Methyl gallate isolated from Mangifera pajang kernel induces proliferation inhibition and apoptosis in MCF-7 breast cancer cells via oxidative stress
Ranneh Yazan, Abu Bakar Mohd Fadzelly✉, Rahim Azlen-Che, Kassim Nur Kartinee, Stanslas Johnson, Teh Yuan-Han, Fadel Abdulmannan, Ellulu Mohammed S
1Department of Technology and Natural Resources, Faculty of Applied Science and Technology, Universiti Tun Hussein Onn Malaysia, Pagoh Campus, KM1 Panchor Road, Muar Johor, Malaysia
2Department of Chemistry, Faculty of Science, Universiti Putra Malaysia, Serdang 43300, Malaysia
3Department of Medicine, Faculty of Medicine and Health Sciences, Universiti Putra Malaysia, Selangor, Malaysia
4Sport and Exercises Sciences School, Faculty of Science, Liverpool John Moores University, Liverpool, UK
5Department of Clinical Nutrition, Faculty of Applied Medical Sciences, Al-Azhar University of Gaza, Gaza City, State of Palestine
ABSTRACT
Objective:To determine the lead bioactive compound in kernel extract of Mangifera pajang and its anti-cancer activity against human breast cancer cell lines with positive estrogen receptor(MCF-7).
Methods:The methanolic extract of dried powder kernel of Mangifera pajang was exposed to column chromatography for isolation. The structural elucidation of the isolated compound was characterized using infrared, nuclear magnetic resonance, mass spectrometry. Furthermore, cytotoxicity, morphological changes,flow cytometry and cell cycle arrest analyses were performed to examine the mechanism of anti-proliferation and apoptosis induced by methyl gallate against MCF-7.
Results:One compound was isolated from the methanolic extract of Mangifera pajang kernel and identified as methyl gallate. The flow cytometric results demonstrated induction of apoptosis in MCF-7 cells by three concentrations of methyl gallate. The cell cycle arrest showed a significant (P<0.05) decrease in cell progression at G2/M phase of MCF-7 after treatment with 100 μM of methyl gallate. The cell percentage of early and late apoptosis was significant at 10 and 100 μM of methyl gallate. Also, methyl gallate treatment induced up-regulation of reactive oxygen species levels in MCF-7 cells with a reduction in superoxide dismutase levels.
Conclusions:These findings indicate that isolated methyl gallate from Mangifera pajang kernel extracts induces growth inhibition and apoptosis in MCF-7 cells via up-regulating oxidative stress pathway.
KEYWORDS: Mangifera pajang; Methyl gallate; Breast cancer;Cell cycle; Apoptosis; Oxidative stress
Significance
Methyl gallate has anti-proliferative activities against MCF-7 cells via apoptosis and oxidative stress. Methyl gallate could be a promising agent for mammary tumor therapy.
1. Introduction
The number of new cases of cancer-related mortalities is expected to increase in the close future[1]. Following lung tumor, breast cancer has been recorded as the second most frequent cancer and the most prevalent cause of death among women. Based on the medical diagnosis, the age-standardized rate of breast cancer occurrence in Malaysia has been 47 per 100 000[2]. The records of the National Registry of Malaysia have shown approximately 21 773 cases who are diagnosed with cancer, while unregistered cases have been expected to reach 10 000 annually[2].
Since breast cancer is not associated with a single cause,environmental and genetic factors are collectively involved in the onset of the tumor. The regulation of genome integrity is controlled by the cell cycle to abolish genetic alterations from being inherited to subsequent generations[3]. However, the dysfunction of cell cycle checkpoints including G1/S and G2/M phases has been strongly associated with the onset of mammary tumors[4]. Therefore, DNA reparation via cell cycle arrest remains the most important biological reaction to inhibit the replication of damaged templates. This biological strategy has been reported previously in eliminating cancer cells[5].
Apoptosis which is an integrated program of cell death is a biological approach to eliminate mammary cancer cells[6]. This defensive mechanism depends on damaging harmful cells before developing into a malignant tumor, regardless of activating inflammatory pathways[7]. Therefore, classical chemotherapeutic drugs such as cisplatin and tamoxifen induce apoptosis and cell cycle arrest to inhibit the proliferation of malignant cells via up-regulating oxidative stress pathways[7]. However, the adverse events due to the administration of chemotherapeutic drugs lead scientists to discover and develop other novel therapeutic agents from natural products.
After the invention of advanced technologies in isolating and identifying bioactive compounds, medicinal plants have been involved massively in the development of new drugs with promising results.Hence, a local Asian plant named, Mangifera pajang (M. pajang)Kosterm was selected due to its effectively biological activities including anti-oxidant, anti-inflammatory, and anti-cancer[8]. Plenty of polyphenol compounds have been identified in M. pajang parts including kernel, peel and pulp[9]. It was reported that M. pajang kernel had a 17 times higher amount of phenolic compounds than the pulp and peel[10]. Moreover, methyl gallate (MG) isolated from M. pajang kernel was the only compound with high antioxidant activity[8]. In addition,M. pajang kernel extract demonstrated inhibitory activity against hepatic, ovarian, and colon cancer cell lines[9]. In the light of previous findings, the current study was designed to evaluate the effectiveness of MG isolated from M. pajang kernel extract against breast cancer cell line (MCF-7) and to unravel the molecular mechanism of actions with regards to its activity on cell cycle and apoptosis.
2. Materials and methods
2.1. Chemicals
Fetal bovine serum, penicillin-streptomycin, RPMI medium,accutase, and Dulbecco’s modified Eagle’s medium were purchased from Invitrogen Co., Carlsbad (CA, USA). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay kit and annexin V-Fluorescein isothiocyanate (FITC) propidium iodide (PI)apoptosis detection kit were purchased from Sigma Aldrich (USA).MCF-7 and MCF-10A cell lines were obtained from the American Type Culture Collection (VA, USA). Cell culture plastic ware was from BD Falcon (USA). All the solvents used for extraction and purification were of the highest purity grade available.
2.2. Collection of plant materials
The fruit of M. pajang was collected from Penampang, Sabah,Malaysia in June-August 2017. The plant was identified by a resident botanist (Dr.Alona Cuevas Linatoc) from Universiti Tun Hussein Onn Malaysia under voucher specimen (No. 012017SBH).The collected sample was washed and the kernel was separated from the whole fruit. The kernel was air-dried and grounded into fine powder for further analysis.
2.3. Extraction and isolation process
Cold maceration method was used for the extraction of compounds from 960 g of ground kernel powder. The kernels were initially soaked in four different organic solvents (hexane,chloroform, ethyl acetate and methanol) successively at room temperature (25 ℃). Solvent 1 (hexane) was removed from kernels after 3 d and the remaining solvent was evaporated with the help of a vacuum rotatory evaporator. Consequently,the previous extraction process was repeatedly performed for chloroform, ethyl acetate, and methanol. The four crude extracts were fractioned by using silica gel Merck Kieselgel 60 PF254Art No. 7734.1000 (70-230 mesh) as the stationary phase for gravity column. Then, the fractions were isolated and purified using silica gel Merck Kieselgel 60 PF254Art No. 9385 (230-400 mesh). The normal phase columns were diluted with a mixture of solvents to increase the polarity. The combination of Sephadex LH-20 with gel permeation chromatography was also used as an adsorbent in the column chromatography, while chloroform and methanol were used as the eluents. Among four extracts, the methanolic crude extract was chosen for further isolation based on the cytotoxic test (data not shown). Initially, fractionation of methanolic extract afforded 23 collections (C1-C23) which were contained in 7 fractions. The fractionation process is illustrated in Supplementary Figure 1. Fractions 2, 3 and 4 were combined into one fraction and purified through mini-column chromatography(Sub fraction 2). The previous process was repeated with fraction 5 and 6 to obtain sub-fraction 3. The ratio of eluting solvent was 40% hexane:60% acetone. The combined fractions were explored using the UV light (254 nm) as a single black spot with an Rfvalue of 0.84. Consequently, the crude methanolic extract was subjected to extensive spectroscopic analyses methods including, infrared(IR) spectra, electron impact-mass spectrometry (EI-MS), nuclear magnetic resonance (NMR),13C NMR, and1H NMR.
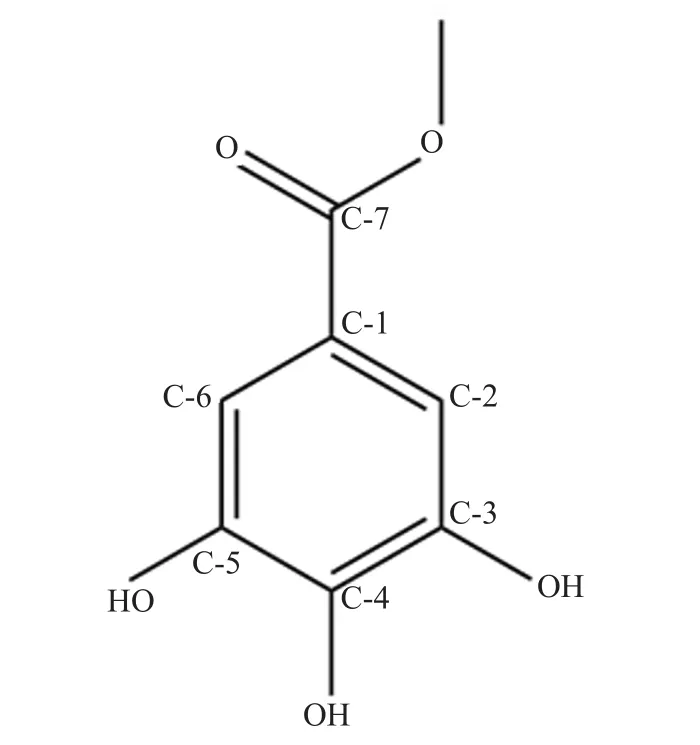
Figure 1. Chemical structure of methyl gallate.
2.4. Cell culture
Human breast cancer cell line with positive estrogen receptor(MCF-7), and non-tumorigenic mammary epithelial cell line (MCF-10A) were maintained in RPMI 1640 with a supplementation of 10% fetal bovine serum and 150 units/mL penicillin-streptomycin mixture (Invitrogen Co., Carlsbad, CA, USA) for growth. All the cultured cells were incubated in a humidified 5% CO2incubator at 37 ℃.
2.5. Cell viability assay
MCF-7, and MCF-10A cells were seeded at a density of 2×103cells per/mL in a 96-well plate. After 24 h, the cells were treated with MG dissolved in 0.1% of dimethyl sulphoxide (DMSO) at 1,10, and 100 μM. After 48 h incubation, the percentage of viable cells was determined by evaluating their ability in metabolizing 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide into formazan. The absorbance of formazan solution was measured at 550 wavelengths by using a VersaMax tunable microplate reader (Molecular Devices, USA). Cell viability (in percent) was calculated by using the following formula:
The dose-response curve was plotted and the 50% inhibitory concentration (IC50) of MG was determined.
2.6. Morphological assessment of cells under an inverted light microscope
Brie fly, 2×103per well of MCF-7 cells was cultured in a 96-well plate and kept for 24 h. The morphological changes of MCF-7 cells were observed under an inverted microscope after 48 h of treatment with serial concentrations (0, 1, 10, 100 μM) of the isolated compound MG dissolved in DMSO. Untreated MCF-7 cells were considered as a control. After the incubation period, the media were discarded and the cells were washed once using phosphate buffer saline (pH 7.4). The morphological changes were observed under a Leica DMI 3000B phase-contrast inverted microscope(Leica Microsystems, Germany) at 400× magnifications for treated cells and 100× magnifications for untreated cells.
2.7. Cell cycle analysis
MCF-7 cells were plated in a six-well plate with a density of 1.5×106cells per/mL. The cells were treated with 0.055% DMSO(vehicle control) and (1, 10, 100 μM) MG for 48 h. Treated cells(1.5×106cells/mL) were fixed with 70% ethanol at -20 ℃ for at least 3 h. Fixed cells were processed with Muse Cell Cycle Kit (Millipore) according to the manufacturer’s instructions and cell cycle profiles were acquired by using a Muse cell analyzer(Millipore).
2.8. Apoptosis assay
MCF-7 cells were plated in a six-well plate with a density of 1.5×106cells per well. The cells were treated with 0.055% DMSO as vehicle control and MG (1, 10, 100 μM) for 48 h. Treated cells were incubated with Muse Annexin Ⅴ & Dead Cell Reagent (Millipore) according to the manufacturer’s protocol. Apoptosis profiles were acquired by Muse cell analyzer (Millipore).
2.9. Cellular parameter of oxidative stress
The production of reactive oxygen species (ROS) in MCF-7 cells treated with different concentrations of MG was gauged as described in Ranneh et al. with slight modifications[3]. At a concentration of 5×103cells/mL, MCF-7 cells were allowed to be attached in 96-well plates for 24 h. Then, the cells were treated with three concentrations (1, 10, 100 μM) of MG and incubated for 48 h. Subsequently, MCF-7 cells were washed and preserved with 100 μL of 10 μM dichloro-dihydro-fluorescein diacetate for 45 min in a dark place at 37 ℃. The fluorescence was read at excitation of 485 nm and emission of 520 nm in a stirred quartz cuvette.
2.10. Cellular intrinsic antioxidant enzymes
After seeding MCF-7 cells (5×103cells/mL) in a 12 well plate for 24 h, three concentrations (1, 10, 100 μM) of MG were added and the cells were incubated for 48 h. Then, the cells were washed thrice with phosphate buffer saline and the cellular lysates were collected using a rubber policeman followed by sonication at high speed using Bioruptor sonicator (Cosmobio, Tokyo, Japan).The cellular antioxidant enzymes including, catalase (CAT),glutathione peroxidase (GPx), and superoxide dismutase (SOD)of cellular lysates were measured using commercially available kits from Cayman Chemical Company (Ann, Arbor, MI) and the experimental procedures were performed according to the manufacturer’s guide.
2.11. Statistical analysis
All the experiments were performed in triplicates for three independent experiments. The results were statistically analyzed by using one-way ANOVA and Tukey’s multiple comparison,using IBM SPSS Statistic software (version 20). The results were presented as mean ± standard deviation (SD).
3. Results
3.1. Extraction and isolation of MG
Ground powder (960 g) from air-dried M. pajang kernel was used for the extraction of the compound through cold maceration.The yield of extracts was 34.93 g, 3.99 g, 3.86 g, and 23.75 g for hexane, chloroform, ethyl acetate, and methanol, respectively. The white solid, 95% pure compound (based on the EI-MS analysis)was obtained from the methanolic extract with a melting point of 192-194 ℃ (literature melting point: 188-189 ℃)[12]. The compound was observed as a white amorphous crystal and was obtained upon purification of fractions 5-12 after washing with hexane. This compound showed a single spot on a TLC plate under a UV lamp with an Rfvalue of 0.84. Based on the chemical structure and IR spectrum analysis, the identified compound was MG (Figure 1 and Figure 2A). The analysis of IR spectrum revealed that the isolated compound contained a broad absorption band of a free hydroxyl group at 3 347 cm–1which overlapped with methyl C-H band from the methoxy group OCH3at 2 700 cm–1. The conjugated carbonyl stretching C=O absorption band occurred at 1 689 cm–1, which indicated the attachment to an aromatic ring, while a C-O stretch band appeared around 1 249 cm–1. The absorption bands around 1 448 cm–1indicate the presence of aromatic C=C as seen in Figure 2A. Furthermore, the EI-MS spectrum was also performed which provides strong evidence regarding the extraction of MG. The molecular ion peak was observed at m/z 184 as shown in Figure 2B. As observed, the molecular weight of MG was 184 g/mol with a molecular formula C8H8O5. Moreover, two more peaks were also observed at m/z 153 and m/z 125 due to the loss of methoxy group(-OCH3) as well as -CO group, respectively (Figure 2B). These fragmentations gave the idea that MG contained ester linkages. In addition to this, small fragment ions at m/z 107, 79, and 51 were due to loss of H2O and -CO, respectively from the benzene ring.This result suggested that MG contained three OH groups on the benzene ring.
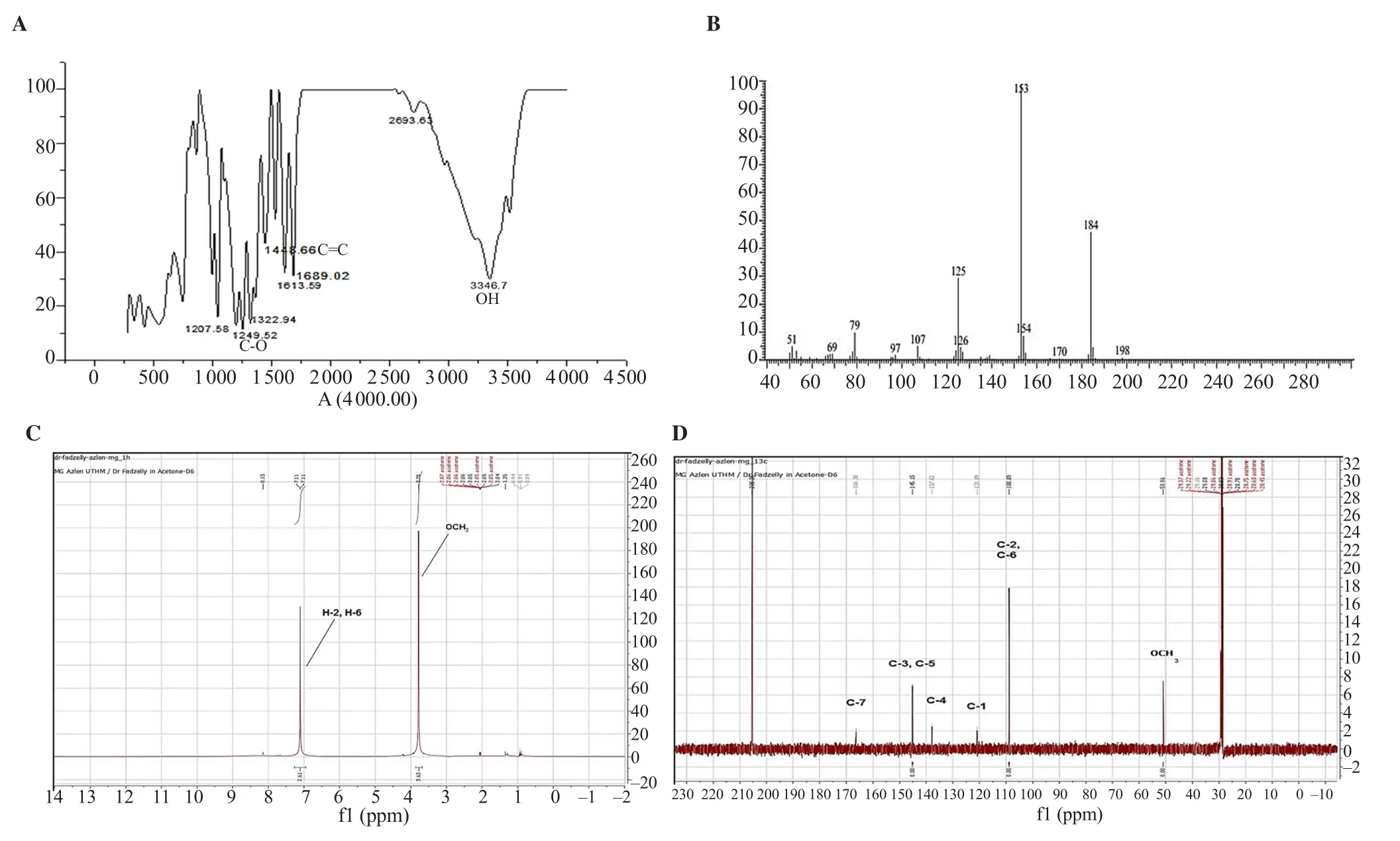
Figure 2. (A) Infrared (IR) spectrum, (B) electron impact-mass spectrometry (EI-MS) spectrum, (C) 1H nuclear magnetic resonance (NMR) spectrum, and (D)13C NMR spectrum of MG isolated from M. pajang kernel methanolic extract.
The1H NMR spectra showed (Figure 2C) two singlet peaks and each one was integrated as 2 and 3. These peaks corresponded to methoxy group, atδ3.76 (3H, s) andδ7.00 (2H, s, H-2, H-6)respectively. Aromatic protons H-2 and H-6 were overlapped due to the symmetrical position of two equivalent protons. Meanwhile,13C NMR spectrum (Figure 2D) showed the presence of six peaks atδ50.96 (OCH3), 108.89 (C-2, C-6), 120.00 (C-4), 137.00 (C-1), 145.15 (C-3, C-5), and 166.30 (C=O). The carbons signals of C-2, C-6 and C-3, C-5 were overlapped more intensely due to the symmetry of the structure. The most deshelled carbon appeared atδ166.40, which corresponded to carbonyl (C=O) group while methoxy group was focused at the more up field region ofδ50.96.The complete assignment of proton and carbon was summarized and presented in Table 1. Peak observed atδ7.11 illustrates two aromatic protons (H) at positions 2 and 6 with symmetrical shape.Hence, the same signals were recorded from both protons. In the higher field region, another peak of proton appeared which was from methoxy protons atδ3.84. Meanwhile, the carbon of13C NMR spectrum of MG exhibited the signals for seven types of carbons(Figure 2D). The peaks were identified as the following; atδ166.30 C=O (carbonyl ester),δ145.15 (two symmetrical oxygenated aromatic carbons at C-3 and C-5),δ137.00 (one oxygenatedaromatic carbon at C-4),δ120.00 (one quaternary aromatic carbon at C-1),δ108.89 (two symmetrical aromatic methane carbons at C-2 and C-6), andδ50.96 (-OCH3).

Table 1. Assignment of 1H -NMR peak position and 13C of methyl gallate from Mangifera pajang kernel and Spondias pinnata[27].
3.2. Cytotoxic effect of MG on MCF-7 and MCF-10A
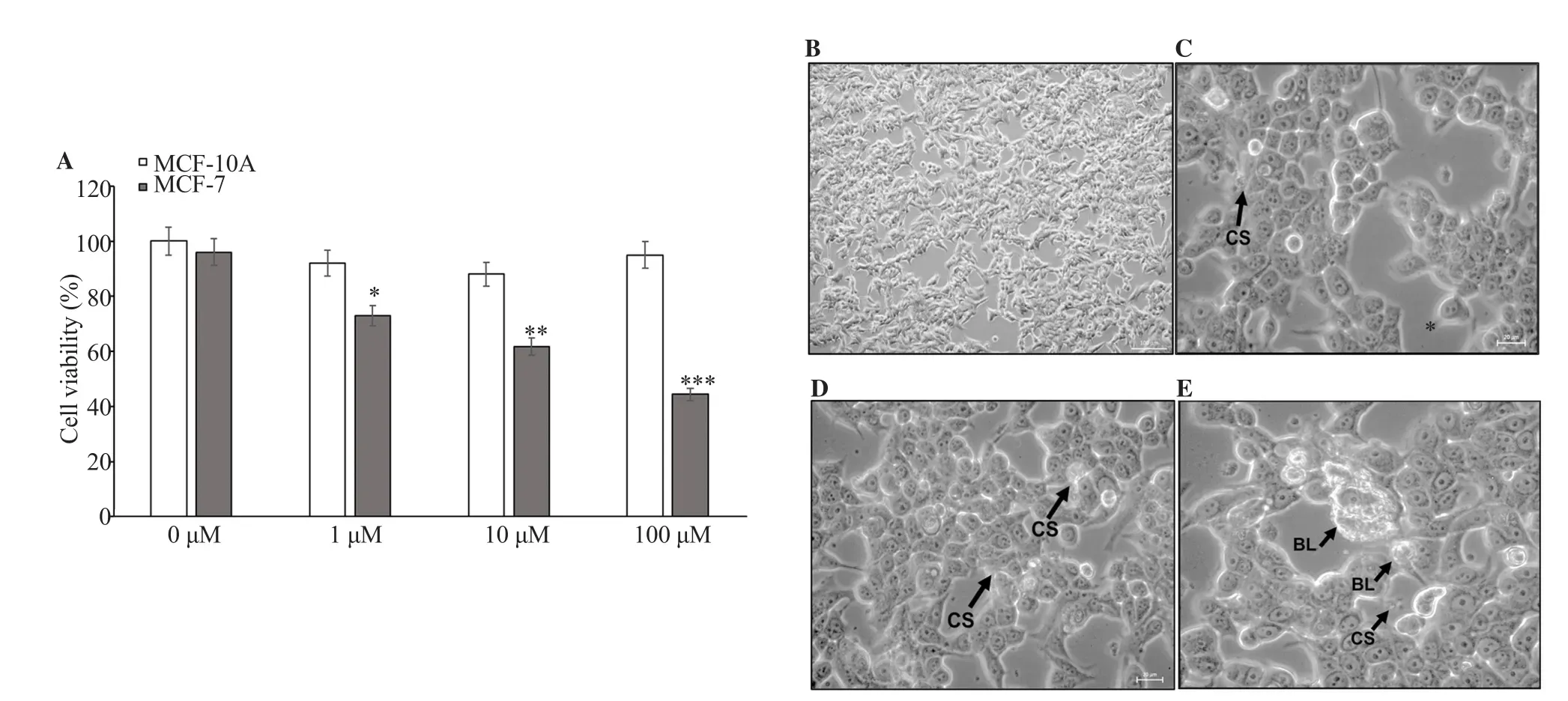
Figure 3. (A) Cytotoxicity of MG on MCF-7 and MCF-10A cells was estimated by MTT assay after 48 h of treatment. Cell viability was examined by MTT assays. (B-E) Morphological changes of MCF-7 cells after 48 h treatment with 0, 1, 10, and 100 μM MG. Apoptosis characteristics, including cell shrinkage(CS) and blebbing of cell membrane (BL) (black arrows) were observed. (B) 100× magnification; (C-E) 400× magnification. *: P<0.05, **: P<0.01, ***: P<0.001 compared with 0 μM.
3.3. Effect of MG on cell cycle in MCF-7 cells
At 48 h, all concentrations of MG reduced the cell population in the G0/G1phase (Figure 4A), meanwhile the reduction of cell percentage was significant (P<0.05) at 100 μM (Figure 4B). The accumulation of cells in the G0/G1phase indicated a prohibition of transition into the S phase induced by MG treatment (Figure 4A). However, MCF-7 cells were able to overcome the effect of MG treatment at 1 μM in the S phase and remained unchanged after 48 h of incubation. A significant decrease of cell population in the S phase was observed at 10 and 100 μM of MG compared with untreated cells (Figure 4B). Surprisingly, MG at 10 μM showed a significant increment in the cell population in the G2/M phase compared to the control. Meanwhile, the G2/M phase cell population was significantly (P<0.05) reduced after 48 h of treatment with 100 μM of MG (Figure 4B).

Figure 4. Cell cycle analysis of MCF-7 breast cancer cells treated with MG isolated from M. pajang at 48 h. Effects of MG on the cell cycle distribution in MCF-7 cells were analyzed using Muse Cell Cycle Analyzer. (A) DNA histogram displays cell cycle phase distribution of control (0 μM of MG) and MG-treated cells at 48 h. (B) Bar charts represent the percentage of cell populations in MCF-7 cells treated with different concentrations of MG at 48 h. Each value represents mean ± standard deviation (SD) (n=3). One-way ANOVA followed by Tukey’s multiple comparison test was used to calculate the statistical differences. *: P<0.05, **: P<0.01 compared with 0 μM.
3.4. Effect of MG on apoptosis in MCF-7 cells
MCF-7 cells were incubated with different concentrations of MG for 48 h and stained with annexin Ⅴ-FITC/propidium, followed by flow-cytometry analysis. As presented in Figure 5, the induction of early and late apoptosis of MCF-7 cells treated with MG was in a dose-dependent manner. Incubating MCF-7 cells with 10 and 100 μM of MG for 48 h significantly increased (P<0.05) the percentage of early apoptotic cells (4.25% and 12.10%, respectively),compared with the control (Figure 5A , 5B). At 1 μM, MG induced significant late apoptosis with 7.60%. When the concentration of MG was increased to 10 and 100 μM, a further significant (P<0.05)increment to 19.35% and 45.00% of late apoptosis was detected(Figure 5A, 5B).
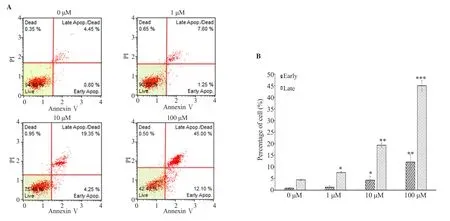
Figure 5. Apoptosis analysis of MCF-7 cells treated with different concentrations of MG. (A) flow cytometry profile of the cells stained with annexin V/propidium iodide (PI) after 48 h of treatment with different concentrations of MG (0-100 μM). (B) Percentage of cells undergoing early apoptosis and late apoptosis. MCF-7 cells grown in 0 μM of MG only was used as the vehicle control. Each value represents mean±SD (n=3). One-way ANOVA followed by Tukey’s multiple comparison test was used to calculate the statistical differences. *: P<0.05, **: P<0.01, ***: P<0.001 compared with 0 μM.
3.5. Effect of MG on intracellular ROS and antioxidant enzymes in MCF-7 cells
MG evoked an upregulation in the production of intracellular ROS in MCF-7 cells, compared to untreated control cells(Figure 6A). After 48 h of treatment with MG, the increment of fluorescence intensity in a dose-response fashion indicated high levels of intracellular ROS. At 100 μM of MG, ROS generation was significantly increased (P<0.001). As illustrated in Figure 6B,CAT level was significantly decreased (P<0.05) at 100 μM of MG,while GPx activity was not affected from 1 μM to 100 μM (Figure 6C). However, MG treatment induced a significant reduction(P<0.05) in SOD enzyme activities at 10 and 100 μM (Figure 6D).

Figure 6. Modulation of intracellular reactive oxygen species (ROS) and antioxidant enzymes in MCF-7 cells post 48 h treatment with different concentrations of MG. (A) Production of intracellular ROS, (B) Catalase (CAT) activity of the cells, (C) Glutathione peroxidase (GPx) activity of the cells, (D) Superoxide dismutase (SOD) activity of the cells. Each value represents mean±SD (n=3). One-way ANOVA followed by Tukey’s multiple comparison test was used to calculate the statistical differences. *: P<0.05, **: P<0.01, ***: P<0.001 compared with 0 μM.
4. Discussion
In the last three decades, the emphasis on discovering natural products as anti-cancerous agents has been continuous[13]. The previous scientific reports of M. pajang extract have documented its ability as a free radical scavenger and inhibitor for cancer cell proliferation[14].However, utilizing the crude extract of M. pajang could be accompanied by other unfunctional compounds which probably cause harm to healthy tissue. Therefore, our current research is designed to isolate and characterize the active anti-breast cancer compound from M. pajang kernel extract. The highest yield extract was obtained from methanol extraction. The fraction of methanolic extract of M. pajang kernel was subjected to different isolation methods and one compound was isolated for further anti-tumor investigations. As previously reported, methanolic extraction of M. pajang kernel improved the extraction yield, which ultimately led to higher antioxidant activity[15].
The methyl ester of gallic acid namely, MG, has been found and identified using different analytical methods such as NMR[16].Compared with previous NMR data of MG, the peaks of the proton(H) of isolated MG from Labisia pumila atδ7.0 showed two protons(H) at positions 2 and 6, while the other proton was atδ3.80 bonded with OCH3[17]. Moreover, MG isolated from seed coats of Givotia rottleriformis Griff. exhibited two protons (H) peaksδ7.26 at positions 2 and 6[18]. Our findings were in agreement with Yuan et al.[19], where the NMR data of isolated MG from Toxicodendron sylvestre, showed peakδ7.1 andδ3.8. These peaks corresponded to protons at positions 2 and 6, and H bonded to OCH3. In the current study, the carbon peaks of13C NMR spectrum of MG exhibited the signals for seven types of carbons. These peaks were in accordance with isolated MG from the methanol extract of Labisia pumila[17], seed coats of Givotia rottleriformis Griff.[18], and Toxicodenron sylvestre[19].
Phenolic compounds, synthesized by plant cells, have shown to be effective against tumor cell proliferation. Gallic acid has been studied extensively against different cancer cells including, the colon, prostate,cervical, and breast. Derived from gallic acid, MG, in previous reports,has caused cell toxicity in EL-4 mouse lymphoma cells, U373 glioma cells, human cervix adenocarcinoma cells (HeLa), and human fibroblast cells (L-132)[20–22]. However, MG activity against breast cancer cell lines has not been extensively studied. In the current study, we observed the cytotoxic effect of MG isolated from M. pajang kernel extract on the estrogen-receptor-positive (MCF-7) cells. Similarly, the isolated MG from Acacia hydaspica R.Parker demonstrated approximately 29.5% cell inhibition against triple-negative breast cancer (MDAMB-231) cell lines at 50 μM after 48 h incubation[23]. The previous results indicated that MG could induce more effective inhibition against MDA-MB-231 than MCF-7. Surprisingly, apoptotic bodies and formation of DNA fragmentation were not observed. The reason behind that is due to the lack of caspase-3, an essential component for apoptotic cascade in MCF-7 cells[24]. Under normal cellular apoptosis,caspase-3 induces certain morphological and biochemical changes associated with apoptosis execution[25].
The involvement of other caspases such as caspase-7 or caspase-6 induced by MG has been speculated in apoptosis and cell cycle arrest.Breast cancer cell proliferation is mainly correlated with four cell cycle phases (sub G1, G1, S, G2/M). To study inhibitory effect against MCF-7 cells by MG isolated from M. pajang kernel extract, we performed cell cycle distribution analysis using Muse cell analyzer with PI staining. The cell cycle arrest by MG was concentration-dependent. As previously reported, MG at 50 μM has inhibited 3T3-L1 adipogenesis cell cycle progression at the G0/G1phase with 36% of cell population after 24 h[26]. However, a serial concentration of MG ranging from 0 to 50 μg/mL failed to induce any cell cycle arrest in EL-4 (mouse lymphoma cell line) after 24 h of treatment[22]. Under similar conditions with our experiments, isolated MG from Spondias pinnata caused a reduction in the G2/M phase after treating U87 cells with 100 μM of MG for 24 h[27]. Gallic acid, which belongs to the same family of MG, has been reported to induce cell cycle arrest in MCF-7 cells at the G0/G1phase via inhibiting cyclin A and cyclin B1[28]. Interestingly,the low cell population at the G2/M phase after treatment with MG indicated DNA damage which prevented the occurrence of mitosis.Taken together, isolated MG from M. pajang kernel extract induced inhibition against MCF-7 cells growth through apoptosis more than cell cycle arrest. Therefore, the apoptotic cell death of MG was further analyzed.
这是诗中“我”作为主体的第二次状态陈述,S2为“长大后”的“我”,O2为价值对象“新娘”。在这一阶段,即“我”的青壮年时期,“我”与“新娘”处于析取状态;
Inducing apoptosis has been considered as an essential key mechanism by which anti-cancer treatment is applicable[29]. FITC-conjoined with annexin Ⅴ and PI iodide has been utilized to explore living cells in early and late apoptosis. Located in the inner membrane of cells, phosphatidylserine is transferred into the external portion where it can be detected by its high affinity for annexin Ⅴ-FITC.However, late apoptosis can be observed during the binding of PI to the cellular DNA after the decomposition of the cell membrane. The current study showed that MG induced early apoptosis in MCF-7 cells.Our results were in agreement with Chaudhuri and his colleagues where isolated MG from Spondias pinnata induced early apoptosis in U87 cells in a dose-response fashion[27]. The regulation of apoptosis has been associated with the expression of Bcl-2 family proteins including, Bcl-2, Bax, Bid, and Bcl-2-extra-large. It has been reported previously that MG down-regulated the expression of Bcl-2 and upregulated the expression of Bax in U87 cancer cells[27]. Moreover, MG induced apoptosis in hepatocellular carcinoma through modulating the levels of Bcl-2, Bax, and Bad-ligands and activating ROS pathways[30].
Oxidative stress is one of the essential cellular metabolic events for inducing apoptosis. The disturbance of pro-oxidant and antioxidant balance in favor of the former elevates the level of free radicals, leading to potential damage[3]. ROS has been continuously associated with carcinogenesis, cancer therapy, and prevention. Therefore, we examined MG isolated from kernel extract of M. pajang on the production of intracellular ROS generation in MCF-7 cells using dichloro-dihydrofluorescein diacetate assay to illustrate the involvement of oxidative stress in cell death. MG evoked an upregulation in the production of intracellular ROS in MCF-7 cells, compared to untreated control cells.Under similar experimental conditions, MG increases ROS levels in hepatocellular carcinoma at 40 μg/mL[30]. Isolated from Syzigium coriaceum leaf extract, MG upregulated ROS production in HepG2 cells at serial concentrations ranging from 20 to 100 μg/mL[31]. In addition, the induction of oxidative stress by chemotherapeutic agents such as cisplatin, anthracyclines, and bleomycin has shown to be effective in certain tumors[32]. The immunotherapy effect of MG has improved the effect of cisplatin in lymphoma treatment with no renal toxicity[33].
In general, phenolic compounds have demonstrated antioxidant and pro-oxidant properties based on pH environment[34]. Despite the reported antioxidant activities of MG[12], its impact on the antioxidant status of MCF-7 cell line has been examined. Our results illustrated that MG decreased CAT and SOD without affecting the levels of GPx in MCF-7 cells. In comparison with our findings, treated HepG2 cells for 48 h with isolated MG from Syzigium coriaceum showed reduced levels of SOD and GPx at 20 μg/mL[31]. Moreover, gallic acid, which is related to MG, inhibited SOD and GPx in Caco-2 cells for 48 h. These findings could be attributed to the ability of phenolic acids in generating phenoxy and superoxide free radicals at alkaline pH. In this sense, malignant cells rely on a higher alkaline intracellular environment for proliferation, compared with non-malignant cells[35].This could explain the pro-oxidant activities of MG in cancer cell lines and specifically in MCF-7 cells. Also, MG was reported to inhibit hepatocellular carcinoma proliferation through oxidative stress[30]. We postulated that MG isolated from M. pajang kernel extract induced oxidative stress which yet was manifested in apoptosis and cell cycle arrest of MCF-7 cell line.
Although the current investigation has found that MG suppressed the proliferation of MCF-7 cells via oxidative stress and apoptosis,this study had a few limitations. First, the MG treatment with MCF-7 cells was short in period (48 h) and a longer incubation of MG with the cells could be more beneficial. Second, apoptotic proteins such as Bcl-2, p53, and Bcl-xL were not detected using Western-blotting in this study, which may have confirmed our apoptosis findings. Gauging the transcription factors associated with oxidative stress such as nuclear factor erythroid 2-related factor 2 would illustrate clearly the mechanism of MG in suppressing the proliferation of MCF-7 cells.Therefore, further investigations are needed to determine the exact mechanism of actions of MG isolated from M. pajang kernel extract.
The results of this experimental work demonstrated that isolated MG from the kernel of M. pajang inhibited the proliferation of MCF-7 breast cancer cells. This bioactive behavior was governed by cell cycle arrest at the G2/M phase, which resulted from apoptosis induction. The alteration of oxidative stress status in treated MCF-7 cells with MG increased ROS levels and decreased CAT and SOD. Moreover, our data suggest that MG could be a potential anti-cancer agent for the therapy of mammary tumors.
Conflict of interest statement
The authors declare no conflict of interest.
Acknowledgments
Universiti Tun Hussein Onn Malaysia, Universiti Putra Malaysia,and Universiti Teknologi Mara are highly acknowledged for use of laboratories and technical assistance.
Funding
This study was funded by Fundamental Research Grant Scheme(FRGS Vot 1560) as well as Universiti Tun Hussein Onn Malaysia Contract grant (H277 & U688).
Authors’ contributions
YR, ACR, and MFAB designed the study. NKK, JS, and YHT carried out the data collection. AF and MSE conducted data analysis. YR and MFAB conducted data interpretation. YR, MFAB and ACR wrote and edited the manuscript.
 Asian Pacific Journal of Tropical Biomedicine2022年4期
Asian Pacific Journal of Tropical Biomedicine2022年4期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Cardiovascular protective properties of gastrodin
- Lipid-lowering effect of Oroxylum indicum (L.) Kurz extract in hyperlipidemic mice
- Analgesic-like activity of perillyl acetate: In vivo and in silico studies
- Anti-obesity effect and UHPLC-QTOF-MS/MS based metabolite profiling of Solanum nigrum leaf extract
