果蔬采后生理代谢变化及调控机制研究进展
唐建新,王佳莉,英丽美,张云鹤,孙炳新
果蔬采后生理代谢变化及调控机制研究进展
唐建新,王佳莉,英丽美,张云鹤,孙炳新
(沈阳农业大学 食品学院,沈阳 110866)
讨论采后果蔬的呼吸代谢、能量代谢,以及活性氧代谢与品质劣变之间的关联性,旨在为采后果蔬保鲜技术的应用提供理论支持和研究思路。综述采后果蔬贮藏期间3种生理代谢的变化及相互调控机制,以及与品质劣变的关联性。采后果蔬的品质劣变与呼吸代谢、能量代谢和活性氧代谢密切相关。采后处理或保鲜技术可通过对果蔬代谢机制进行调控来减少褐变、营养物质流失、软化、病原菌侵染、冷害等不良现象的发生。呼吸代谢、能量代谢和活性氧代谢共同构成了一个复杂的生理代谢网络,相互作用共同调控果蔬的生理代谢,进而影响采后果蔬的生理品质。
果蔬保鲜;呼吸代谢;能量代谢;活性氧代谢;品质劣变
我国果蔬产量目前位居世界首位,蔬菜种植面积约占世界总量的30%,产量约占世界总量的50%[1]。果蔬采后仍保持旺盛的生理代谢,极易发生氧化褐变、组织软化、腐烂变质和营养物质流失等一系列不良品质变化,造成经济损失和资源浪费。随着人们对果蔬品质要求的不断提升,果蔬采后保鲜具有十分重要的意义。采后果蔬的贮藏品质与呼吸代谢、能量代谢和活性氧代谢密切相关。文中拟阐述采后果蔬呼吸代谢、能量代谢与活性氧代谢变化的作用机制及其与品质劣变之间的关联性,分析不同采后处理或保鲜技术对果蔬采后生理代谢的影响,以期为果蔬贮藏保鲜技术的应用提供理论依据和应用指导。
1 呼吸代谢
呼吸代谢是采后果蔬生理代谢的中枢,能够为生命活动和理化反应提供能量和反应中间体,而较高的呼吸作用会加速营养物质的消耗,产生活性氧(ROS)会加速细胞膜的氧化损伤,从而进一步加速采后果蔬的品质劣变和衰老[2]。果蔬的呼吸作用与变质程度呈正相关,与货架期呈负相关[3],因此降低呼吸强度对于维持果蔬采后品质具有重要意义。
1.1 呼吸途径
植物的呼吸途径包括糖酵解途径(EMP)、磷酸戊糖途径(HMP)、三羧酸循环(TCA)、发酵途径以及线粒体电子传递链中的细胞色素途径(CCP)和替代途径(AP)[4]。呼吸途径会随外界环境和采后处理技术的变化而发生不同程度的变化,采后果蔬的品质劣变与呼吸速率的高低以及呼吸途径的比例密切相关[3]。EMP 途径作为呼吸代谢的首要环节,是植物体内蛋白质、脂质和糖类等有机物氧化分解的主要途径,较高的EMP途径会加速底物消耗和果蔬衰老[5]。EMP-TCA和HMP途径是植物体内重要的有氧呼吸途径,可为机体提供能量。此外,HMP途径的增强可以提高植物对非生物胁迫的耐受能力[6]。KONG等[7]指出TCA、EMP和HMP途径的比例下调将造成细胞能量供应不足,导致细胞膜的修复能力被削弱和对逆境胁迫的抗性降低。当环境中O2浓度过低时,植物组织会进行无氧呼吸,产生乙醛和乙醇等发酵代谢产物,发酵代谢的激活被指出可能会降低有氧呼吸的比例,从而减少细胞的能量供应,对采后果蔬贮藏期品质产生重要影响[8]。适度厌氧代谢产物积累有利于维持采后果蔬的贮藏期品质,而过度积累会对细胞产生毒性,加速褐变和异味等不良现象的出现[9]。CCP和AP途径是线粒体电子传递链中主要的呼吸途径,可为机体提供能量和抑制ROS的产生。CCP途径的末端氧化酶即细胞色素氧化酶(CCO)的活性可反映组织的呼吸活动和线粒体功能[10]。功能失调的CCO会扰乱线粒体电子传递链(ETC),导致ATP产量减少和能量供应不足[11]。O2(80%)+ CO2(20%)处理可通过抑制双孢蘑菇COX活性的下降来延缓CCP比例的下降,进一步减轻组织受冷害的程度[4]。AP途径主要通过ROS与ATP转换解偶联来抑制ROS的产生,从而提高机体对不良环境的抗逆性,且交替氧化酶(AOX)的活性以及AP基因的表达可由非生物胁迫(如低温或冻害)诱导升高[12]。
1.2 呼吸代谢对细胞能量水平的调控
线粒体是生物体进行呼吸代谢和能量合成的场所,有机物在细胞内通过ETC完成一系列的氧化还原反应,最终生成CO2和H2O,并释放出能量。呼吸途径的调控对于能量供给水平具有重要影响。褪黑素处理可通过降低EMP途径和增加HMP途径比例来减少呼吸底物消耗,为细胞提供充足的能量供应,从而延缓白菜叶片的衰老[3]。纳米复合包装通过抑制呼吸强度来限制ATP水平的下降,从而维持双孢蘑菇的能量状态和改善采后品质[13]。过度的呼吸代谢会消耗大量的代谢物质,从而加速果蔬的成熟和衰老[5]。苹果和梨等呼吸跃变型果实在呼吸跃变时ATP含量会发生剧烈变化,降低呼吸高峰强度可减少呼吸底物消耗,更好地维持贮藏期的品质[14]。由此可见,维持适当的呼吸强度和充足的能量供应对维持采后果蔬货架期品质具有重要意义。
1.3 呼吸代谢对活性氧代谢的影响
线粒体在进行能量供应的同时,其ETC也是重要的ROS发生器。植物ETC见图1[15],其中复合体Ⅰ(NADH脱氢酶)和Ⅲ(CCO)是产生ROS的主要位点,植物组织中大约1%~2%的呼吸代谢总氧消耗会转变为ROS产物,加速组织氧化损伤,进而导致采后果蔬出现褐变、腐烂、软化、风味和营养物质流失等一系列品质劣变问题。CCO和AOX是ETC中2种重要的末端氧化酶,二者不完全氧化会加速ROS的产生。在存在丙酮酸的情况下,AOX可竞争不饱和细胞色素链中的电子来稳定ETC,进而最大程度地减少ROS的产生,从而提高组织抵御逆境胁迫的能力[16]。CHEN等[17]指出,抑制AOX活性可诱导烟草在极端干旱环境中产生ROS,从而加剧组织的氧化损伤。此外,呼吸代谢中吡啶核苷酸的含量也对活性氧代谢具有重要影响。烟酰胺腺嘌呤二核苷酸(磷酸)(NAD(P))在ETC中被还原为还原型烟酰胺腺嘌呤二核苷酸(磷酸)(NAD(P)H),用来支持细胞中的生物合成反应和维持组织免受氧化应激所必需的氧化还原电位[18]。较高的NAD(P)H/NAD(P)比例可导致ETC中电子的泄露,从而加速ROS的产生和对机体的氧化损伤[19]。鲜切甜瓜中较高的NAD(P)H/NAD(P)比例可加速O2−的产生,使组织遭受更高的氧化应激,从而加剧细胞膜氧化损伤和品质劣变[19]。NO处理可通过提高6-磷酸葡萄糖脱氢酶和6-磷酸葡萄糖酸脱氢酶的活性来维持细胞中NAD(P)H的含量,进一步保护香蕉果实免受低温诱导的氧化胁迫,从而提高香蕉的耐寒性[20]。由此可见,呼吸代谢可通过调控呼吸途径关键酶活性和吡啶核苷酸的含量来影响活性氧代谢。
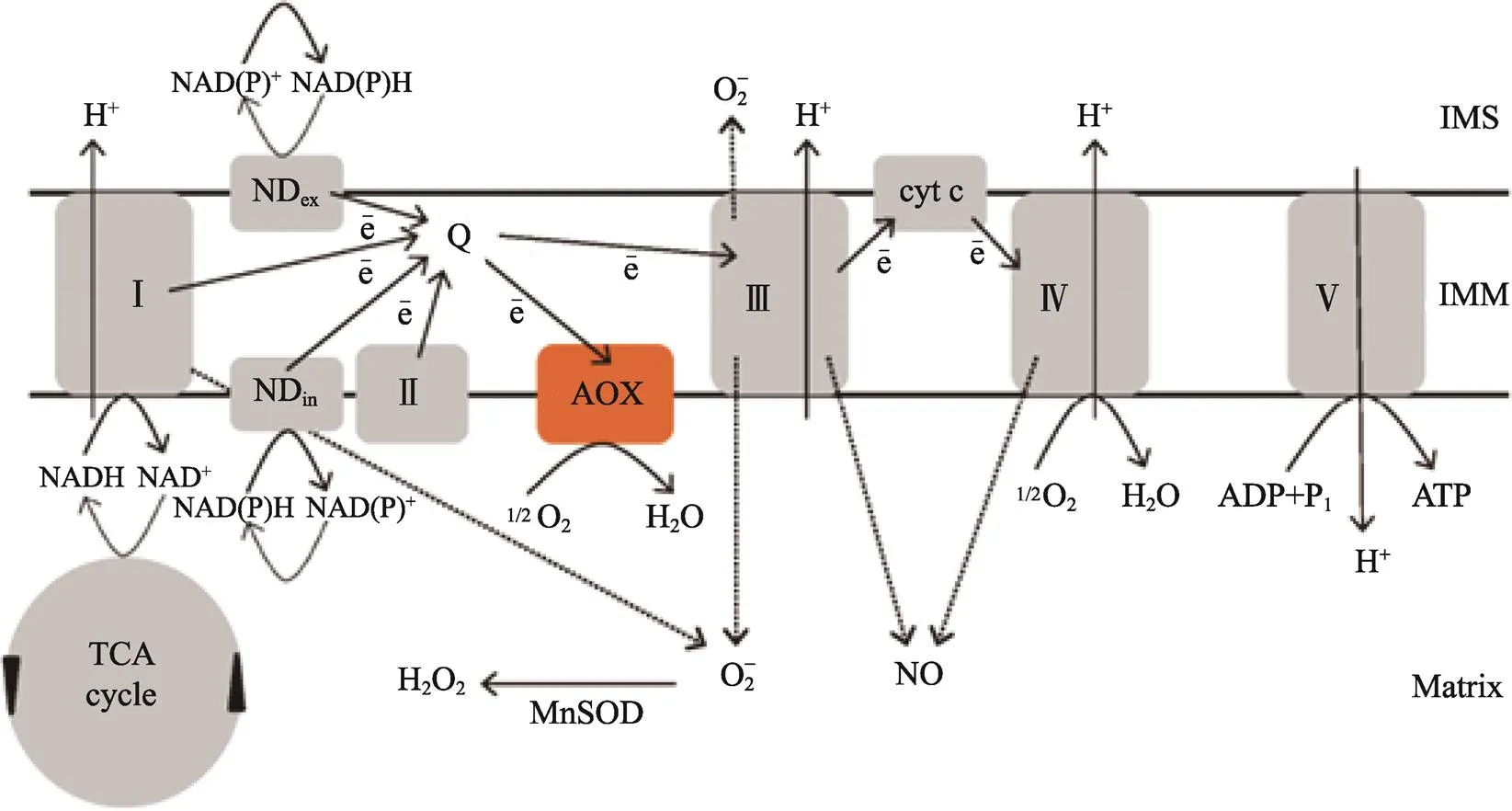
注:复合体Ⅰ(NADH 脱氢酶)催化NADH 氧化生成泛醌(辅酶 Q),复合体Ⅱ(琥珀酸脱氢酶)是泛醌进一步的电子供应,泛醇(泛醌还原型)中的电子被传递到复合体Ⅲ(细胞色素氧化酶),然后经细胞色素c(cyt c)最终传递到复合体Ⅳ(细胞色素氧化酶),复合体Ⅳ催化 O2与电子结合生成H2O,复合体Ⅴ(ATP合成酶)利用复合体Ⅰ、Ⅲ、Ⅳ产生的质子动力势将 ADP 磷酸化生成ATP。复合体Ⅰ和Ⅲ是电子泄露的主要场所
1.4 呼吸代谢对采后果蔬生理品质的调控
呼吸代谢对于采后果蔬的生理品质具有重要影响。较高的呼吸强度会加速营养物质消耗,从而引发一系列品质劣变问题的出现,降低呼吸强度和延缓呼吸跃变型果实呼吸高峰的出现是采后果蔬保鲜技术应用的核心。其中,呼吸跃变型果实在呼吸跃变期间主要以替代途径为主,跃变之后主要以细胞色素氧化途径为主,因此可通过调控呼吸跃变型果实中的替代比例来降低总呼吸强度,从而延缓果蔬衰老[21]。此外,替代途径的抑制也可以降低乙烯的产生量,进一步延缓采后果蔬的成熟和衰老[22]。呼吸强度和呼吸途径关键酶活性对于采后果蔬的颜色、质地、营养、风味物质、抗逆性等具有重要影响。壳聚糖/柠檬酸复合涂膜通过降低呼吸强度,以有效提高胡萝卜的贮藏品质[23]。NO处理可通过抑制EMP途径减少底物的消耗,促进HMP和AP途径,提高细胞抗逆性,进而提高桃的抗氧化性和耐寒性[3]。
2 能量代谢
能量是维持机体理化反应和生理代谢的重要物质。近年来,关于能量代谢的研究主要集中在膜脂合成相关的ATP阈值、高ATP水平调控的采后果蔬保鲜技术、高ATP水平抑制果蔬褐变以及细胞外核苷酸(eATP和eADP)在植物信号转导中的调节作用等方面[24]。采后果实在收获后能量电荷逐渐减少,细胞能量代谢与呼吸代谢、活性氧代谢和果蔬采后品质劣变密切相关,能量状态是影响果蔬采后成熟和衰老的重要因素。
2.1 能量的形式
ATP是储存和运输能量的载体,主要由线粒体氧化磷酸化产生。当果蔬遭遇逆境胁迫或衰老时,组织细胞可通过调控ATP的合成来维持能荷的稳定和线粒体电子传递链的正常运行[21]。过低的能量水平会加速 ROS 的产生和细胞膜的氧化损伤,尤其是当ATP水平降至阈值浓度(0.01 mol/L)以下时,细胞代谢和线粒体功能发生紊乱,导致膜完整性逐渐丧失,从而加速采后果蔬的品质劣变[25]。细胞整体能量状态通常用细胞能量电荷来反映,能荷水平越高,则细胞能量水平越高[26],更有利于采后果蔬品质的维持。纳米复合包装中较高的能荷水平可延缓双孢蘑菇的腐烂和维持细胞的微观结构[13]。此外,ATP/ADP以及AMP/ADP的比值被指出与线粒体的功能和组织衰老密切相关[27]。高ATP/ADP比值可通过抑制EMP途径来减少采后果实的底物消耗和避免生理代谢紊乱的发生[28-29]。由此可见,应用合适的采后保鲜技术将细胞能量状态调控在合适水平是维持采后果蔬品质的关键因素。
2.2 能量状态对采后果蔬呼吸代谢的影响
果蔬在采后仍保持旺盛的生理代谢和细胞活性,植物呼吸代谢强弱主要受到细胞能荷状态的调控。ATP需求通过改变ATP、ADP和AMP之间的比例来控制呼吸速率,从而导致糖酵解酶的变构调节和线粒体电子转运的反馈调节[30]。高水平的ATP可通过降低呼吸代谢来抑制ATP的合成,低水平的ATP可通过增强呼吸代谢来激发ATP合成途径,为机体提供能量,以维持细胞正常生理代谢活动[31]。SHU等[31]指出褪黑素处理可通过维持较高的ATP和ADP水平以及较低的AMP水平来降低呼吸代谢,达到维持大白菜贮藏期品质和延缓衰老的作用。此外,低水平的ATP被指出可能阻断ETC末端的电子转移,从而抑制呼吸代谢和能量供应[32]。由此可见,采后果蔬贮藏期间呼吸和能量代谢的相互调节机制仍需进一步研究。
2.3 能量状态对活性氧积累的影响
采后果蔬的能量代谢与ROS积累密切相关。当果蔬遭遇逆境胁迫或衰老时,线粒体加速运转会在为机体提供能量的同时加速ROS的产生。高水平的ATP可触发受损果实中ROS的产生,但对激活抗氧化系统的作用效果较弱[19]。例如,高水平的ATP可触发信号分子ROS的产生,可诱导鲜切甜瓜伤口处酚 类物质的积累,从而增强细胞对切割创伤的应激能 力[33];外源ATP可诱导拟南芥受害叶片中O2−的积累,从而加速其品质的劣变进程[34]。也有相关报道称,高ATP水平可通过诱导抗氧化酶活性升高和内源抗氧化物质积累来激活抗氧化防御系统,从而减少ROS的产生和细胞氧化损伤。CHEN等[35]指出较高的能量状态可诱导抗氧化酶活性的升高,从而使采后黄瓜免受低温氧化损伤。由此可见,采后果蔬贮藏期间能量状态对ROS的产生是抑制作用还是促进作用仍需要进一步研究。
2.4 能量亏缺对细胞膜完整性的损伤
细胞膜是生物体的重要组成部分,膜的完整性对维持细胞的功能性具有重要意义。膜的完整性和细胞膜内外渗透压的维持、膜脂功能蛋白的合成、跨膜K+和Na+的转运均依赖于ATP合成[36]。当果实遭遇逆境胁迫或衰老时,ATP合成能力降低导致能量亏缺,而能量亏缺会加速细胞膜水解,破坏细胞内外渗透压,改变细胞膜组分和结构,从而导致细胞膜修复功能的失调,以及细胞膜脂质过氧化和细胞膜通透性的增加,细胞膜完整性受损。例如,涂膜和热处理可通过维持葡萄果实中较高的能荷和ATP水平来维持细胞膜的完整性,从而延缓果实的自溶软化[37];外源ATP有助于维持果蔬不饱和脂肪酸水平和膜的完整性,从而抑制荔枝果实的疾病发展[38]。
能量状态的变化对脂质过氧化的影响通过间接影响膜脂肪酸整体状态实现。这是因为膜脂质过氧化主要是不饱和脂肪酸的氧化,而不饱和脂肪酸的氧化可促进饱和脂肪酸的积累,进而扰乱细胞膜稳态和损伤细胞膜的完整性和功能性[39]。蓝莓果实在低温贮藏期间较低的能荷水平不足以促进磷脂的合成以及修复已被脂质过氧化损伤的细胞膜,从而导致应激反应中苯丙氨酸解氨酶和脂氧合酶活性增加,进一步加速膜完整性和功能性的损伤[40]。2,4-二硝基苯酚处理可通过降低ATP含量来诱导磷脂酶D、脂肪酶和脂肪氧合酶活性的升高,磷脂酰胆碱、磷脂酰肌醇和不饱和脂肪酸含量的下降,以及磷脂酸和饱和脂肪酸含量的升高,加速细胞膜降解和膜氧化损伤,从而加剧龙眼果实褐变和病害的发生[41]。
2.5 能量代谢对采后果蔬生理品质的调控
采后果蔬组织褐变、病原体感染和冷害的发生与细胞能量状态密切相关。高水平的ATP对于抑制苹果[42]和蘑菇[43]等果蔬的褐变具有积极效应。线粒体能量代谢是影响园艺作物病原体感染的关键因素,组织能量亏缺会加速细胞膜的氧化损伤,导致细胞区室化丧失和加速病原体的发育,从而降低细胞的抗病性[44]。β-氨基丁酸处理可通过维持较高的ATP水平来提高桃果实对根霉腐烂病的抗病性[45]。茉莉酸甲酯处理也可通过维持较高的ATP水平来减少枇杷果实炭疽病的发生[46]。细胞能量可通过直接影响膜脂的生物合成和细胞膜的修复来介导冷藏果实的抗寒性[47]。Ca2+-ATPase是细胞内主要的Ca2+转运蛋白,可维持细胞内的Ca2+稳态,从而减轻冷敏感植物在冷胁迫下的代谢功能障碍和结构损伤[48],因此高ATP水平有助于减轻采后果蔬贮藏期间冷害的发生。JIN等[49]指出H+-ATPase和Ca2+-ATPase活性的增强有助于提高桃果实在冷藏期间的耐冷性,且高ATP水平对于缓解西葫芦[49]和桃[50]等果蔬的冷害也表现出积极效应。
3 活性氧代谢
采后果实衰老是一种氧化现象,ROS是植物氧化损伤的主要介质,过量ROS积累会引发氧化胁迫作用,导致采后果蔬呼吸代谢紊乱、能量供应不足和生物膜功能丧失,进一步加速采后果蔬衰老、冷害、褐变、软化等品质劣变的发生。适度的ROS积累可作为信号分子激活抗氧化防御系统,从而维持细胞稳态和减少细胞膜氧化损伤,因此采用合适的贮藏保鲜技术来减少ROS产生,以维持细胞内ROS稳态,对于减轻细胞氧化损伤和降低品质劣变具有重要意义。
3.1 活性氧产生与清除系统
采后果蔬贮藏期间的生物和非生物胁迫会导致ETC中末端氧化酶COX和AOX不完全氧化,从而加速ROS的产生。ROS主要包括O2−、H2O2和·OH等,H2O2是抗氧化胁迫反应中的重要信号分子和启动衰老的重要因子,可引发抗氧化级联反应和植物的程序化死亡[51-52]。·OH是细胞中毒性最强的自由基,可导致核酸和蛋白质等分子变性。孙志栋等[53]指出采前喷施30 mg/L Nα-月桂酰-L-精氨酸乙酯盐酸盐可通过提高·OH的清除率来提高冷藏蓝莓的好果率。此外,·OH可提高乙烯合成酶活性,进而诱导内源乙烯的合成,从而加速果蔬的成熟和衰老进程[54]。由于ROS对植物的生长代谢也表现出一定的积极作用,低浓度的ROS在细胞信号传递过程中可作为第二信使诱导植物对激素和环境胁迫产生多种应答反应,从而提高组织抗逆性[55]。此外,ROS也参与了细胞壁与蛋白质的交联以及细胞壁的木质化过程,可加强细胞壁的防御性,以阻止病原菌入侵,因此可作为抗菌剂直接使用[56]。
超氧化物歧化酶(SOD)、过氧化氢酶(CAT)、过氧化物酶(POD)和抗坏血酸过氧化物酶(APX)等抗氧化酶,以及总酚、黄酮、抗坏血酸和花青素等内源抗氧化物质可构成ROS清除系统,维持细胞内的ROS代谢平衡和减轻细胞氧化损伤。应用合适的贮藏保鲜技术以提高细胞的抗氧化性对于维持采后果蔬品质具有重要影响。甘氨酸甜菜碱处理可通过诱导抗氧化酶活性提高来减轻桃果实的氧化损伤和维持更好的采后品质[57]。短期厌氧处理可通过提高细胞抗氧化性来延缓脂质过氧化作用,进一步抑制枇杷果实褐变和腐烂发生[58]。
3.2 活性氧代谢对呼吸代谢的影响
当果蔬遭受逆境胁迫或衰老时,过量ROS积累会加速脂质过氧化和细胞膜通透性的增加,使细胞膜完整性受损,而线粒体功能主要依赖于线粒体膜的完整性,因此活性氧代谢对于呼吸速率、呼吸途径关键酶活性和吡啶核苷酸含量具有重要影响。鲜切甜瓜中较高的ROS积累将导致CCO功能障碍,降低氧化磷酸化过程中O2的利用能力,从而减少ATP的产生,导致更多的O2−积累,从而加速细胞氧化损伤[59]。在O2(80%)+CO2(20%)的气调环境下,可通过抑制ROS和NO的产生诱导EMP和CCP途径关键酶活性的降低,从而减少底物的消耗,更有利于双孢蘑菇贮藏期间的品质维持[2]。
3.3 活性氧代谢对能量代谢的影响
ROS水平对线粒体的结构和功能具有重要影响。植物组织中超过95%的ATP由位于线粒体内膜的呼吸代谢氧化磷酸化产生,过量ROS积累会加剧线粒体的功能性障碍,使氧化磷酸化解偶联,不能满足细胞正常代谢的能量供应,严重时会导致细胞程序性死亡[60]。低水平的ROS积累可通过减轻细胞膜氧化损伤,从而维持细胞膜的完整性,进一步避免能量代谢紊乱现象的发生,从而延缓桃果实的品质劣变[61]。纳米复合包装可通过减轻由ROS积累引起的氧化损伤来维持双孢蘑菇的正常能量代谢,更好地维持双孢蘑菇贮藏期间的微观结构[13]。此外,ZHAO等[60]研究发现,拟南芥中ROS的产生可作为信号分子来调节能量代谢,从而减少采后果实的品质劣变。
3.4 活性氧代谢对细胞膜完整性的影响
生物膜的稳定性受膜脂降解酶活性、磷脂组分、膜脂脂肪酸组成及含量等多方面因素的影响。ROS积累是引起细胞膜损伤和代谢失调的重要原因。组织中ROS的过量积累会攻击细胞膜的多价不饱和脂肪酸,导致蛋白质和核酸(酶)等大分子的分解,引起原生质膜脱脂化和过氧化[62]。膜脂过氧化作用会进一步破坏线粒体呼吸和氧化磷酸化,导致细胞膜通透性增大和流动性降低,离子转运机制紊乱,亚油酸、亚麻酸等不饱和脂肪酸含量降低,膜结合酶功能受到影响,细胞氧化损伤加剧[62]。此外,膜脂过氧化作用还会产生脱落酸和茉莉酸等植物激素,促进乙烯的产生,从而加速果实的成熟和衰老进程[63]。外源施用H2O2可通过降低抗氧化酶活性和内源性抗氧化物质积累来降低细胞清除ROS的能力,增加O2−的产生,从而进一步加速细胞膜不饱和脂肪酸降解和脂质过氧化,对采后龙眼果实的品质产生不良影响[64]。2,4-二硝基苯酚处理可诱导采后龙眼果实中ROS积累、脂氧化酶活性增加和膜脂的不饱和脂肪酸降解,进而导致细胞膜结构完整性和细胞区室化消失,多酚氧化酶和/或过氧化物酶与酚类底物接触形成棕色聚合物,从而加速果皮褐变[44]。此外,外源性施用ROS清除剂没食子酸丙酯可提高细胞清除ROS的能力,从而降低ROS的产生,延缓脂质过氧化和细胞膜通透性的增加,更好地维持细胞膜结构的完整性,从而抑制采后龙眼果实果皮褐变的发生[65]。
3.5 活性氧对采后果蔬生理品质的调控
ROS代谢对于采后果蔬的褐变、冷害以及组织抗病性具有重要影响。RUENROENGKLIN等[66]指出,荔枝果实果皮褐变与H2O2和·OH含量的快速增加密切相关。细胞膜是冷害发生的主要部位,细胞膜脂质不饱和度的降低会加速采后果实低温贮藏期间冷害的发生[67]。茉莉酸甲酯处理可通过降低细胞膜不饱和/饱和脂肪酸比例来减轻枇杷果实冷害的发 展[68]。此外,细胞抗氧化性的提高被指出也可减轻采后果实冷害的发生。抗氧化酶活性的提高可减少青 椒[69]和番茄[70]等果实低温贮藏期间冷害的发生。采后果蔬病害的发生也与细胞ROS水平密切相关,过量ROS积累产生的强烈氧化胁迫会造成果蔬组织和拮抗菌细胞内氧化还原状态失衡,蛋白质和核酸等生物分子变性,拮抗菌的拮抗能力降低,从而提高果蔬采后病害的发生率;由于低水平的ROS可作为第二信使诱导宿主细胞抗病性的提高,抑制病原菌的侵染,从而降低果蔬采后病害发生率[71]。0.1 mol/L硅酸钠处理可通过诱导H2O2和O2-等ROS的积累来引发采后甜瓜对玫瑰单端孢霉产生防御和应激反应,从而降低病害的发生[72]。由此可见,应用低浓度ROS提高果蔬抗病性有望成为降低果蔬采后病害发生率的有效技术[73]。
4 结语
采后果蔬的呼吸代谢、能量代谢和活性氧代谢与品质劣变密切相关。呼吸代谢在为采后果蔬的生理代谢提供能量的同时,会产生ROS对细胞造成氧化损伤,从而加速品质劣变。细胞能量亏缺会促进呼吸代谢来为机体提供能量,同时也会加速ROS的产生,对机体造成氧化损伤,进一步加速采后果蔬贮藏期间褐变、冷害和病原菌侵染的发生。ROS在采后生理代谢中表现出两方面的作用,适度的ROS积累可作为信号分子激活抗氧化防御系统,从而维持细胞ROS稳态和减少细胞氧化损伤,而ROS的过量积累会加速细胞膜的氧化损伤,导致线粒体结构和功能障碍,进一步影响细胞的呼吸和能量代谢。由此可见,植物组织的呼吸代谢、能量代谢和活性氧代谢共同构成了一个复杂的生理代谢网络,相互影响共同调控采后果蔬的生理品质。应用合适的贮藏保鲜技术将采后果蔬的生理代谢活动调控在合适水平是提高果蔬贮藏保鲜效果的关键。
[1] 王志伟. 果蔬加工技术现状与发展探讨[J]. 现代农业研究, 2021, 27(6): 135-136.
WANG Zhi-wei. Present Situation and Development of Fruit and Vegetable Processing Technology[J]. Modern Agriculture Research, 2021, 27(6): 135-136.
[2] LI Ling, KITAZAWA H, WANG Xiang-you, et al. Regulation of Respiratory Pathway and Electron Transport Chain in Relation to Senescence of Postharvest White Mushroom (Agaricus Bisporus) under High O2/CO2Controlled Atmospheres[J]. Journal of Agricultural and Food Chemistry, 2017, 65(16): 3351-3359.
[3] TAN Xiao-li, FAN Zhong-qi, ZENG Ze-xiang, et al. Exogenous Melatonin Maintains Leaf Quality of Postharvest Chinese Flowering Cabbage by Modulating Respiratory Metabolism and Energy Status[J]. Postharvest Biology and Technology, 2021, 177: 111524.
[4] LI Ling, KITAZAWA H, ZHANG Rong-fei, et al. New Insights into the Chilling Injury of Postharvest White Mushroom (Agaricus Bisporus) Related to Mitochondria and Electron Transport Pathway under High O2/CO2Controlled Atmospheres[J]. Postharvest Biology and Technology, 2019, 152: 45-53.
[5] LI Ling, LV Feng-yan, GUO Yan-yin, et al. Respiratory Pathway Metabolism and Energy Metabolism Associated with Senescence in Postharvest Broccoli (Brassica Oleracea L. Var. Italica) Florets in Response to O2/CO2Controlled Atmospheres[J]. Postharvest Biology and Technology, 2016, 111: 330-336.
[6] SLASKI J J, ZHANG Gui-chang, BASU U, et al. Aluminum Resistance in Wheat (Triticum Aestivum) is Associated with Rapid, Al-Induced Changes in Activities of Glucose-6-Phosphate Dehydrogenase and 6-Phosphogluconate Dehydrogenase in Root Apices[J]. Physiologia Plantarum, 1996, 98(3): 477-484.
[7] KONG Qing-jun, QI Jian-rui, AN Pei-pei, et al. Melaleuca Alternifolia Oil can Delay Nutrient Damage of Grapes Caused by Aspergillus Ochraceus through Regulation of Key Genes and Metabolites in Metabolic Pathways[J]. Postharvest Biology and Technology, 2020, 164: 111152.
[8] LI Dong, LI Li, XIAO Gong-nian, et al. Effects of Elevated CO2on Energy Metabolism and Γ-Aminobutyric Acid Shunt Pathway in Postharvest Strawberry Fruit[J]. Food Chemistry, 2018, 265: 281-289.
[9] FEYGENBERG O, HERSHKOVITZ V, BEN-ARIE R, et al. Postharvest Use of Organic Coating for Maintaining bio-Organic Avocado and Mango Quality[J]. Acta Horticulturae, 2005(682): 507-512.
[10] LIN Yi-fen, LIN Yi-xiong, LIN He-tong, et al. Application of Propyl Gallate Alleviates Pericarp Browning in Harvested Longan Fruit by Modulating Metabolisms of Respiration and Energy[J]. Food Chemistry, 2018, 240: 863-869.
[11] LIN Yi-xiong, LIN He-tong, CHEN Yi-hui, et al. The Role of ROS-Induced Change of Respiratory Metabolism in Pulp Breakdown Development of Longan Fruit during Storage[J]. Food Chemistry, 2020, 305: 125439.
[12] WANG Jia, RAJAKULENDRAN N, AMIRSADEGHI S, et al. Impact of Mitochondrial Alternative Oxidase Expression on the Response of Nicotiana Tabacum to Cold Temperature[J]. Physiologia Plantarum, 2011, 142(4): 339-351.
[13] WU Yuan-yue, HU Qiu-hui, LI Zhi-xiao, et al. Effect of Nanocomposite-Based Packaging on Microstructure and Energy Metabolism of Agaricus Bisporus[J]. Food Chemistry, 2019, 276: 790-796.
[14] SAQUET A A, STREIF J, BANGERTH F. Energy Metabolism and Membrane Lipid Alterations in Relation to Brown Heart Development in ‘Conference’ Pears during Delayed Controlled Atmosphere Storage[J]. Postharvest Biology and Technology, 2003, 30(2): 123-132.
[15] VANLERBERGHE G C. Alternative Oxidase: A Mitochondrial Respiratory Pathway to Maintain Metabolic and Signaling Homeostasis during Abiotic and Biotic Stress in Plants[J]. International Journal of Molecular Sciences, 2013, 14(4): 6805-6847.
[16] MOLLER I M. Plant Mitochondria and Oxidative Stress: Electron Transport, NADPH Turnover, and Metabolism of Reactive Oxygen Species[J]. Annual Review of Plant Physiology and Plant Molecular Biology, 2001, 52: 561-591.
[17] CHEN Yi-hui, SUN Jun-zheng, LIN He-tong, et al. Salicylic Acid Reduces the Incidence of Phomopsis Longanae Chi Infection in Harvested Longan Fruit by Affecting the Energy Status and Respiratory Metabolism[J]. Postharvest Biology and Technology, 2020, 160: 111035.
[18] KRUGER N J, VON SCHAEWEN A. The Oxidative Pentose Phosphate Pathway: Structure and Organisation[J]. Current Opinion in Plant Biology, 2003, 6(3): 236-246.
[19] WU Zhang-fei, TU Ming-mei, YANG Xing-ping, et al. Effect of Cutting on the Reactive Oxygen Species Accumulation and Energy Change in Postharvest Melon Fruit during Storage[J]. Scientia Horticulturae, 2019, 257: 108752.
[20] WANG Yan-sheng, LUO Zi-sheng, KHAN Z U, et al. Effect of Nitric Oxide on Energy Metabolism in Postharvest Banana Fruit in Response to Chilling Stress[J]. Postharvest Biology and Technology, 2015, 108: 21-27.
[21] 郭芹, 李庆鹏, 靳婧, 等. 交替氧化酶在果蔬抗氰呼吸途径的调控机制[J]. 生物技术进展, 2013, 3(6): 412-415.
GUO Qin, LI Qing-peng, JIN Jing, et al. Regulation Mechanism of Alternative Oxidase in Cyanide-Resistant Respiration Pathway of Fruits and Vegetables[J]. Current Biotechnology, 2013, 3(6): 412-415.
[22] GUO Qin, LV Xin, XU Fei, et al. Chlorine Dioxide Treatment Decreases Respiration and Ethylene Synthesis in Fresh-Cut 'Hami' Melon Fruit[J]. International Journal of Food Science & Technology, 2013, 48(9): 1775-1782.
[23] 董文丽, 巩雪, 侯理达, 等. 壳聚糖/柠檬酸复合涂膜对胡萝卜的保鲜效果[J]. 包装工程, 2021, 42(9): 72-78.
DONG Wen-li, GONG Xue, HOU Li-da, et al. Effects of Chitosan and Citric Acid Composite Film on Preservation of Carrot[J]. Packaging Engineering, 2021, 42(9): 72-78.
[24] ZHOU Qian, ZHANG Chun-lei, CHENG Shun-chang, et al. Changes in Energy Metabolism Accompanying Pitting in Blueberries Stored at Low Temperature[J]. Food Chemistry, 2014, 164: 493-501.
[25] RAWYLER A, ARPAGAUS S, BRAENDLE R. Impact of Oxygen Stress and Energy Availability on Membrane Stability of Plant Cells[J]. Annals of Botany, 2002, 90(4): 499-507.
[26] JIANG Y, JIANG Y, QU H, et al. Energy Aspects in Ripening and Senescence of Harvested Horticultural Crops[J]. Stewart Postharvest Review, 2007, 3(2): 1-5.
[27] WANG Wen-gong, YANG Xiao-ling, LÓPEZ DE SILANES I, et al. Increased AMP: ATP Ratio and AMP-Activated Protein Kinase Activity during Cellular Senescence Linked to Reduced HuR Function[J]. Journal of Biological Chemistry, 2003, 278(29): 27016-27023.
[28] MALDONADO E N, LEMASTERS J J. ATP/ADP Ratio, the Missed Connection between Mitochondria and the Warburg Effect[J]. Mitochondrion, 2014, 19: 78-84.
[29] SAQUET A A, STREIF J, BANGERTH F. Changes in ATP, ADP and Pyridine Nucleotide Levels Related to the Incidence of Physiological Disorders in 'Conference' Pears and 'Jonagold' Apples during Controlled Atmosphere Storage[J]. The Journal of Horticultural Science and Biotechnology, 2000, 75(2): 243-249.
[30] GEIGENBERGER P, RIEWE D, FERNIE A R. The Central Regulation of Plant Physiology by Adenylates[J]. Trends in Plant Science, 2010, 15(2): 98-105.
[31] SHU Chang, ZHANG Wan-li, ZHAO Han-dong, et al. Chlorogenic Acid Treatment Alleviates the Adverse Physiological Responses of Vibration Injury in Apple Fruit through the Regulation of Energy Metabolism[J]. Postharvest Biology and Technology, 2020, 159: 110997.
[32] PARTRIDGE R S, MONROE S M, PARKS J K, et al. Spin Trapping of Azidyl and Hydroxyl Radicals in Azide-Inhibited Rat Brain Submitochondrial Particles[J]. Archives of Biochemistry and Biophysics, 1994, 310(1): 210-217.
[33] LI Xiao-an, LONG Qing-hong, GAO Fan, et al. Effect of Cutting Styles on Quality and Antioxidant Activity in Fresh-Cut Pitaya Fruit[J]. Postharvest Biology and Technology, 2017, 124: 1-7.
[34] SONG C J, STEINEBRUNNER I, WANG Xuan-zhi, et al. Extracellular ATP Induces the Accumulation of Superoxide via NADPH Oxidases in Arabidopsis[J]. Plant Physiology, 2006, 140(4): 1222-1232.
[35] CHEN Bing-xia, YANG Hu-qing. 6-Benzylaminopurine Alleviates Chilling Injury of Postharvest Cucumber Fruit through Modulating Antioxidant System and Energy Status[J]. Journal of the Science of Food and Agriculture, 2013, 93(8): 1915-1921.
[36] PRADET A, RAYMOND P. Adenine Nucleotide Ratios and Adenylate Energy Charge in Energy Metabolism[J]. Annual Review of Plant Physiology, 1983, 34: 199-224.
[37] 张群, 周文化, 谭欢, 等. 葡萄果实采后自溶软化与细胞膜完整性及线粒体内能量代谢的关系[J]. 现代食品科技, 2016, 32(12): 45-54.
ZHANG Qun, ZHOU Wen-hua, TAN Huan, et al. Changes in Cell Membrane Integrity and Mitochondrial Energy Metabolism of Postharvest Grape Fruits during Aril Breakdown[J]. Modern Food Science and Technology, 2016, 32(12): 45-54.
[38] YI C, QU H X, JIANG Y M, et al. ATP-Induced Changes in Energy Status and Membrane Integrity of Harvested Litchi Fruit and Its Relation to Pathogen Resistance[J]. Journal of Phytopathology, 2008, 156(6): 365-371.
[39] HUANG Hua, GUO Li-fang, WANG Ling, et al. 1-Methylcyclopropene (1-MCP) Slows Ripening of Kiwifruit and Affects Energy Status, Membrane Fatty Acid Contents and Cell Membrane Integrity[J]. Postharvest Biology and Technology, 2019, 156: 110941.
[40] YI Chun, JIANG Yue-ming, SHI J, et al. ATP-Regulation of Antioxidant Properties and Phenolics in Litchi Fruit during Browning and Pathogen Infection Process[J]. Food Chemistry, 2010, 118(1): 42-47.
[41] LIN Yi-fen, CHEN Meng-yin, LIN He-tong, et al. Phomopsis Longanae-Induced Pericarp Browning and Disease Development of Longan Fruit can Be Alleviated or Aggravated by Regulation of ATP-Mediated Membrane Lipid Metabolism[J]. Food Chemistry, 2018, 269: 644-651.
[42] 王志华, 贾朝爽, 王文辉, 等. 低温贮藏对‘金红’苹果能量代谢和品质的影响[J]. 园艺学报, 2020, 47(12): 2277-2289.
WANG Zhi-hua, JIA Chao-shuang, WANG Wen-hui, et al. Effects of Low Temperature Storage on Energy Metabolism, Related Physiology and Quality in 'Jinhong' Apple Fruit[J]. Acta Horticulturae Sinica, 2020, 47(12): 2277-2289.
[43] 蔺凯丽, 黄琦, 黄琦辉, 等. 麦角硫因抑制双孢蘑菇褐变及其与能量代谢关系[J]. 中国农业科学, 2018, 51(8): 1568-1576.
LIN Kai-li, HUANG Qi, HUANG Qi-hui, et al. Browning Inhibition and Energy Metabolism Mechanism of Agaricus Bisporus by Ergothioneine Treatment[J]. Scientia Agricultura Sinica, 2018, 51(8): 1568-1576.
[44] 陈艺晖, 林河通, 林艺芬, 等. 拟茎点霉侵染对采后龙眼果皮LOX活性和膜脂脂肪酸组分的影响[J]. 热带亚热带植物学报, 2011, 19(3): 260-266.
CHEN Yi-hui, LIN He-tong, LIN Yi-fen, et al. Effects of Phomopsis Longanae Chi Infection on Lipoxygenase Activity and Fatty Acid Constituents of Membrane Lipids in Pericarp of Harvested Longan Fruits[J]. Journal of Tropical and Subtropical Botany, 2011, 19(3): 260-266.
[45] WANG Jing, CAO Shi-feng, WANG Lei, et al. Effect of Β-Aminobutyric Acid on Disease Resistance Against Rhizopus Rot in Harvested Peaches[J]. Frontiers in Microbiology, 2018, 9: 1505.
[46] CAO Shi-feng, CAI Yu-ting, YANG Zhen-feng, et al. Effect of MeJA Treatment on Polyamine, Energy Status and Anthracnose Rot of Loquat Fruit[J]. Food Chemistry, 2014, 145: 86-89.
[47] JIN Peng, ZHU Hong, WANG Lei, et al. Oxalic Acid Alleviates Chilling Injury in Peach Fruit by Regulating Energy Metabolism and Fatty Acid Contents[J]. Food Chemistry, 2014, 161: 87-93.
[48] JIAN Ling-cheng, LI Ji-hong, CHEN Wen-ping, et al. Cytochemical Localization of Calcium and Ca2+-ATPase Activity in Plant Cells under Chilling Stress: A Comparative Study between the Chilling-Sensitive Maize and the Chilling-Insensitive Winter Wheat[J]. Plant and Cell Physiology, 1999, 40(10): 1061-1071.
[49] 张苗, 姜玉, 汤静, 等. 冷激结合甜菜碱处理对西葫芦冷害及能量代谢的影响[J]. 食品科学, 2020, 41(7): 184-190.
ZHANG Miao, JIANG Yu, TANG Jing, et al. Effects of Cold Shock Combined with Glycine Betaine Treatment on Chilling Injury and Energy Metabolism of Zucchini[J]. Food Science, 2020, 41(7): 184-190.
[50] 赵颖颖, 陈京京, 金鹏, 等. 低温预贮对冷藏桃果实冷害及能量水平的影响[J]. 食品科学, 2012, 33(4): 276-281.
ZHAO Ying-ying, CHEN Jing-jing, JIN Peng, et al. Effect of Low Temperature Conditioning on Chilling Injury and Energy Status in Cold-Stored Peach Fruit[J]. Food Science, 2012, 33(4): 276-281.
[51] SPYCHALLA J P, DESBOROUGH S L. Superoxide Dismutase, Catalase, and Alpha-Tocopherol Content of Stored Potato Tubers[J]. Plant Physiology, 1990, 94(3): 1214-1218.
[52] DESIKAN R, REYNOLDS A, HANCOCK J T, et al. Harpin and Hydrogen Peroxide both Initiate Programmed Cell Death but Have Differential Effects on Defence Gene Expression in Arabidopsis Suspension Cultures[J]. The Biochemical Journal, 1998, 330(Pt 1): 115-120.
[53] 孙志栋, 李共国, 王利平, 等. 采前喷施LAE提高蓝莓冷藏品质及其通径分析[J]. 包装工程, 2021, 42(7): 28-34.
SUN Zhi-dong, LI Gong-guo, WANG Li-ping, et al. Path Analysis of Improving Cold Storage Quality of Blueberry by Preharvest Spraying LAE[J]. Packaging Engineering, 2021, 42(7): 28-34.
[54] 柯德森, 王爱国, 罗广华. 成熟香蕉果实活性氧与乙烯形成酶活性的关系[J]. 植物生理学报, 1998, 24(4): 313-319.
KE De-sen, WANG Ai-guo, LUO Guang-hua. The Relationship between Active Oxygen and the Activity of Ethylene-Forming Enzyme in Ripening Banana Fruit[J]. Acta Photophysiologica Sinica, 1998, 24(4): 313-319.
[55] WANG Di, LI Wen-xuan, LI Dong, et al. Effect of High Carbon Dioxide Treatment on Reactive Oxygen Species Accumulation and Antioxidant Capacity in Fresh-Cut Pear Fruit during Storage[J]. Scientia Horticulturae, 2021, 281: 109925.
[56] HÜCKELHOVEN R, KOGEL K H. Reactive Oxygen Intermediates in Plant-Microbe Interactions: Who is who in Powdery Mildew Resistance? [J]. Planta, 2003, 216(6): 891-902.
[57] 王懿, 侯媛媛, 马钰晴, 等. 甘氨酸甜菜碱处理对桃果实冷害及抗坏血酸-谷胱甘肽循环代谢的影响[J]. 食品科学, 2021, 42(13): 158-165.
WANG Yi, HOU Yuan-yuan, MA Yu-qing, et al. Effect of Glycine Betaine Treatment on Chilling Injury and Ascorbic Acid-Glutathione Cycle Metabolism in Peach Fruit[J]. Food Science, 2021, 42(13): 158-165.
[58] GAO Hai-yan, TAO Fei, SONG Li-li, et al. Effects of Short-Term N2treatment on Quality and Antioxidant Ability of Loquat Fruit during Cold Storage[J]. Journal of the Science of Food and Agriculture, 2009, 89(7): 1159-1163.
[59] SRINIVASAN S, AVADHANI N G. Cytochrome c Oxidase Dysfunction in Oxidative Stress[J]. Free Radical Biology and Medicine, 2012, 53(6): 1252-1263.
[60] ZHAO Yan-nan, YU Hong, ZHOU Jian-min, et al. Malate Circulation: Linking Chloroplast Metabolism to Mitochondrial ROS[J]. Trends in Plant Science, 2020, 25(5): 446-454.
[61] KAN Juan, WANG Hong-mei, JIN Chang-hai. Changes of Reactive Oxygen Species and Related Enzymes in Mitochondrial Respiration during Storage of Harvested Peach Fruits[J]. Agricultural Sciences in China, 2011, 10(1): 149-158.
[62] INZÉ D, MONTAGU M V. Oxidative Stress in Plants[J]. Current Opinion in Biotechnology, 1995, 6(2): 153-158.
[63] PUKACKA S, RATAJCZAK E. Production and Scavenging of Reactive Oxygen Species in Fagus Sylvatica Seeds during Storage at Varied Temperature and Humidity[J]. Journal of Plant Physiology, 2005, 162(8): 873-885.
[64] LIN Yi-fen, LIN He-tong, ZHANG Shen, et al. The Role of Active Oxygen Metabolism in Hydrogen Peroxide-Induced Pericarp Browning of Harvested Longan Fruit[J]. Postharvest Biology and Technology, 2014, 96: 42-48.
[65] LIN Yi-fen, LIN Yi-xiong, LIN He-tong, et al. Inhibitory Effects of Propyl Gallate on Browning and Its Relationship to Active Oxygen Metabolism in Pericarp of Harvested Longan Fruit[J]. LWT - Food Science and Technology, 2015, 60(2): 1122-1128.
[66] RUENROENGKLIN N, YANG Bao, LIN He-tong, et al. Degradation of Anthocyanin from Litchi Fruit Pericarp by H2O2and Hydroxyl Radical[J]. Food Chemistry, 2009, 116(4): 995-998.
[67] MARANGONI A G, PALMA T, STANLEY D W. Membrane Effects in Postharvest Physiology[J]. Postharvest Biology and Technology, 1996, 7(3): 193-217.
[68] CAO Shi-feng, ZHENG Yong-hua, WANG Kai-tuo, et al. Methyl Jasmonate Reduces Chilling Injury and Enhances Antioxidant Enzyme Activity in Postharvest Loquat Fruit[J]. Food Chemistry, 2009, 115(4): 1458-1463.
[69] 张萌, 曹婷婷, 程紫薇, 等. 高湿贮藏对青椒果实冷害和抗氧化活性的影响[J]. 食品科学, 2021, 42(3): 243-250.
ZHANG Meng, CAO Ting-ting, CHENG Zi-wei, et al. Effect of High Relative Humidity Storage on Chilling Injury and Antioxidant Activity of Green Pepper Fruits[J]. Food Science, 2021, 42(3): 243-250.
[70] 乔勇进, 王梦晗, 王凯晨, 等. 酵母多糖处理提高樱桃番茄抗冷性的机制分析[J]. 食品科学, 2016, 37(10): 240-245.
QIAO Yong-jin, WANG Meng-han, WANG Kai-chen, et al. Mechanism Underlying the Improvement of Cold Resistance in Cherry Tomato Treated with Yeast Saccharide[J]. Food Science, 2016, 37(10): 240-245.
[71] CHEN Z, RICIGLIANO J W, KLESSIG D F. Purification and Characterization of a Soluble Salicylic Acid-Binding Protein from Tobacco[J]. Proceedings of the National Academy of Sciences of the United States of America, 1993, 90(20): 9533-9537.
[72] LYU Liang, BI Yang, LI Shen-ge, et al. Sodium Silicate Prime Defense Responses in Harvested Muskmelon by Regulating Mitochondrial Energy Metabolism and Reactive Oxygen Species Production[J]. Food Chemistry, 2019, 289: 369-376.
[73] WANG Yi-fei, WANG Peng, XIA Jin-dan, et al. Effect of Water Activity on Stress Tolerance and Biocontrol Activity in Antagonistic Yeast Rhodosporidium Paludigenum[J]. International Journal of Food Microbiology, 2010, 143(3): 103-108.
Advances in Physiological Metabolism Changes and Regulation Mechanism of Harvested Fruits and Vegetables
TANG Jian-xin, WANG Jia-li, YING Li-mei, ZHANG Yun-he, SUN Bing-xin
(College of Food Science, Shenyang Agricultural University, Shenyang 110866, China)
The work aims to discuss the relationship respectively between the respiratory metabolism, energy metabolism and reactive oxygen species metabolism and quality deterioration of harvested fruits and vegetables, in order to provide theoretical support and guidance for the application of harvested fruits and vegetables preservation technology. The changes and mutual regulation mechanisms of three physiological metabolisms during postharvest storage were summarized, and the relationship with quality deterioration was reviewed. The quality deterioration of harvested fruits and vegetables was closely related to respiratory metabolism, energy metabolism and reactive oxygen species metabolism. Postharvest treatments or preservation technologies reduced browning, nutrient loss, softening, pathogen infection, and cold injure by regulating the metabolism of fruits and vegetables. Respiratory metabolism, energy metabolism and reactive oxygen species metabolism form a complex physiological metabolic network and interact with each other to regulate the physiological metabolism of fruits and vegetables and further affect the physiological quality.
fruits and vegetables preservation; respiratory metabolism; energy metabolism; reactive oxygen species metabolism; quality deterioration
TS255.3
A
1001-3563(2022)05-0091-09
10.19554/j.cnki.1001-3563.2022.05.013
2021-07-31
辽宁省科技厅揭榜挂帅科技攻关专项(2021JH1/10400035);辽宁省教育厅课题(LSNQN202009)
唐建新(1995—),女,沈阳农业大学硕士生,主攻食用菌保鲜技术。
孙炳新(1981—),男,博士,沈阳农业大学副教授,主要研究方向为食品包装与农产品贮藏保鲜。