic cooperative surgery (LECS) that combines the strongest points of interventional endoscopy and laparoscopic surgery for the removal of gastric wall tumors was developed by Hikiet al[33 ]. In the original procedure of LECS, the stomach wall is opened for resection of the tumor, and the lumen is exposed to intraperitoneal space. LECS involves precutting around the tumor with an endoscope and artificial perforation of the gastric wall. Next,excision of the tumor with laparoscopy and repair of the gastric wall with a stapler are performed[34 ,35 ].
The indications for LECS are currently limited to lesions that are normally managed by ESD but are technically challenging to resectviaESD because of the presence of ulcers, or if the tumor size is more than 30 mm in diameter[36 ].
When a LECS procedure and sentinel node biopsy were combined, an extremely minimally invasive procedure that is adequate for radical oncological resection of EGC is achieved. Sentinel node navigation surgery is an ideal surgical option for preservation of most parts of the stomach and consequent maintenance of normal gastric function to improve quality of life in patients with EGC.Although many previous studies and clinical trials have demonstrated the safety and feasibility of the sentinel node concept in gastric cancer, the clinical application of sentinel node navigation surgery is debatable. Several issues regarding technical standardization and oncological safety need to be resolved.Recently several studies to resolve these problems are being actively performed, and sentinel node navigation surgery might be an important surgical option in the treatment of gastric cancer in the future.
Other techniques (Table 3 ) have been developed to avoid spread of the tumor to the peritoneum[37 -39 ]. When performed by expert teams they show a lot of promise and achieve solid oncologic results[40 ,41 ]. Use and standardization of these minimally invasive surgical procedures contributes to reduction of unnecessary gastrectomy for gastric submucosal tumors[42 ]. Most of the clinical experiences are Japanese, and the small number of treated cases does not allow a comparison with the longest used endoscopic and surgical techniques. More research and clinical trials about LECS for EGC are expected.Furthermore, surgeons need to select one of these minimally invasive procedures according to the characteristics of the tumor, the localization of the tumor in the stomach, the personal surgical experience and the technological characteristics of the health institution.
In the case of EGC without the above features, total or partial gastrectomy with a free margin of at least 2 cm is the treatment of choice. Adequate lymphadenectomy offers a high chance of curing patientswith EGC[43 -45 ]. The lymph nodes of the stomach are classified into stations numbered as in Table 4 .The regional stations are the lymph node stations 1 -12 and LN station 14 v (Figure 1 A). The remnant stations are considered as distant stations, and metastases to these nodes are classified as M1 . The JGCA defined the extent of systematic lymphadenectomy according to the type (total or distal) of gastrectomy indicated (Table 5 ).

Table 3 Laparoscopic endoscopic cooperative procedures
Surgical treatment is also mandatory when there is a lack of training in complex endoscopic procedures. In hospitals with a low volume of advanced endoscopic procedures, gastrectomy remains the gold standard for treatment of EGC.
As already known, tumor grading and vascular-lymphatic invasion were found to be significant risk factors. A study on 517 patients with EGC found that LNMs are present in 22 % of cases (114 patients),directly related to an ulcerated lesion of more than 2 cm diameter. Furthermore, the risk of LNMs is higher in males (P= 0 .03 ), elderly patients (P = 0 .01 ), in the presence of depressed tumor (P = 0 .01 ) and with submucosal invasion (P= 0 .03 ). Further risk factors include tumor localization in the body (P=0 .04 ) or at the angulus (P = 0 .02 ). In these areas, the submucosa is thinner, and the lymphatic vessels more widespread in the lamina propria of the mucosa.
Similar results have been documented in a recent paper, where age, gender, tumor size, type of differentiation, Lauren classification and lympho-vascular and perineural invasion showed a significant correlation with the rate of LNMs in EGC by univariate and multivariate analyses in 1033 patients who underwent radical gastrectomy with lymphadenectomy. Patients with T1 b gastric cancer had an older age, a higher proportion of proximal lesions, larger tumor size, more frequent vascular lymphatic invasion, perineural invasion and more LNMs than patients with T1 a gastric cancer[48 -51 ].
In a series of 5265 Japanese patients that underwent gastrectomy and lymphadenectomy, 3016 lesions were intramucosal EGCs, and 2249 were infiltrating the submucosa. LNMs were present in 65 (2 .2 %) of the patients with intramucosal EGC and in 402 (17 .9 %) of those with penetrating EGC. The authorsdocumented that in the intramucosal cancer, the risk of LNMs is higher in depressed or ulcerated lesions, in lesions bigger than 30 mm, in those with an undifferentiated type or in those with vascular and lymphatic invasion. In submucosal EGCs a higher incidence of LNMs was documented in submucosal invasion ≥ 500 µm (23 .7 %) compared to submucosal invasion < 500 µm (8 .8 %, P < 0 .0001 ),when there was vascular lymphatic invasion (36 .6 % vs 9 .8 % if no infiltration, P < 0 .0001 ), in case of elevated EGC (24 .8 %) compared to depressed type (17 .1 %, P = 0 .0003 ) and in tumors larger than 30 mm(P<0 .0001 ). Moreover, extra-gastric LNMs (level II) were found in 10 % of the cases, in the absence of involvement of the peri-gastric lymph nodes.

Table 4 Anatomical definitions of lymph node stations

Table 5 Extent of systematic lymphadenectomy according to the type (total or distal) of gastrectomy indicated

Figure 1 Lymph node removal along the upper border of the pancreas (lymph node station N. 12 a) (A), along the common hepatic artery(lymph node station N. 8 a) and along the left gastric artery (lymph node station N. 9 ) (B). Personal experience: Consecutive patients were recruited by senior surgeons (MMC, GB).
To optimize lymphadenectomy, the sentinel node search has been proposed. Said procedure is now standardized in breast cancer surgery but still experimental in the case of EGC[52 ,53 ]. Two endoscopically administered tracers are used sequentially: the 99 Tc radioisotope (at least 3 h before the intervention) and the blue isosulphan dye (during the intervention). Since skip LNMs are frequent(about 20 % of cases in EGCs), a negative sentinel node does not exclude the possibility that subsequent lymph node stations are involved in the disease. The usefulness of the sentinel lymph node would therefore not consist in excluding LNMs, as initially proposed, but in identifying the lymphatic drainage basin that must be removed. Thus, the sentinel lymph node would therefore become an aid to identify the relationship between gastric anatomy and gastric lymphatic drainage.
LNM strongly affects the prognosis[14 ,54 ]. This is a topic of not negligible importance because EGC is believed to be a curable albeit malignant disease. In T1 a EGCs, the 10 -year survival rates are about 100 %in patients undergoing gastric resection and extended (D2 ) lymphadenectomy[55 ]. Five-year and tenyear survival in these patients was 2 %–3 % higher than in those with limited (D1 ) lymphadenectomy. In the case of submucosal EGC, the 10 -year survival rate was about 100 % in patients undergoing gastric resection and extended lymphadenectomy. Five-year and ten-year survival was 10 % higher in these patients than in those with limited lymphadenectomy. Looking at the postoperative lymph node positive (pN+) patient group, 10 -year survival was 87 % if extended lymphadenectomy was performed,and 55 % if lymphadenectomy was limited.
In patients with a radical resection, when a D2 lymphadenectomy (Figure 1 B) was performed and the duodenum was excluded in the intestinal reconstruction, cholecy-stectomy, considered by some to be a non-essential measure, is necessary to avoid gallstone formation and its complications.
The use of 300 mg or 600 mg of ursodeoxycholic acid compared with placebo resulted in a significantly decreased proportion of patients developing gallstones within 12 mo after surgery for gastric cancer (5 .3 % in the 300 mg group, 4 .3 % in the 600 mg group and 16 .7 % in the placebo group)[56 ]. Gallstone formation and its complications may be prevented using ursodeoxycholic acid rather than prophylactic cholecystectomy. However, the results raise some questions. As Pitt and Nakeeb[57 ]maintained, the study was conducted in patients undergoing gastrectomy for stomach cancer but follow-up did not last more than a year. Furthermore, more than 80 % of patients have EGCs with a presumptively satisfactory oncological prognosis, given that 84 % of cancers occurred in the distal stomach and 57 % of lymphadenectomies were D2 [56 ]. In this setting, we believe that prophylactic cholecystectomy is necessary for patients with a good cancer prognosis, as suggested by Pitt and Nakeeb[57 ].
Studies on the subject conclude that prophylactic cholecystectomy does not have a significant impact on the natural course of the disease[58 ]. However, it leads to a reduction in the number of biliary complications (which may affect up to 15 % of the operated patients) and does not induce an increase in mortality and morbidity rates. In one study, a mortality rate of 1 .8 % was reported in the case of cholecystectomy performed during an intervention other than that of the gastric cancer treatment.Prophylactic cholecystectomy seems to be unnecessary only in cases where the continuity of the digestive tract involves the use of the duodenum[59 ]. In a study on 969 patients, after gastrectomy,gallbladder stones developed in 6 .1 % of patients (59 out of 969 ) with an incidence of cholecystitis/cholangitis of 1 .2 % of cases (12 /969 ). It has been found that the method used to restore intestinal continuity, with preservation of the duodenal transit or excluding the duodenum, is an independent risk factor for both the development of cholelithiasis (P= 0 .018 ) and cholecystitis and cholangitis (P=0 .006 ). It has been also confirmed that in patients who develop cholelithiasis, the incidence of cholecystitis and cholangitis is particularly high when the duodenal transit was excluded (31 .3 %)compared to those with duodenal transit maintained (7 .4 %).
CONCLUSION
In agreement with the JGCA treatment guidelines, D1 lymphadenectomy can be sufficient to treat EGCs not suitable for endoscopic treatment when the regional nodes are clinically free from disease. It is important to point out, however, that a relevant percentage of EGCs in Western countries are of the diffuse type and therefore associated with a high risk of LNMs, especially when the submucosa is invaded. Moreover, in Western countries, endoscopic resections are not very common, and preoperative diagnosis of lymph node status has some limitations, despite advancements in radiological techniques.Considering these limitations, D2 lymphadenectomy in EGCs not susceptible to radical endoscopic treatment is the surgical treatment of choice. In this area, based on a good prognosis and intestinal reconstruction that excludes the duodenum from gastrointestinal transit, we believe that prophylactic cholecystectomy is necessary.
FOOTNOTES
Author contributions:Chiarello MM and Brisinda G conceived the original idea; Fico V, Pepe G, Altieri G and Tropeano G performed a comprehensive review of all available literature and synthesized the data; Chiarello MM,Fico V, Adams NJ and Brisinda G wrote the manuscript; Chiarello MM, Fico V, Pepe G, Altieri G, Tropeano G and Brisinda G contributed to the study design, manuscript structure and performed a final critical appraisal of the manuscript; Chiarello MM, Fico V, Pepe G, Tropeano G, Adams NJ, Altieri G and Brisinda G read and approved the final manuscript.
Conflict-of-interest statement:The authors declare that they have no conflict of interest.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4 .0 ) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4 .0 /
Country/Territory of origin:Italy
ORCID number:Maria Michela Chiarello 0000 -0003 -3455 -0062 ; Valeria Fico 0000 -0003 -1619 -4164 ; Gilda Pepe 0000 -0001 -9852 -6243 ; Giuseppe Tropeano 0000 -0001 -9006 -5040 ; Neill James Adams 0000 -0002 -2813 -8648 ; Gaia Altieri 0000 -0002 -0324 -2430 ; Giuseppe Brisinda 0000 -0001 -8820 -9471 .
S-Editor:Ma YJ
L-Editor:Filipodia
P-Editor:Ma YJ
 World Journal of Gastroenterology2022年7期
World Journal of Gastroenterology2022年7期
- World Journal of Gastroenterology的其它文章
- Clinical online nomogram for predicting prognosis in recurrent hepatolithiasis after biliary surgery: A multicenter, retrospective study
- Effect of Bifidobacterium longum 35624 on disease severity and quality of life in patients with irritable bowel syndrome
- Crohn’s disease-related ‘gastrocnemius myalgia syndrome’ successfully treated with infliximab: A case report
- Will the collaboration of surgery and external radiotherapy open new avenues for hepatocellular carcinoma with portal vein thrombosis?
- Stereotactic radiotherapy and the potential role of magnetic resonance-guided adaptive techniques for pancreatic cancer
