Circadian Rhythm and Intersexual Differences in Auditory Frequency Sensitivity in Emei Music Frogs
Bicheng ZHU ,Xiaoqian SUN, ,Haodi ZHANG, ,Yezhong TANG and Jianguo CUI*
1 CAS Key Laboratory of Mountain Ecological Restoration and Bioresource Utilization, Ecological Restoration and Biodiversity Conservation Key Laboratory of Sichuan Province,Chengdu Institute of Biology,Chinese Academy of Sciences,Chengdu 610041,Sichuan,China
2 University of Chinese Academy of Sciences,Beijing 100049,China
Abstract In anurans,calling behaviour is strongly seasonal and circadian.Previous studies have revealed that auditory sensitivity in frogs exhibits seasonal plasticity,and electroencephalographic signals exhibit highly correlated circadian patterns;of which,the circadian rhythm remains unknown.In this study,the circadian rhythm and intersexual differences of auditory sensitivity were tested in the Emei music frog (Nidirana daunchina).This was achieved by comparing thresholds and latencies of auditory brainstem responses (ABRs) evoked by tones and clicks stimuli between male and female frogs during the day and at night,respectively.Our results revealed that both auditory thresholds and latencies had no differences between day and night except the latencies in 3.5-4.0 kHz frequencies.However,the thresholds of tone pip evoked ABRs differed significantly between male and female frogs from 2.5 to 5.0 kHz.This demonstrated that the auditory sensitivity of Emei music frogs exhibits sexual dimorphism at high frequencies,with female frogs exhibiting greater auditory sensitivity than that of male frogs.Simultaneously,the power spectra of male advertisement calls are matched well with the frequency range of auditory sensitivity in male and female frogs,which supports the matched filter hypothesis.Our study enhances the understanding of circadian plasticity and sexual dimorphism of auditory sensitivity in frogs.
Keywords auditory brainstem response,auditory plasticity,day-night rhythm,frog,sexual auditory dimorphism,the matched filter hypothesis
1.Introduction
Rhythms profoundly influence the activities of humans and non-human animals.The courtship behaviours of several animals are seasonally and/or diurnally plastic,e.g.most anurans only call at night in their reproductive season.Call stimulation has a significant effect on the level of gonadal steroids in frogs (Lynch and Wilczynski,2006;O’Bryant and Wilczynski,2010).Although the auditory system is often considered relatively stable,there is evidence that the peripheral auditory responses can change in threshold across the reproductive state (Sisneros and Bass,2003;Goense and Feng,2005;Lucaset al.,2007;Zhanget al.,2012;Gallet al.,2013).Hormonally regulated expression of large-conductance calciumactivated potassium channels mediates seasonally or socially induced changes in auditory sensitivity (Rohmannet al.,2013).However,whether the auditory sensitivity exhibits circadian plasticity remains unknown.
The auditory brainstem response (ABR) is a form of auditory evoked potential that represents the summed output of synchronized neural activity in the auditory nerve and brainstem (Katbamnaet al.,2006;Hall,2007).The ABR is an effective method to study the auditory sensitivity in frogs (Wanget al.,2016;Cuiet al.,2017;Zhaoet al.,2017;Zhuet al.,2017;Womacket al.,2018;Yanget al.,2018;Sunet al.,2019),lizards (Brittan-Powellet al.,2010;Chenet al.,2016),turtles (Wanget al.,2019) and other taxa (Gallet al.,2011;Schrodeet al.,2014).Jerger and Hall (1980) reported that the ABRs in women have larger amplitudes and shorter latencies than those in men.Previous auditory studies performed on non-human species did not consider sex (Lucaset al.,2002;Brittan-Powell and Dooling,2004;Brittan-Powellet al.,2005) or found limited evidence of sexual differences (Zhouet al.,2006;Caraset al.,2010;Schrodeet al.,2014).However,several studies on ABRs of anurans and turtles have revealed a significant sexual difference in auditory sensitivity (Wanget al.,2016;Zhaoet al.,2017;Zhuet al.,2017;Wanget al.,2019).Therefore,the question of whether sexual auditory dimorphism is widespread remains controversial and must be verified in other species.
The emergence and maintenance of animal communication systems requires the co-evolution of signal sender and receiver.The ‘matched filter hypothesis’ has been proposed for acoustic communication systems of anurans (Capranica and Moffat,1983;Gerhardt and Schwartz,2001),arguing that receivers should gain an advantage from being selectively tuned to the vocalization frequencies of senders.The matching relationship between call frequency and frequency sensitivity of the inner ear affects both the efficiency of intraspecific vocal communication and speciation through interspecific reproductive isolation (Ryan,1986;Ryan and Wilczynski,1988;Yanget al.,2018).Numerous studies support this hypothesis in different taxa (Kostarakoset al.,2009;Chenet al.,2016;Zhuet al.,2017;Yanget al.,2018).In addition,there is experimental evidence that call frequency and auditory frequency sensitivity does not match (Goutteet al.,2017;Zhaoet al.,2017).Most studies have only analyzed the correlation between female auditory sensitivity and male call frequency considering that females usually choose their mates based on male calls (Moreno-Gómezet al.,2013).The matching relationship between male auditory sensitivity and male call frequency has been neglected,especially after considering the importance of males listening to their rivals in a chorus.Therefore,both male and female ABRs and male vocalizations were recorded in order to determine the general matching relationships between female and male auditory sensitivity and male vocalizations in the Emei music frog (Nidirana daunchina).
From May to August,male music frogs build mud-based burrows in muddy areas under the shadow of weeds,and produce calls of varying lengths consisting of a series of ‘musical’ notes from inside the burrows ‘inside call’,or outside the burrows ‘outside call’ (Chenet al.,2011;Cuiet al.,2012;Figure 1).A previous study observed seasonal plasticity of auditory frequency sensitivity in Emei music frogs,which may be adaptive to seasonal reproductive behaviour (Zhanget al.,2012).In Emei music frogs,vocal activity follows a circadian rhythm associated with daily changes in temperature and relative humidity (Cuiet al.,2011).

Figure 1 A male Emei music frog (N.daunchina) prepares to call inside the burrow.
In the present study,subdermal recordings of the ABRs were used to compare the auditory sensitivity during the day and at night between male and female frogs to analyze the following:(1) whether the auditory sensitivity exhibits circadian rhythm,(2) whether the auditory sensitivity exhibits sexual dimorphism,and (3) the matching relationships between male and female auditory sensitivity and male vocalizations.
2.Materials and Methods
2.1.Study site and call analysisThe study site was located in the area of Mount Emei (29.36° N and 103.22° E,elevation of 941 m above sea level),Sichuan,China.We extracted the relative amplitudes of male inside calls (N=31) and outside calls (N=21) in 0.1 kHz steps from 0.2 to 6.0 kHz,using PRAAT software script (Boersma and Weeninkk,version 5.1.11,University of Amsterdam) (Boersma,2002).All sound data were derived from our previous study (Cuiet al.,2012).
2.2.ABR measurementsIn total,14 male and 19 female adult frogs (body mass,7.57-14.04 g;snout-vent length,45-55 mm) were captured from ponds on Mount Emei in August 2013.The frogs were maintained in tanks (L × W × H,55 cm × 35 cm × 30 cm) containing water (2-cm deep) and aquatic plants with natural temperature and photoperiod.Each tank contained 4-6 frogs.Male and female frogs were separated and fed live crickets and individually marked with passive integrated transponder (PIT) tags (Hongteng,Inc.China) to prevent recapture.This was performed as per the Guidelines for the Use of Live Amphibians and Reptiles in Field Research (Beaupreet al.,2004).The frogs were tested at noon (temperature,26.3 ± 1.3℃;relative humidity,72.0% ± 5.8%) or midnight (temperature,25.6 ± 0.9℃;relative humidity,74.6% ± 3.7%) randomly.Each frog was tested twice with 36-hours intervals.After experiments,the frogs were returned to their original habitats immediately.Frogs were maintained in captivity on an average of 2 days.
The ABR measurements were conducted in a sound-proof acoustic chamber (0.5 m × 0.5 m × 0.5 m).The subjects were lightly anesthetized via water immersion with a 0.2% solution of MS-222 (tricaine methane sulphonate) before recording.The equipment,stimulus presentations,ABR acquisition and data management have been described previously (Cuiet al.,2017).In all cases,frogs were anesthetized to a level at which they no longer responded to a toe pinch (5-7 min).The frogs were placed in a natural posture and directly facing a speaker (SMEAFS,Saul Mineroff Electronics,Elmont,NY),at a 10-cm distance.
For each frog,three subdermal steel electrodes (Rochester Electro-Medical,Inc.FL,USA) were inserted directly under the skin at the vertex (non-inverting),above the tympanum (inverting) and in the ipsilateral front leg (ground),respectively.The recording electrodes were connected to an amplifier (PA4 and RA4,20x gain) via wires wrapped in tin foil,and the amplified signals were transmitted to a RM2 digital processor (Tucker-Davis Technologies,Gainesville,USA) via fibre optic cables.The RM2 was linked via a USB to a computer running custom software (Open ABR) developed by Ed Smith (The University of Maryland,USA) based on MATLAB 2009a (The MathWorks,Inc.USA).Two types of stimuli,tone pips and spectrally broadband clicks (as control),were generated by Open ABR and delivered through a portable amplified field speaker driven by the RM2 and positioned in front of the frog’s head.Stimuli were synthesized digitally from 0.5 kHz to 6.0 kHz,at 0.5-kHz intervals with a stimulation duration of 1-ms rise/fall time (linear ramp),3-ms plateau time and sample rate of 24 414 Hz.The frequency response of the speaker was flat (< 2 dB) in the investigated frequency range.The 0.01-ms broadband clicks were synthesized and used as stimuli to verify the presence of a biological signal in response to sound were maintained during a recording session.Visual inspection of these click-evoked ABRs indicated there were no response changes throughout each recording session.
Prior to the ABR recording,the sound pressure level of all the stimuli was calibrated using a G.R.A.S.46BE 1/4-inch microphone (G.R.A.S.Sound and Vibration,Denmark) with CCP Supply (Type 12AL,G.R.A.S.Sound and Vibration,Denmark) positioned at the frog’s head (10 cm from the speaker).ABR stimuli were delivered randomly with a varying frequency from 0.5 kHz to 6.0 kHz.The presentation rate was 4/s.All stimuli ran two replications.Stimuli were initiated at 45 dB re.20 μPa and increased in 5-dB steps based on the assumption that all ABR thresholds in this species were below 90 dB (re.20 μPa).All biological signals were notch filtered at 50 Hz during data collection.
The thresholds of ABR were determined using methods described in a previous study (Cuiet al.,2017).Threshold measurements were defined as the lowest stimulus level for which no repeatable responses could be recognized.ABR latencies were measured between stimulus onset and the first ABR waveform valley using manually placed cursors in MATLAB (version 2009a).
2.3.Analysis and statisticsThe ABR thresholds and latencies obtained from male and female music frogs in response to tone and click stimuli were sorted and analyzed using Sigma Plot 11.0 software (Systat Software Inc.,San Jose,USA).We analyzed the temporal (note number) and spectral (minimum frequency,maximum frequency,bandwidth) characteristics of male inside calls and outside calls using Adobe Audition 3.0 (California,USA).All data were examined for assumptions of normality and homogeneity of variance,using Shapiro-Wilk and Levene tests,respectively.The t-test or Mann-Whitney Rank Sum Test were used to compare the difference between the power spectra,temporal and spectral characteristics of inside calls and outside calls,and the temperature between day and night.The differences of ABR thresholds and latency during the day and at night between male and female frogs were assessed using Two-way repeated measures Analysis of Variance (ANOVA) (rhythm,sex and frequency as factors),followed by the Holm-Sidak post-hoc test.Spearman rank order correlation was used to analyze the matching relationships between female and male auditory sensitivity and male vocalizations,the correlation between temperature and auditory sensitivity.Mann-WhitneyUtest were also used to verify the effect of body mass on auditory sensitivity.Data were expressed as Mean ± SD;P≤ 0.05 was considered to be statistically significant.
2.4.Ethics StatementAll applicable international,national,and/or institutional guidelines for the care and use of animals were followed.All procedures performed in studies involving animals were in accordance with the ethical standards of the Animal Care and Use Committee of Chengdu Institute of Biology,CAS (CIB2013031008).
3.Results
3.1.Circadian rhythm of auditory sensitivityNo statistically significant interaction was observed between rhythm and sex at each frequency (two-way repeated measures ANOVA,P> 0.05;Tables 1-2).
Although there is a general trend that auditory thresholds are slightly lower at night than during the day (Figure 2A),the thresholds were not different during the day and at night (two-way repeated measures ANOVA,P> 0.05;Table 2).The difference in auditory thresholds between day and night wasclose to being significant only at 2.5 kHz and 3.5 kHz frequencies (two-way repeated measures ANOVA;2.5 kHz,F=4.048,P=0.053;3.5 kHz,F=3.340,P=0.077;Figure 2A;Tables 1-2).
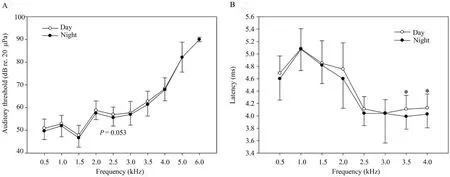
Figure 2 Circadian rhythm of auditory sensitivity.Auditory threshold (A) and latency (B) were recorded during the day and at night.Two-way repeated measures ANOVA,*P < 0.05.Day,open circles,n=31;Night,filled circles,n=31.
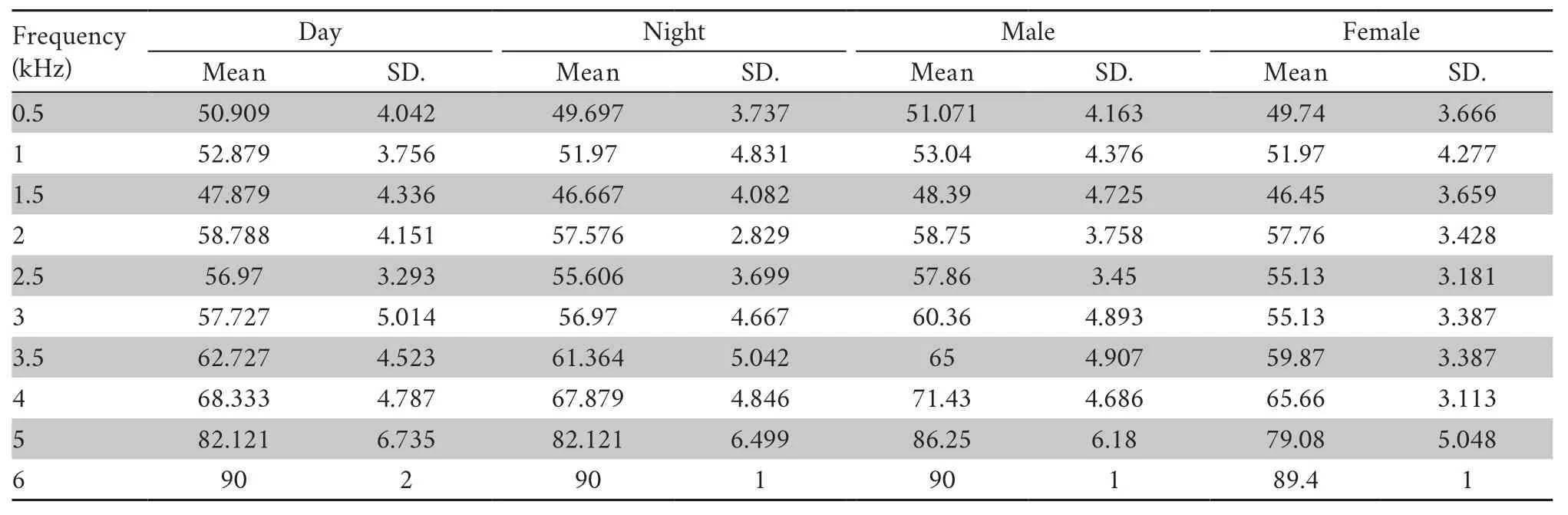
Table 1 The auditory thresholds (dB) of Emei music frogs during the day and at night between male and female frogs respectively.
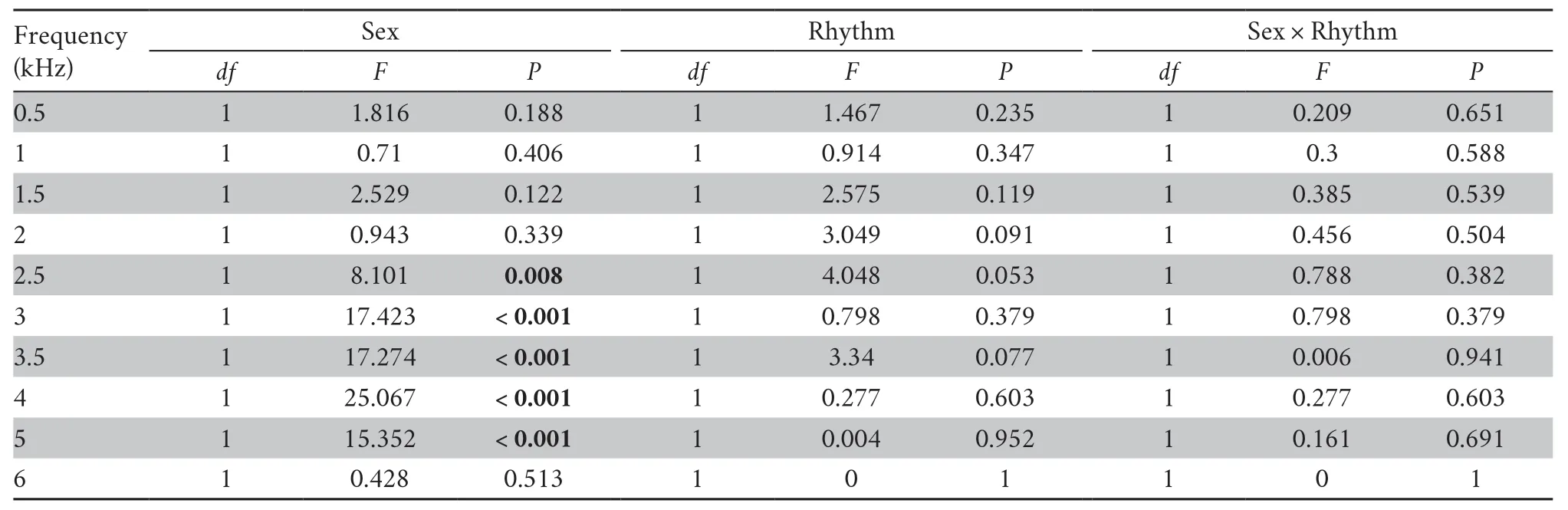
Table 2 The statistical results of auditory thresholds between sex and rhythm at each frequency.
The ABR latencies differed significantly between day and night at 3.5-4.0 kHz frequencies (two-way repeated measures ANOVA;3.5 kHz,F=6.142,P=0.019;4.0 kHz,F=7.412,P=0.012;Figure 2B).
The temperature at night is significantly lower than that during the day (t-test,t=2.299,df=64,P=0.025).Therefore,we analyzed the correlation between temperature and auditory sensitivity using Pearson product moment correlation.Temperature was negatively correlated with auditory sensitivity threshold at 3.0 kHz and latency at 2.5-4.0 kHz (Table 3).
3.2.Sexual dimorphism of auditory sensitivityThe possibility that ABR thresholds differ between male and female frogs was investigated.As presented in Figure 3A and Table 1,we observed that female frogs were more sensitive than male frogs across the 2.5-5.0 kHz frequency range (two-way repeated measures ANOVA,P< 0.01;Table 2),although the differences were not significant at 1.0-2.0 kHz frequencies (twoway repeated measures ANOVA,P> 0.05;Table 2).The finding that auditory sensitivity did not differ between large and small body mass in female or male frogs (Mann-WhitneyUtest,P> 0.05) provided evidence against a strong influence of body size,although it is not possible to rule out the effect of body size in determining the threshold differences between the sexes completely.

Table 3 Correlations between temperature and auditory sensitivity.
Significant latency differences were observed between male and female frogs at 2.5-3.0 kHz frequencies (two-way repeated measures ANOVA;2.5 kHz,F=4.532,P=0.046;3.0 kHz,F=5.079,P=0.038;Figure 3B).These results demonstrated that female music frogs had a more sensitive audition at high frequencies than that of male frogs.
In addition,we compared the spectral characteristics of male inside calls and outside calls to testify whether having an auditory advantage at high frequencies contributes to femalefrogs distinguishing male inside calls from outside calls to some extent.Significant differences were observed in the minimum frequency (Fmin,Mann-WhitneyUtest,T=12518.000,P< 0.001),the maximum frequency (Fmax,Mann-WhitneyUtest,T=12196.000,P< 0.001) and bandwidth (Mann-WhitneyUtest,T=12037.000,P< 0.001) between male inside calls and outside calls,although no difference was observed in the note number between them (Mann-WhitneyUtest,T=473.500,P=0.543;Figure 4).Male outside calls had a higher minimum frequency,maximum frequency,and wider frequency band than those of inside calls.
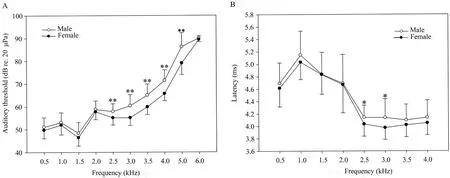
Figure 3 Sexual dimorphism of auditory sensitivity.Auditory threshold (A) and latency (B) were recorded between male and female frogs.Two-way repeated measures ANOVA,*P < 0.05,**P < 0.01.Male,open circles,n=28;Female,filled circles,n=38.
3.3.Matching relationship between auditory sensitivity and male vocalizationsWe analyzed the relative amplitude of male inside calls and outside calls.The relative amplitude ranges of these two kinds of calls were both concentrated at 0.5-3.5 kHz frequencies (Figure 5).The relative amplitude of inside calls revealed a single energy peak (an inverted ‘V’-shaped) which was different from that of the outside calls having two energy peaks (an ‘M’-shaped).Significant differences were observed between the relative amplitude of inside and outside calls (Mann-WhitneyUtest,T=3152.000,P=0.012;Figure 5).
The ABRs of male and female frogs to tone pip and click stimuli were characterized by valley-peak waveforms,which were visualized during the day and at night.The multiunit audiograms of Emei music frogs were characterized by two regions of enhanced sensitivity:a low-frequency region (1.0-2.0 kHz) and a high-frequency region (2.0-3.0 kHz),with a decreasing sensitivity above 3.0 kHz (Figure 4).The best threshold of the low-and high-frequency region had a mean of 47.9 dB (re.20 μPa) at 1.5 kHz and 56.9 dB (re.20 μPa) at 2.5 kHz,respectively.
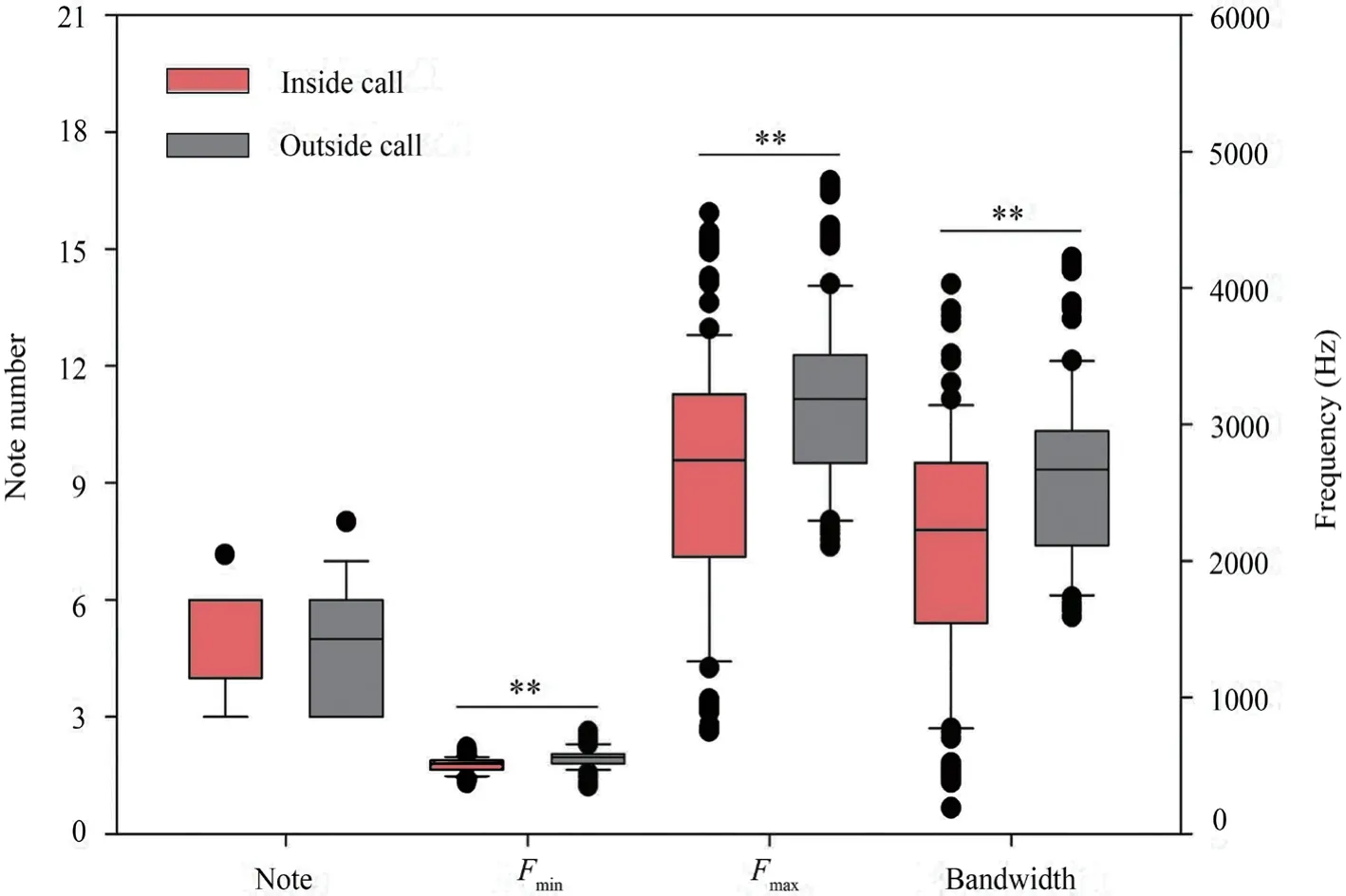
Figure 4 Comparison of the temporal and spectral characteristics of male inside and outside calls.Fmin,minimum frequency in harmonics of the call (i.e.the lowest frequency that is visible in the spectrogram);Fmax,maximum frequency (i.e.the highest frequency that is visible in the spectrogram).Mann-Whitney U test,**P < 0.001.Inside call,light-red box,n=31;Outside call,gray box,n=21.
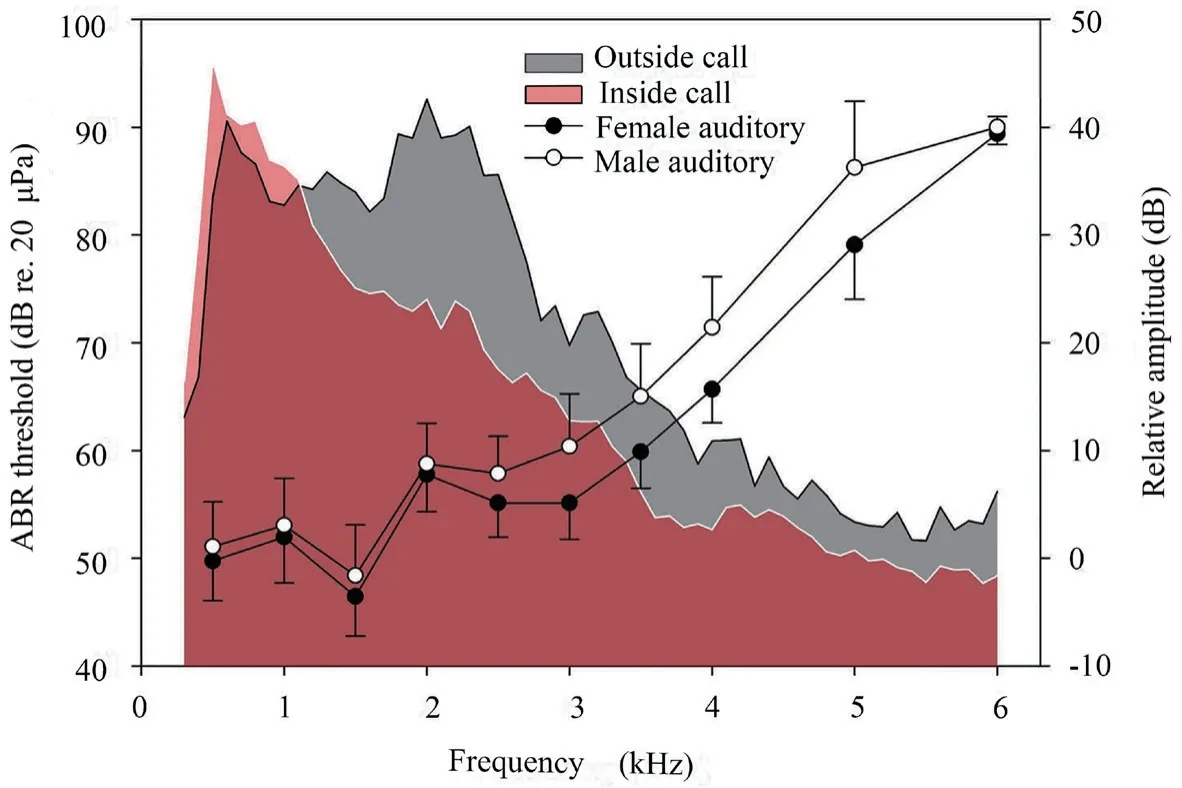
Figure 5 The audiogram of Emei music frogs (y-axis on left) and power spectrum of male advertisement vocalizations (y-axis on right).Relative amplitude,outside call (gray area),inside call (light-red area);ABR threshold,female (filled circles),male (open circles).
Spearman rank order correlation was used for measuring the degree to which the audiograms and call spectra matched.Both male and female auditory thresholds were significantly correlated with the relative amplitude of male inside and outside calls (Table 4).Remarkably,the matched relationship between female auditory threshold and relative amplitude of male inside calls (r=-0.924,P< 0.001,n=10) was more significant than the relationship between female auditory threshold and relative amplitude of male outside calls (r=-0.711,P=0.019,n=10;Table 4).
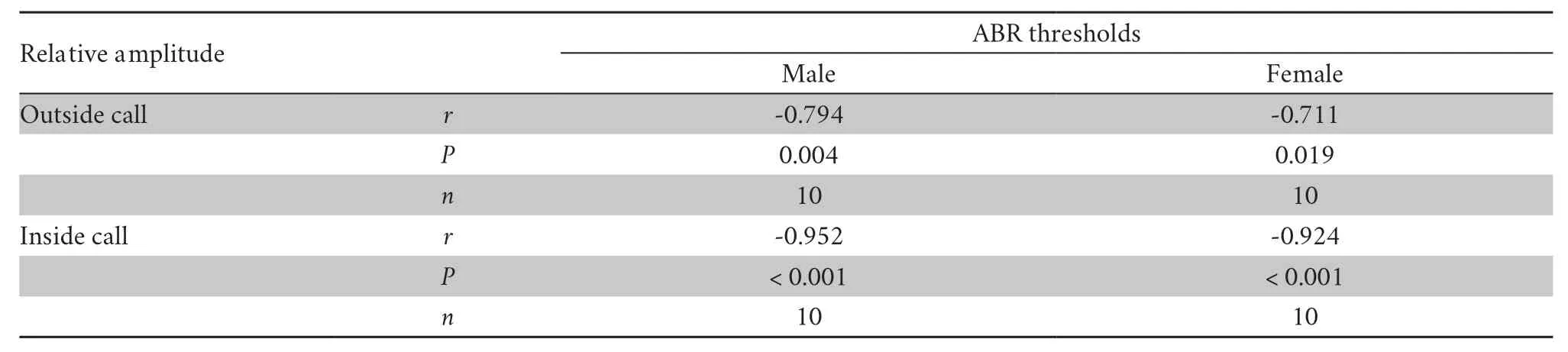
Table 4 Results of Spearman rank order correlation analyses between call amplitude and ABR thresholds.
4.Discussion
Our results demonstrated the following:(1) both auditory thresholds and latencies had no differences between day and night except the latencies at partial frequencies,(2) female music frogs have a more sensitive audition at high frequencies than that of male frogs,and (3) the frequency distribution of male advertisement calls was matched well with the frequency range of female and male auditory thresholds.
4.1.Circadian rhythm of auditory sensitivityThe circadian rhythm is found in almost every organism,from cyanobacteria to plants and humans (Bameset al.,1977;Pando,2002;Ramilet al.,2011).Exposure to several nights of a simulated chorus lowered the auditory thresholds of green treefrogs,Hyla cinerea(Gall and Wilczynski,2015).However,our results demonstrated that the auditory sensitivity of Emei music frogs did not exhibit a circadian rhythm,although the latency differed significantly at partial frequencies during the day and at night.Therefore,the auditory sensitivity of music frogs can be adjusted seasonally (Zhanget al.,2012) but not with a marked diel cycle.
Anurans possess two inner ear organs that are particularly sensitive to airborne sounds:an amphibian papilla (AP) and a basilar papilla (BP) (Zakon and Wilczynski,1988).Fibers innervating the AP are sensitive to low-and mid-frequency sounds;fibers innervating the BP are sensitive to higher frequencies (Narinset al.,2006).The approximate ‘W’-shaped audiogram with two sensitive regions of auditory thresholds (1.0-1.5 kHz and 2.0-3.0 kHz) in Emei music frogs probably reflects the sensitivity of AP and BP,respectively.AP tuning is considered more malleable than BP tuning (Narins,2001;Gall and Wilczynski,2015;Sunet al.,2019).However,in this study,we observed that the latencies of ABRs differed significantly between day and night at high frequencies,probably arising from BP,which indicated greater plasticity at frequencies associated with BP than at frequencies associated with AP in the circadian rhythm of auditory plasticity.
Our years of field observations reveal that the amplexus and oviposition of Emei music frogs occur primarily at night.There may be relatively less adaptive value for extensive auditory processing of sexual vocal signals at noon.Therefore,decreasing auditory sensitivity to save energy may be adaptive at noon (Zhanget al.,2012).A slight decline in auditory latency at night may increase (to some extent) the salience of male mating calls or the distance which it can be detected by female music frogs (Gall and Wilczynski,2015).In this study,the difference in auditory latency between day and night was less than 0.2 ms.Whether such a slight decline in auditory latency is biologically significant remains to be analyzed.
Certainly,the diel differences in auditory sensitivity were non-significant over most frequency ranges.Although exposure to several nights of a simulated chorus decreased the auditory thresholds of green treefrogs (Gall and Wilczynski,2015),there is no evidence that the auditory thresholds of frogs can change in 12 hours.Meanwhile,Emei music frogs call both during the day and at night (Cuiet al.,2011).The circadian rhythm of auditory sensitivity will be examined in our subsequent study in a purely nocturnal frog.
4.2.Sexual dimorphism of auditory sensitivityPrevious studies on sexual dimorphism in anurans focused on morphological traits (Arak,1988;Jean-Matthieu and Michael,2002).However,our results demonstrated that auditory sensitivity exhibited sexual dimorphism.Female frogs exhibit greater auditory sensitivity than that of male frogs at high frequencies,probably arising from BP.The presence of sexual differences in anuran auditory tunings was observed in BP rather than in AP (Liuet al.,2014;Gall and Wilczynski,2015).We discovered that the peak amplitude of BP was greater in female frogs than in male frogs.The sexual auditory dimorphism in Emei music frogs may reflect different reproductive strategies between male and female frogs.Compared with male frogs,female frogs bear the repercussion of mating owing to much greater investment in reproduction.Therefore,female frogs are expected to be pickier in mate choice (Forstmeieret al.,2014).In this study,when the distance was doubled,sound pressure in the acoustic far-field attenuated by 6 dB (re.20 μPa) at partial frequencies.This means,female frogs may detect conspecific male calls at twice the distance (and four times the area) than the males could,which may have important biological implications.
Numerous studies have demonstrated that female frogs have a more sensitive auditory function than that of males (Narins and Capranica,1976;Schrodeet al.,2014;Wanget al.,2016).This was observed in music frogs with female frogs having a more sensitive auditory function than that of male frogs at relatively high frequencies (≥ 2.5 kHz).However,the females of the Chinese tiger frogs (Hoplobatrachus chinensis) and Cope’s grey treefrogs (H.chrysoscelis) have more sensitive audition than that of males only at low frequencies (tiger frogs,≤ 1.6 kHz;grey treefrogs,≤ 2.0 kHz) (Schrodeet al.,2014;Wanget al.,2016).This difference is probably related to structural differences in the inner ear between male and female frogs.Differences in the inner ear organ structure (particularly BP) can affect the sensitivity to high-frequency sounds (Gerhardt and Schwarz,2001).
The serrate-legged small treefrogs (Kurixalus odontotarsus) and American bullfrogs (Rana catesbeiana) have similar sexual auditory differences with Emei music frogs (Hetherington,1994;Masonet al.,2003;Werneret al.,2009;Zhuet al.,2017),suggesting that this is not an isolated case.However,previous studies have not explained why female frogs have a more sensitive auditory function than that of male frogs only at high frequencies.Considering that the burrows may act as a low-pass filter (working like a quarter-wavelength resonator),male inside calls have a lower frequency range than that of outside calls,which seems to explain the genuine and possibly biologically meaningful difference between the two types of calls.Having an auditory advantage at high frequencies may contribute to female frogs distinguishing male inside calls from outside calls,leading to female frogs choosing male frogs with nests (Cuiet al.,2012).Therefore,we compared the spectral characteristics of male inside calls and outside calls,and observed that the frequency band of outside calls was significantly higher than that of inside calls (Figure 4).The only different frequency range between male inside and outside calls (2.6-3.2 kHz) coincided with the frequency at which differences were observed in auditory sensitivity between male and female frogs (2.5 kHz).Such a coincidence supported our conjecture to some extent.In general,our study provides a new insight into why female frogs have more sensitive auditory function than that of male frogs at high frequencies.
4.3.Matching relationship between auditory sensitivity and male vocalizationsThe relative amplitude of male vocalization matches the female auditory threshold in Emei music frogs,which supports the matched filters hypothesis (Gerhardt and Schwartz,2001).On this basis,an organism may choose an appropriate behavioural action that ultimately maximizes its fitness.A tuned sensitivity filter should result in strong stabilizing selection.Therefore,it is believed that selection should favour the tuning of the frequency of male speciesspecific advertisement calls.The spectral energy distribution of male acoustic signals is always matched well with female auditory sensitivity (Doolinget al.,1979;Wilczynskiet al.,1992;Ladich and Yan,1998;Witteet al.,2005;Kostarakoset al.,2009;Zhuet al.,2017;Yanget al.,2018),although evidence suggests that,call frequency and auditory frequency sensitivity does not match in some species (Goutteet al.,2017;Zhaoet al.,2017).
In addition,it is essential for male frogs to listen to their neighbours,especially when they aggregate in choruses.However,only a few studies have been reported on the matching relationship between the male auditory threshold and the relative amplitude of male vocalizations.Our study revealed that,similar to that in female frogs,the relative amplitude of male vocalizations matched male auditory sensitivity,which is essential for male frogs to locate and assess nearby competitors.In conclusion,the matching relationships between female and male auditory thresholds and the relative amplitude of male vocalizations in Emei music frogs enriched the content of matched filters hypothesis.
Both species recognition and the efficiency of intraspecific vocal communication are affected by the matching relationship between auditory threshold and the relative amplitude of vocalizations (Ryan,1986;Yanget al.,2018).Remarkably,we observed that the matched relationship between female auditory threshold and the relative amplitude of male inside calls is more significant than the relationship between female auditory threshold and the relative amplitude of male outside calls.These results suggested that the auditory system of Emei music frogs may be more sensitive to the male inside calls than to the male outside calls,which may help female music frogs to distinguish the inside calls from outside calls and explain why female music frogs prefer to select males with nests (Cuiet al.,2012).This behaviour is necessary to improve the efficiency and fidelity of intraspecific communication.
AcknowledgementsWe thank Pengbo GONG for the help in field experiments,Yue YANG and Xia QIU for the help in drawing the figures.This work was supported by National Natural Science Foundation of China (31772464),Youth Innovation Promotion Association CAS (2012274) to Jianguo CUI.
 Asian Herpetological Research2022年1期
Asian Herpetological Research2022年1期
- Asian Herpetological Research的其它文章
- Comparative Osteology of Two Far Eastern Species of Ratsnakes (Serpentes:Colubridae),Elaphe dione (Pallas,1773) and E.schrenckii (Strauch,1873),for the Purpose of Palaeontological Studies
- Offspring Sex Is Not Determined by Gestation Temperature in a Viviparous Lizard (Eremias multiocellata) from the Desert Steppe of Inner Mongolia
- Sex But Not Altitude,Modulates Phenotypic Covariations Between Growth and Physiological Traits in Adult Asiatic Toads
- High-elevation Adaptation of Motion Visual Display Modifications in the Toad-Headed Agamid Lizards (Phrynocephalus)
- An Annotated List of Lizards (Sauria:Squamata) Recorded from the People’s Republic of China
- Appendix 1
